A Multi-Host Approach to Quantitatively Assess the Role of Dogs as Sentinels for Rift Valley Fever Virus (RVFV) Surveillance in Madagascar
Abstract
1. Introduction
2. Materials and Methods
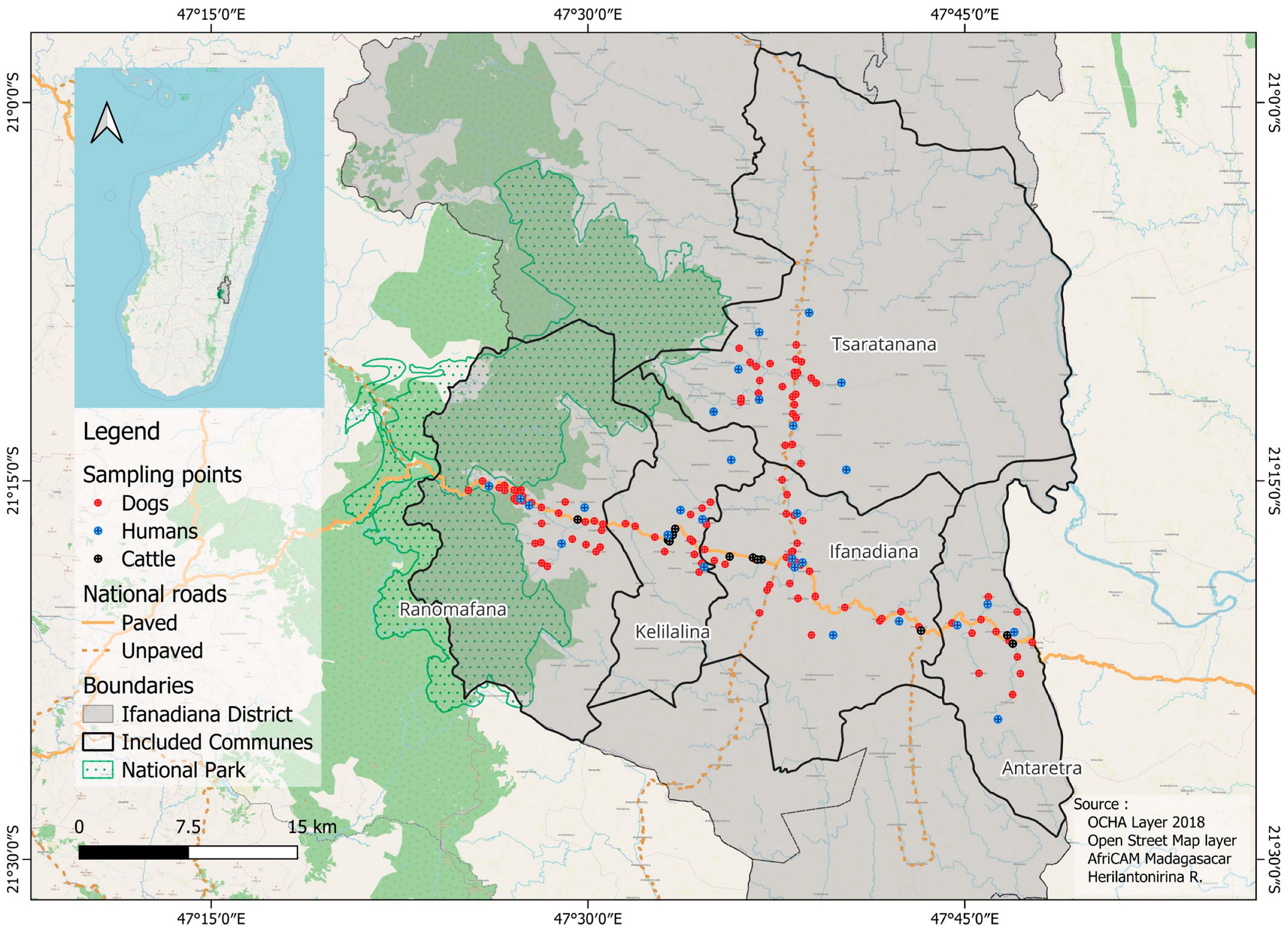
2.1. Study Site and Population
2.2. Serological Data
2.2.1. Dog Data
2.2.2. Cattle Data
2.2.3. Human Data
2.3. Serological Analyses
2.3.1. Dog and Cattle Sera
2.3.2. Human Dry Blood Spots
2.4. Serocatalytic Models
2.5. Hypotheses
2.6. Model Fitting and Comparison
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACF | Autocorrelation Function |
| cELISA | competitive Enzyme-Linked Immunosorbent Assay |
| CI | Confidence intervals |
| CrI | Credible Intervals |
| DBS | Dry Blood Spot |
| DENV | Dengue Virus |
| DIC | Deviance Information Criterion |
| FOI | Force Of Infection |
| H0 | null hypothesis |
| H1 | first alternative hypothesis |
| H2 | second alternative hypothesis |
| IHOPE | Ifanadiana Health Outcomes and Prosperity longitudinal Evaluation |
| IgG | Immunoglobulins of type G |
| INSTAT | Institut National de la Statistique |
| IPM | Institut Pasteur de Madagascar |
| JEV | Japanese Encephalitis Virus |
| MIA | microsphere-based immunoassay |
| MCMC | Markov Chain Monte Carlo |
| NEFF | Effective Sample Size |
| NP | Nucleoprotein |
| RVF | Rift Valley Fever |
| RVFV | Rift Valley Fever Virus |
| WNV | West Nile Virus |
References
- World Bank. World Bank Navigating Two Decades of High Poverty and Charting a Course for Change in Madagascar: Poverty and Equity Assessment, February 2024; World Bank: Washington, DC, USA, 2024; pp. 14–52. [Google Scholar]
- Bonds, M.H.; Ouenzar, M.A.; Garchitorena, A.; Cordier, L.F.; McCarty, M.G.; Rich, M.L.; Andriamihaja, B.; Haruna, J.; Farmer, P.E. Madagascar Can Build Stronger Health Systems to Fight Plague and Prevent the next Epidemic. PLoS Negl. Trop. Dis. 2018, 12, e0006131. [Google Scholar] [CrossRef]
- Neo, J.P.S.; Tan, B.H. The Use of Animals as a Surveillance Tool for Monitoring Environmental Health Hazards, Human Health Hazards and Bioterrorism. Vet. Microbiol. 2017, 203, 40–48. [Google Scholar] [CrossRef]
- Halliday, J.E.B.; Meredith, A.L.; Knobel, D.L.; Shaw, D.J.; Bronsvoort, B.M.d.C.; Cleaveland, S. A Framework for Evaluating Animals as Sentinels for Infectious Disease Surveillance. J. R. Soc. Interface 2007, 4, 973–984. [Google Scholar] [CrossRef]
- Resnick, M.P.; Grunenwald, P.; Blackmar, D.; Hailey, C.; Bueno, R.; Murray, K.O. Juvenile Dogs as Potential Sentinels for West Nile Virus Surveillance. Zoonoses Public Health 2008, 55, 443–447. [Google Scholar] [CrossRef]
- Bowser, N.H.; Anderson, N.E. Dogs (Canis familiaris) as Sentinels for Human Infectious Disease and Application to Canadian Populations: A Systematic Review. Vet. Sci. 2018, 5, 83. [Google Scholar] [CrossRef]
- Davoust, B.; Leparc-Goffart, I.; Demoncheaux, J.-P.; Tine, R.; Diarra, M.; Trombini, G.; Mediannikov, O.; Marié, J.-L. Serologic Surveillance for West Nile Virus in Dogs, Africa. Emerg. Infect. Dis. 2014, 20, 1415–1417. [Google Scholar] [CrossRef]
- Durand, B.; Haskouri, H.; Lowenski, S.; Vachiery, N.; Beck, C.; Lecollinet, S. Seroprevalence of West Nile and Usutu Viruses in Military Working Horses and Dogs, Morocco, 2012: Dog as an Alternative WNV Sentinel Species? Epidemiol. Infect. 2016, 144, 1857–1864. [Google Scholar] [CrossRef]
- Thongyuan, S.; Kittayapong, P. First Evidence of Dengue Infection in Domestic Dogs Living in Different Ecological Settings in Thailand. PLoS ONE 2017, 12, e0180013. [Google Scholar] [CrossRef]
- Shimoda, H.; Ohno, Y.; Mochizuki, M.; Iwata, H.; Okuda, M.; Maeda, K. Dogs as Sentinels for Human Infection with Japanese Encephalitis Virus. Emerg. Infect. Dis. 2010, 16, 1137–1139. [Google Scholar] [CrossRef]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef]
- Daubney, R.; Hudson, J.R.; Garnham, P.C. Enzootic Hepatitis or Rift Valley Fever. An Undescribed Virus Disease of Sheep Cattle and Man from East Africa. J. Pathol. Bacteriol. 1931, 34, 545–579. [Google Scholar] [CrossRef]
- Budasha, N.; Gonzalez, J.; Sebhatu, T.; Ezama, A. Rift Valley Fever Seroprevalence and Abortion Frequency among Livestock of Kisoro District, South Western Uganda (2016): A Prerequisite for Zoonotic Infection. BMC Vet. Res. 2018, 14, 271. [Google Scholar] [CrossRef]
- Anywaine, Z.; Lule, S.A.; Hansen, C.; Warimwe, G.; Elliott, A. Clinical Manifestations of Rift Valley Fever in Humans: Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2022, 16, e0010233. [Google Scholar] [CrossRef]
- Fontenille, D. Etude Des Circuits de Vection d’arbovirus, à Madagascar; Institut Pasteur de Madagascar: Antananarivo, Madagascar, 1989. [Google Scholar]
- Morvan, J.; Saluzzo, J.F.; Fontenille, D.; Rollin, P.; Coulangés, P. Rift valley fever on the east coast of Madagascar. Res. Virol. 1991, 142, 475–482. [Google Scholar] [CrossRef]
- Morvan, J.; Rollin, P.; Laventure, S.; Rakotoarivony, I.; Guillot, J. Rift Valley fever epizootic in the central highlands of Madagascar. Res. Virol. 1992, 143, 407–415. [Google Scholar] [CrossRef]
- Andriamandimby, S.F.; Randrianarivo-Solofoniaina, A.E.; Jeanmaire, E.M.; Ravololomanana, L.; Razafimanantsoa, L.T.; Rakotojoelinandrasana, T.; Razainirina, J.; Hoffmann, J.; Ravalohery, J.-P.; Rafisandratantsoa, J.-T.; et al. Rift Valley Fever during Rainy Seasons, Madagascar, 2008 and 2009. Emerg. Infect. Dis. 2010, 16, 963–970. [Google Scholar] [CrossRef]
- Harimanana, A.; Andriamandimby, S.; Ranoaritiana, D.; Randrianasolo, L.; Irinantenaina, J.; Ranoelison, N.; Rafisandrantatsoa, J.; Ankasitrahana, M.; Raherinandrasana, A.; Andriamahatana, M.; et al. The Re-Emergence of Rift Valley Fever in Mananjary District, Madagascar in 2021: A Call for Action. Pathogens 2024, 13, 257. [Google Scholar] [CrossRef]
- Ratsitorahina, M.; Rasambainarivo, J.H.; Raharimanana, S.; Rakotonandrasana, H.; Andriamiarisoa, M.-P.; Rakalomanana, F.A.; Richard, V. Dog Ecology and Demography in Antananarivo, 2007. BMC Vet. Res. 2009, 5, 21. [Google Scholar] [CrossRef]
- Kshirsagar, A.R.; Applebaum, J.W.; Randriana, Z.; Rajaonarivelo, T.; Rafaliarison, R.R.; Farris, Z.J.; Valenta, K. Human-Dog Relationships across Communities Surrounding Ranomafana and Andasibe-Mantadia National Parks, Madagascar. J. Ethnobiol. 2020, 40, 483–498. [Google Scholar] [CrossRef]
- Cleaveland, S.; Meslin, F.X.; Breiman, R. Dogs Can Play Useful Role as Sentinel Hosts for Disease. Nature 2006, 440, 605. [Google Scholar] [CrossRef]
- Becquart, P.; Bohou Kombila, L.; Mebaley, T.N.; Paupy, C.; Garcia, D.; Nesi, N.; Olive, M.-M.; Vanhomwegen, J.; Boundenga, L.; Mombo, I.M.; et al. Evidence for Circulation of Rift Valley Fever Virus in Wildlife and Domestic Animals in a Forest Environment in Gabon, Central Africa. PLoS Negl. Trop. Dis. 2024, 18, e0011756. [Google Scholar] [CrossRef]
- Hoogstraal, H.; Meegan, J.M.; Khalil, G.M.; Adham, F.K. The Rift Valley Fever Epizootic in Egypt 1977–1978. 2. Ecological and Entomological Studies. Trans. R. Soc. Trop. Med. Hyg. 1979, 73, 624–629. [Google Scholar] [CrossRef]
- Ihantamalala, F.A.; Bonds, M.H.; Randriamihaja, M.; Rakotonirina, L.; Herbreteau, V.; Révillion, C.; Rakotoarimanana, S.; Cowley, G.; Andriatiana, T.A.; Mayfield, A.; et al. Geographic Barriers to Establishing a Successful Hospital Referral System in Rural Madagascar. BMJ Glob. Health 2021, 6, e007145. [Google Scholar] [CrossRef]
- Evans, M.V.; Bonds, M.H.; Cordier, L.F.; Drake, J.M.; Ihantamalala, F.; Haruna, J.; Miller, A.C.; Murdock, C.C.; Randriamanambtsoa, M.; Raza-Fanomezanjanahary, E.M.; et al. Socio-Demographic, Not Environmental, Risk Factors Explain Fine-Scale Spatial Patterns of Diarrhoeal Disease in Ifanadiana, Rural Madagascar. Proc. R. Soc. B Biol. Sci. 2021, 288, 20202501. [Google Scholar] [CrossRef]
- CREAM. Monographie de la Région Vatovavy Fitovinany; Centre de Recherches, d’Etudes et d’Appui à l’analyse Economique à Madagascar: Antananarivo, Madagascar, 2013; pp. 20–34. [Google Scholar]
- Cordier, L.F.; Kalaris, K.; Rakotonanahary, R.J.L.; Rakotonirina, L.; Haruna, J.; Mayfield, A.; Marovavy, L.; McCarty, M.G.; Aina, A.T.; Ratsimbazafy, B.; et al. Networks of Care in Rural Madagascar for Achieving Universal Health Coverage in Ifanadiana District. Health Syst. Reform 2020, 6, e1841437. [Google Scholar] [CrossRef]
- Miller, A.C.; Garchitorena, A.; Rabeza, V.; Randriamanambintsoa, M.; Rahaniraka Razanadrakato, H.-T.; Cordier, L.; Ouenzar, M.A.; Murray, M.B.; Thomson, D.R.; Bonds, M.H. Cohort Profile: Ifanadiana Health Outcomes and Prosperity Longitudinal Evaluation (IHOPE). Int. J. Epidemiol. 2018, 47, 1394–1395e. [Google Scholar] [CrossRef]
- Olive, M.-M.; Chevalier, V.; Grosbois, V.; Tran, A.; Andriamandimby, S.-F.; Durand, B.; Ravalohery, J.-P.; Andriamamonjy, S.; Rakotomanana, F.; Rogier, C.; et al. Integrated Analysis of Environment, Cattle and Human Serological Data: Risks and Mechanisms of Transmission of Rift Valley Fever in Madagascar. PLoS Negl. Trop. Dis. 2016, 10, e0004827. [Google Scholar] [CrossRef]
- Miller, A.C.; Ramananjato, R.H.; Garchitorena, A.; Rabeza, V.R.; Gikic, D.; Cripps, A.; Cordier, L.; Razanadrakato, H.-T.R.; Randriamanambintsoa, M.; Hall, L.; et al. Baseline Population Health Conditions Ahead of a Health System Strengthening Program in Rural Madagascar. Glob. Health Action 2017, 10, 1329961. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, pdb.prot102269. [Google Scholar] [CrossRef]
- Melnykov, V.; Maitra, R. Finite Mixture Models and Model-Based Clustering. Stat. Surv. 2010, 4, 80–116. [Google Scholar] [CrossRef]
- Pérez-Ramírez, E.; Cano-Gómez, C.; Llorente, F.; Adžić, B.; Al Ameer, M.; Djadjovski, I.; Hage, J.; Mellouli, F.; Goletić, T.; Hovsepyan, H.; et al. External Quality Assessment of Rift Valley Fever Diagnosis in 17 Veterinary Laboratories of the Mediterranean and Black Sea Regions. PLoS ONE 2020, 15, e0239478. [Google Scholar] [CrossRef] [PubMed]
- Pédarrieu, A.; Mellouli, F.; Khallouki, H.; Zro, K.; Sebbar, G.; Sghaier, S.; Madani, H.; Bouayed, N.; Lô, M.; Diop, M.; et al. External Quality Assessment of Rift Valley Fever Diagnosis in Countries at Risk of the Disease: African, Indian Ocean and Middle-East Regions. PLoS ONE 2021, 16, e0251263. [Google Scholar] [CrossRef]
- Berguido, F.J.; Settypalli, T.B.K.; Mbuyi, C.G.T.; Bakhom, M.T.; van Vuren, P.J.; Pawęska, J.T.; Cattoli, G.; Grabherr, R.; Lamien, C.E. Development of a Luminex-Based Assay for the Detection of Anti-Capripoxvirus and Rift Valley Fever Virus Antibodies in Domestic Ruminants. Virol. J. 2024, 21, 335. [Google Scholar] [CrossRef]
- Hens, N.; Aerts, M.; Faes, C.; Shkedy, Z.; Lejeune, O.; Damme, P.V.; Beutels, P. Seventy-Five Years of Estimating the Force of Infection from Current Status Data. Epidemiol. Infect. 2010, 138, 802–812. [Google Scholar] [CrossRef]
- Brown, R.D.; Scott, G.R.; Dalling, T. Persistence of Antibodies to Rift Valley Fever in Man. Lancet 1957, 270, 345. [Google Scholar] [CrossRef]
- Matiko, M.K.; Salekwa, L.P.; Kasanga, C.J.; Kimera, S.I.; Evander, M.; Nyangi, W.P. Serological Evidence of Inter-Epizootic/Inter-Epidemic Circulation of Rift Valley Fever Virus in Domestic Cattle in Kyela and Morogoro, Tanzania. PLoS Negl. Trop. Dis. 2018, 12, e0006931. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Kortekaas, J.; Bowden, T.A.; Warimwe, G.M. Rift Valley Fever: Biology and Epidemiology. J. Gen. Virol. 2019, 100, 1187–1199. [Google Scholar] [CrossRef]
- Wright, D.; Allen, E.R.; Clark, M.H.A.; Gitonga, J.N.; Karanja, H.K.; Hulswit, R.J.G.; Taylor, I.; Biswas, S.; Marshall, J.; Mwololo, D.; et al. Naturally Acquired Rift Valley Fever Virus Neutralizing Antibodies Predominantly Target the Gn Glycoprotein. iScience 2020, 23, 101669. [Google Scholar] [CrossRef]
- Hay, J.A.; Routledge, I.; Takahashi, S. Serodynamics: A Primer and Synthetic Review of Methods for Epidemiological Inference Using Serological Data. Epidemics 2024, 49, 100806. [Google Scholar] [CrossRef]
- Cucunubá, Z.M.; Nouvellet, P.; Conteh, L.; Vera, M.J.; Angulo, V.M.; Dib, J.C.; Parra-Henao, G.J.; Basáñez, M.G. Modelling Historical Changes in the Force-of-Infection of Chagas Disease to Inform Control and Elimination Programmes: Application in Colombia. BMJ Glob. Health 2017, 2, e000345. [Google Scholar] [CrossRef] [PubMed]
- Jeanmaire, E.; Rabenarivahiny, R.; Biarmann, M.; Rabibisoa, L.; Ravaomanana, F.; Randriamparany, T.; Andriamandimby, S.; Diaw, C.; Fenozara, P.; De La Rocque, S.; et al. Prevalence of Rift Valley Fever Infection in Ruminants in Madagascar After the 2008 Outbreak. Vector-Borne Zoonotic Dis. 2011, 11, 395–402. [Google Scholar] [CrossRef]
- Gray, G.; Anderson, B.; LaBeaud, A.; Héraud, J.; Fèvre, E.; Andriamandimby, S.; Cook, E.; Dahir, S.; de Glanville, W.; Heil, G.; et al. Seroepidemiological Study of Interepidemic Rift Valley Fever Virus Infection Among Persons with Intense Ruminant Exposure in Madagascar and Kenya. Am. J. Trop. Med. Hyg. 2015, 93, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.; Warimwe, G.; Di Nardo, A.; Lyons, N.; Gubbins, S. Systematic Literature Review of Rift Valley Fever Virus Seroprevalence in Livestock, Wildlife and Humans in Africa from 1968 to 2016. PLoS Negl. Trop. Dis. 2018, 12, e0006627. [Google Scholar] [CrossRef]
- Ahmed Kamal, S. Observations on Rift Valley Fever Virus and Vaccines in Egypt. Virol. J. 2011, 8, 532. [Google Scholar] [CrossRef]
- Hartman, A. Rift Valley Fever. Clin. Lab. Med. 2017, 37, 285–301. [Google Scholar] [CrossRef]
- Chevalier, V.; Pépin, M.; Plée, L.; Lancelot, R. Rift Valley Fever—A Threat for Europe? Eurosurveillance 2010, 15, 19506. [Google Scholar] [CrossRef]
- Richards, S.L.; Ponnusamy, L.; Unnasch, T.R.; Hassan, H.K.; Apperson, C.S. Host-Feeding Patterns of Aedes albopictus (Diptera: Culicidae) in Relation to Availability of Human and Domestic Animals in Suburban Landscapes of Central North Carolina. J. Med. Entomol. 2006, 43, 543–551. [Google Scholar] [CrossRef]
- Ponlawat, A.; Harrington, L.C. Blood Feeding Patterns of Aedes aegypti and Aedes albopictus in Thailand. J. Med. Entomol. 2005, 42, 844–849. [Google Scholar] [CrossRef]
- Garcia-Rejon, J.E.; Blitvich, B.J.; Farfan-Ale, J.A.; Loroño-Pino, M.A.; Chi Chim, W.A.; Flores-Flores, L.F.; Rosado-Paredes, E.; Baak-Baak, C.; Perez-Mutul, J.; Suarez-Solis, V.; et al. Host-Feeding Preference of the Mosquito, Culex Quinquefasciatus, in Yucatan State, Mexico. J. Insect Sci. 2010, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, J.; Kant, J.; Vloet, R.; Cêtre-Sossah, C.; Marianneau, P.; Lacôte, S.; Banyard, A.; Jeffries, C.; Eiden, M.; Groschup, M.; et al. European Ring Trial to Evaluate ELISAs for the Diagnosis of Infection with Rift Valley Fever Virus. J. Virol. Methods 2012, 187, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Surtees, R.; Stern, D.; Ahrens, K.; Kromarek, N.; Lander, A.; Kreher, P.; Weiss, S.; Hewson, R.; Punch, E.K.; Barr, J.N.; et al. Development of a Multiplex Microsphere Immunoassay for the Detection of Antibodies against Highly Pathogenic Viruses in Human and Animal Serum Samples. PLoS Negl. Trop. Dis. 2020, 14, e0008699. [Google Scholar] [CrossRef]
- Geering, W.A.; Davies, F.G.; Martin, V. Preparation of Rift Valley Fever Contingency Plans; FAO Animal Health Manual: Rome, Italy, 2002; ISBN 978-92-5-104821-4. [Google Scholar]
- Chevalier, V.; Rakotondrafara, T.; Jourdan, M.; Héraud, J.; Andriamanivo, H.; Durand, B.; Ravaomanana, J.; Rollin, P.; Rakotondravao, R. An Unexpected Recurrent Transmission of Rift Valley Fever Virus in Cattle in a Temperate and Mountainous Area of Madagascar. PLoS Negl. Trop. Dis. 2011, 5, e1423. [Google Scholar] [CrossRef]
- Nicolas, G.; Durand, B.; Rakotoarimanana, T.; Lacôte, S.; Chevalier, V.; Marianneau, P. A 3-Year Serological and Virological Cattle Follow-Up in Madagascar Highlands Suggests a Non-Classical Transmission Route of Rift Valley Fever Virus. Am. J. Trop. Med. Hyg. 2013, 90, 265–266. [Google Scholar] [CrossRef][Green Version]
- Olive, M.-M.; Grosbois, V.; Tran, A.; Nomenjanahary, L.A.; Rakotoarinoro, M.; Andriamandimby, S.-F.; Rogier, C.; Heraud, J.-M.; Chevalier, V. Reconstruction of Rift Valley Fever Transmission Dynamics in Madagascar: Estimation of Force of Infection from Seroprevalence Surveys Using Bayesian Modelling. Sci. Rep. 2017, 7, 39870. [Google Scholar] [CrossRef]
- Tantely, L.; Rakotoniaina, J.-C.; Tata, E.; Andrianaivolambo, L.; Razafindrasata, F.; Fontenille, D.; Élissa, N. Biology of Mosquitoes That Are Potential Vectors of Rift Valley Fever Virus in Different Biotopes of the Central Highlands of Madagascar. J. Med. Entomol. 2013, 50, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Toma, B. La fonction sentinelle en epidemiologie. Epidémiol. et santé anim. 2009, 56, 5–14. [Google Scholar]
- Broban, A.; Olive, M.-M.; Tantely, M.L.; Dorsemans, A.-C.; Rakotomanana, F.; Ravalohery, J.-P.; Rogier, C.; Heraud, J.-M.; Andriamandimby, S.F. Seroprevalence of IgG Antibodies Directed against Dengue, Chikungunya and West Nile Viruses and Associated Risk Factors in Madagascar, 2011 to 2013. Viruses 2023, 15, 1707. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, V.; Marsot, M.; Molia, S.; Rasamoelina, H.; Rakotondravao, R.; Pedrono, M.; Lowenski, S.; Durand, B.; Lecollinet, S.; Beck, C. Serological Evidence of West Nile and Usutu Viruses Circulation in Domestic and Wild Birds in Wetlands of Mali and Madagascar in 2008. Int. J. Environ. Res. Public. Health 2020, 17, 1998. [Google Scholar] [CrossRef]
- Rajerison, M.; Andrianaivoarimanana, V.; Ratsitorahina, M.; Rahelinirina, S.; Chanteau, S.; Telfer, S.; Rahalison, L. Field Assessment of Dog as Sentinel Animal for Plague in Endemic Foci of Madagascar. Integr. Zool. 2021, 16, 886–892. [Google Scholar] [CrossRef] [PubMed]



| DOGS | CATTLE | HUMANS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Birth Year | Tested | Positive | % Positive [95% CI] | Tested | Positive | % Positive [95% CI] | Tested | Positive | % Positive [95% CI] |
| 2011– 2016 | 64 | 3 | 4.69 [0.1–13.1] | 11 | 4 | 36.4 [10.1–69.2] | 305 | 56 | 18.4 [14.2–23.2] |
| 2017 | 18 | 0 | 0[0–18.6] | 9 | 1 | 11.1 [0.3–48.3] | 56 | 10 | 17.9 [8.9–30.4] |
| 2018 | 26 | 0 | 0[0–13.2] | 13 | 5 | 38.46 [13.9–68.4] | 42 | 7 | 16.7 [6.9–31.4] |
| 2019 | 31 | 5 | 16.1 [5.4–33.7] | 17 | 6 | 35.3 [14.2–61.7] | 37 | 5 | 13.5 [4.5–28.8] |
| 2020 | 61 | 5 | 8.2 [2.7–18.1] | 11 | 4 | 36.4 [10.9–69.2] | 38 | 5 | 13.2 [4.4–28.1] |
| 2021 | 58 | 6 | 10.3 [3.9–21.2] | 23 | 8 | 34.8 [16.4–57.3] | 8 | 3 | 37.5 [8.5–75.5] |
| 2022 | 139 | 4 | 2.88 [0.8–7.2] | 19 | 3 | 15.8 [3.4–39.6] | 0 | 0 | - |
| 2023 | 116 | 0 | 0[0–3.1] | 32 | 2 | 6.3 [0.8–20.8] | 0 | 0 | - |
| TOTAL | 513 | 23 | 4.5 [2.9–6.7] | 135 | 33 | 24.4 [17.5–32.6] | 486 | 86 | 17.7 [14.4–21.9] |
| Model | Description of the Relationship of RVFV FOI in Dogs to | Number of Estimated Parameters | DIC | |
|---|---|---|---|---|
| RVFV FOI in Humans | RVFV FOI in Cattle | |||
| Model 0 | H0 | H0 | 23 | 810.7 |
| Model 1 | H1 | H0 | 18 | 801.8 |
| Model 2 | H2 | H0 | 26 | 819.9 |
| Model 3 | H0 | H1 | 16 | 798.9 |
| Model 4 | H1 | H1 | 11 | 790.3 |
| Model 5 | H2 | H1 | 19 | 809.4 |
| Model 6 | H0 | H2 | 24 | 818.2 |
| Model 7 | H1 | H2 | 19 | 813.8 |
| Model 8 | H2 | H2 | 19 | 809.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramaroson, H.S.; Garchitorena, A.; Lacoste, V.; Andriamandimby, S.F.; Schoenhals, M.; Bastard, J.; Albrechtova, K.; Chevalier, L.J.G.; Rakotomanana, D.; Rasamoel, P.d.V.; et al. A Multi-Host Approach to Quantitatively Assess the Role of Dogs as Sentinels for Rift Valley Fever Virus (RVFV) Surveillance in Madagascar. Viruses 2025, 17, 1461. https://doi.org/10.3390/v17111461
Ramaroson HS, Garchitorena A, Lacoste V, Andriamandimby SF, Schoenhals M, Bastard J, Albrechtova K, Chevalier LJG, Rakotomanana D, Rasamoel PdV, et al. A Multi-Host Approach to Quantitatively Assess the Role of Dogs as Sentinels for Rift Valley Fever Virus (RVFV) Surveillance in Madagascar. Viruses. 2025; 17(11):1461. https://doi.org/10.3390/v17111461
Chicago/Turabian StyleRamaroson, Herilantonirina Solotiana, Andres Garchitorena, Vincent Lacoste, Soa Fy Andriamandimby, Matthieu Schoenhals, Jonathan Bastard, Katerina Albrechtova, Laure J. G. Chevalier, Domoina Rakotomanana, Patrick de Valois Rasamoel, and et al. 2025. "A Multi-Host Approach to Quantitatively Assess the Role of Dogs as Sentinels for Rift Valley Fever Virus (RVFV) Surveillance in Madagascar" Viruses 17, no. 11: 1461. https://doi.org/10.3390/v17111461
APA StyleRamaroson, H. S., Garchitorena, A., Lacoste, V., Andriamandimby, S. F., Schoenhals, M., Bastard, J., Albrechtova, K., Chevalier, L. J. G., Rakotomanana, D., Rasamoel, P. d. V., Raliniaina, M., Andriamahefa, H. F., Andriamananjara, M. A., Rasoloharimanana, L. T., Razafimahatratra, S. L., Ratsimbasoa, C. A., Durand, B., & Chevalier, V. (2025). A Multi-Host Approach to Quantitatively Assess the Role of Dogs as Sentinels for Rift Valley Fever Virus (RVFV) Surveillance in Madagascar. Viruses, 17(11), 1461. https://doi.org/10.3390/v17111461





