Functional Analysis of the Pathogenesis-Related Protein 1 (CaPR1) Gene in the Pepper Response to Chilli veinal mottle virus (ChiVMV) Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Viral Inoculation
2.2. RNA Isolation and cDNA Synthesis
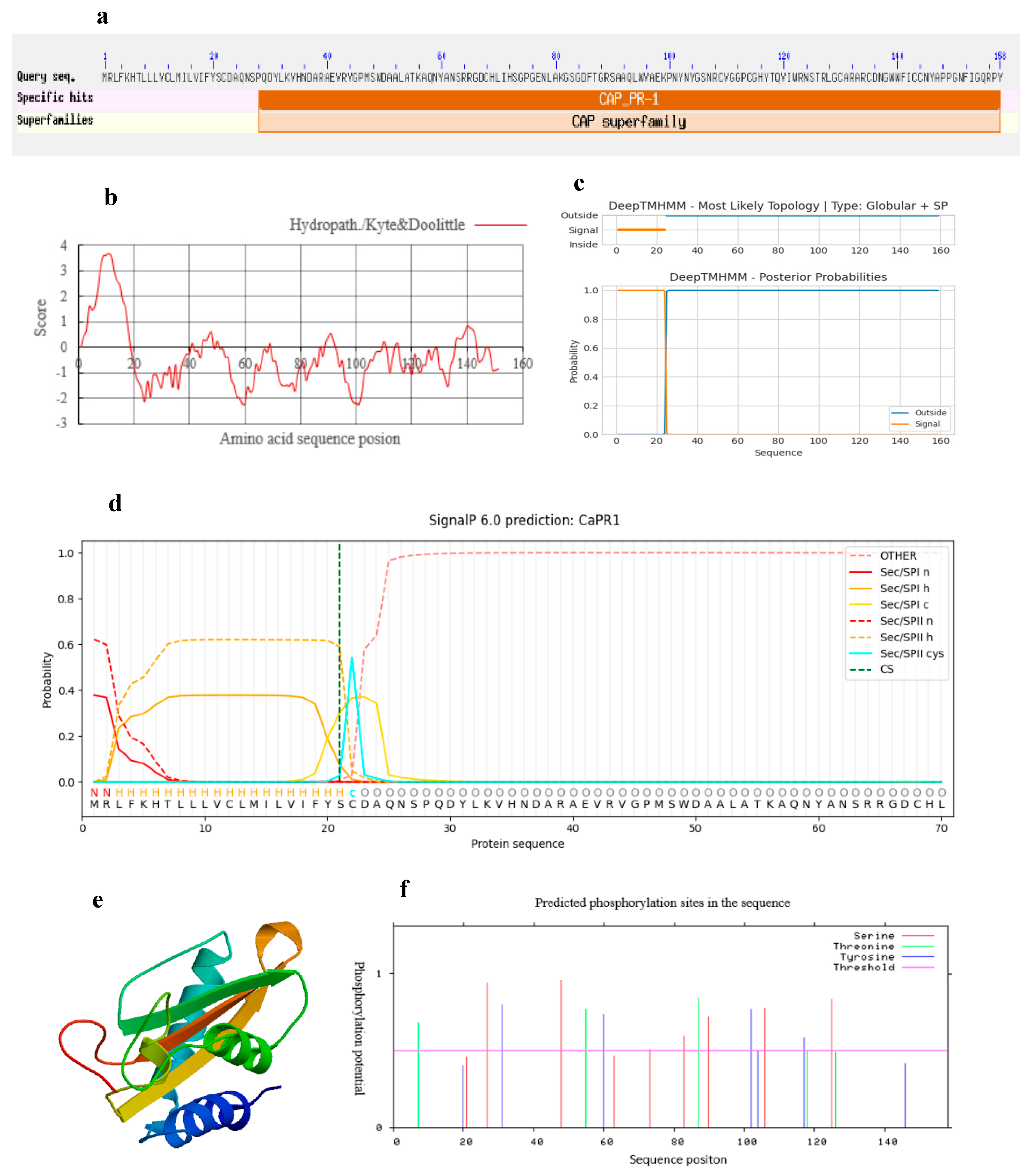
2.3. Gene Cloning and Bioinformatics Analysis
2.4. CaPR1 Subcellular Localization
2.5. Overexpression Vector Establishment and Genetic Transformation of Tobacco
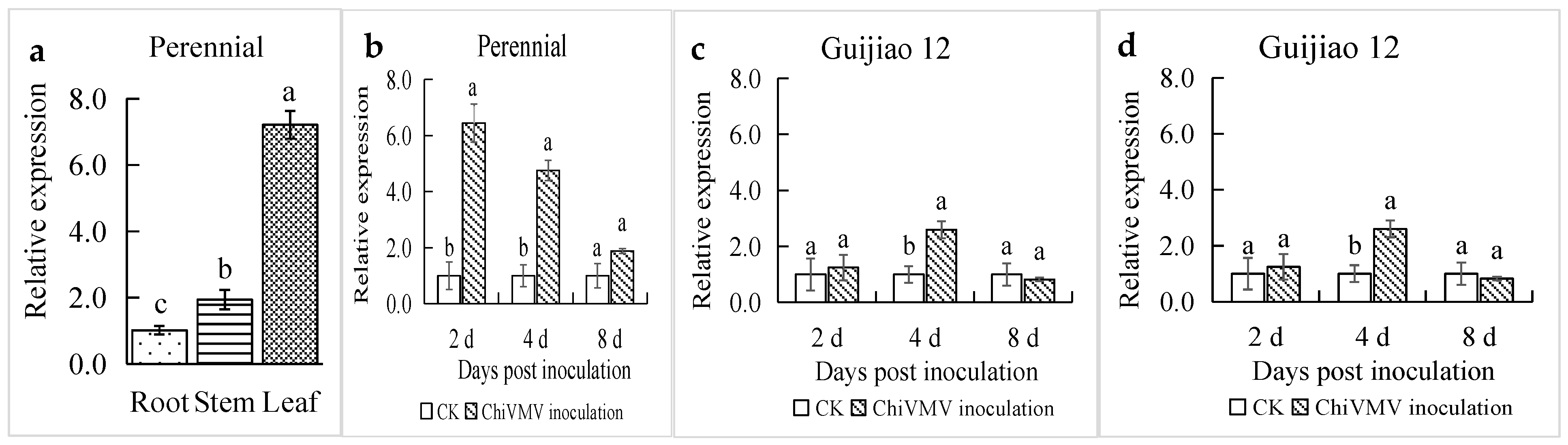
2.6. Gene Expression Analysis
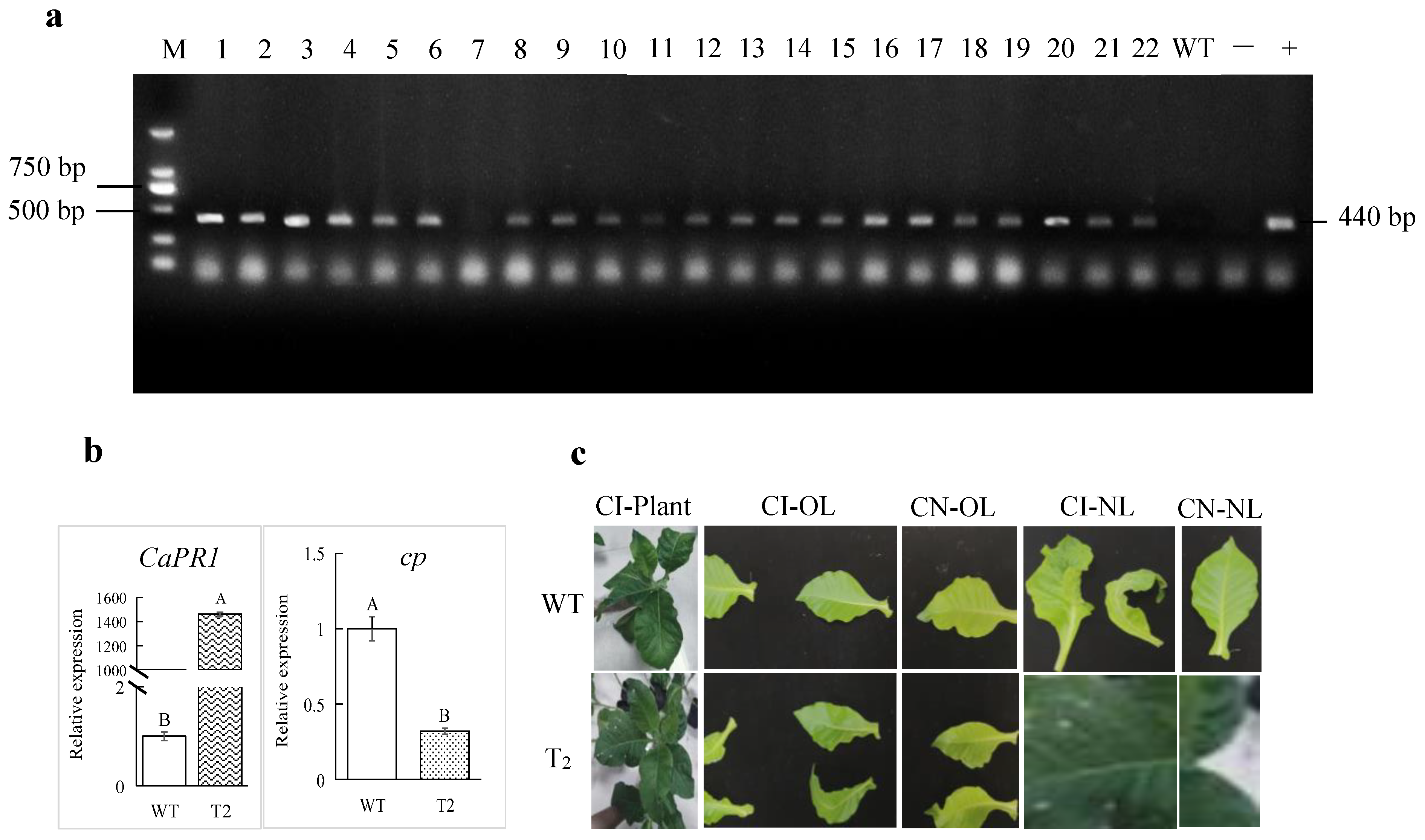
2.7. Assessment of ChiVMV Resistance in Transgenic Tobacco
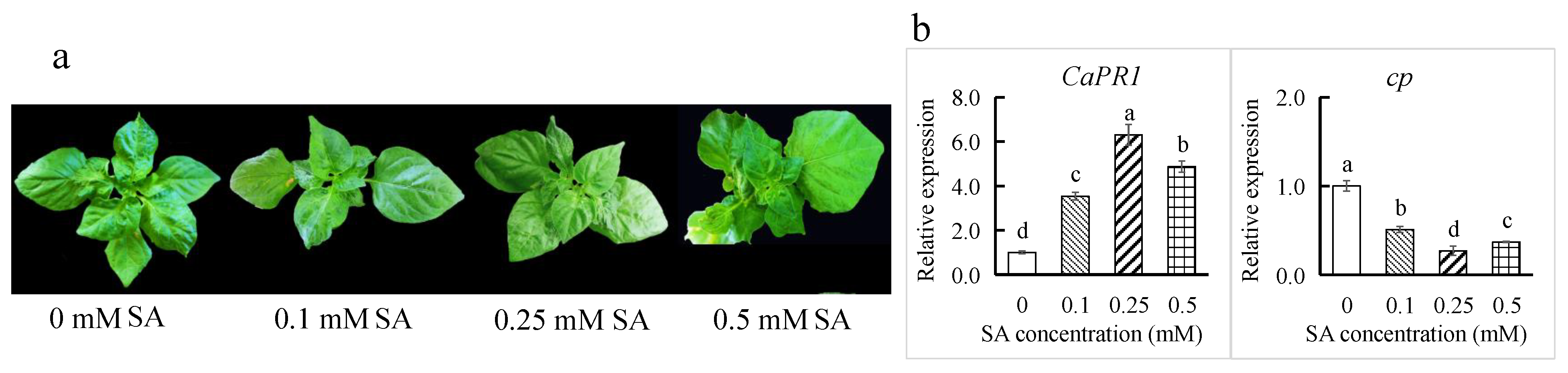
2.8. Treatment with Salicylic Acid
2.9. Statistical Analysis
3. Results
3.1. Cloning and Bioinformatic Analysis of the CaPR1 Gene
3.2. CaPR1 Expression Analysis
3.3. Subcellular Localization Analysis of CaPR1
3.4. Overexpression of CaPR1 Enhanced Resistance to ChiVMV
3.5. SA Treatment Enhanced Resistance to ChiVMV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zou, X.; Ma, Y.; Dai, X.; Li, X.; Yang, S. Spread and industry development of pepper in China. Acta Hortic. Sin. 2020, 47, 1715–1726. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Tan, G.; Xiao, L.; Zhou, W.; Lan, P.; Chen, X.; Liu, Y.; Li, R.; Li, F. A multiyear survey and identification of pepper- and tomato-infecting viruses in Yunnan Province China. Front. Microbiol. 2021, 12, 623875. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Niu, S.; Peng, M.; Wang, D.; Weng, Z.; Xiong, Z. Natural occurrence of Chilli veinal mottle virus on Capsicum chinense in China. Plant Dis. 2006, 90, 377. [Google Scholar] [CrossRef]
- Tsai, W.S.; Huang, Y.C.; Zhang, D.Y.; Reddy, K.; Hidayat, S.H.; Srithongchai, W.; Jan, F.J. Molecular characterization of the CP gene and 3’UTR of Chilli veinal mottle virus from South and Southeast Asia. Plant Pathol. 2008, 57, 408–416. [Google Scholar] [CrossRef]
- Rao, S.; Chen, X.; Qiu, S.; Peng, J.; Zheng, H.; Lu, Y.; Wu, G.; Chen, J.; Jiang, W.; Zhang, Y.; et al. Identification of two new isolates of Chilli veinal mottle virus from different regions in China: Molecular diversity phylogenetic and recombination analysis. Front. Microbiol. 2020, 11, 3307. [Google Scholar] [CrossRef]
- Hwang, J.; Li, J.; Liu, W.Y.; An, S.J.; Cho, H.; Her, N.H.; Yeam, I.; Kim, D.; Kang, B.C. Double mutations in eIF4E and eIFiso4E confer recessive resistance to Chilli veinal mottle virus in pepper. Mol. Cells 2009, 27, 329–336. [Google Scholar] [CrossRef]
- Schwessinger, B.; Ronald, C.P. Plant innate immunity: Perception of conserved microbial signatures. Annu. Rev. Plant Biol. 2012, 63, 451–482. [Google Scholar] [CrossRef]
- Koornneef, A.; Pieterse, C.M.J. Cross talk in defense signaling. Plant Physiol. 2008, 146, 839–844. [Google Scholar] [CrossRef]
- Zribi, I.; Ghorbel, M.; Haddaji, N.; Besbes, M.; Brini, F. Genome-wide identification and expression profiling of pathogenesis-related protein 1 (PR-1) genes in durum wheat (Triticum durum Desf.). Plants 2023, 12, 1998. [Google Scholar] [CrossRef]
- Van Loon, L.; Van Kammen, A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana Tabacum var. ‘Samsun’ and ‘Samsun NN’: II. changes in protein constitution after infection with Tobacco mosaic virus. Virology 1970, 40, 199–211. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Du, Y.; Amin, N.; Ahmad, N.; Zhang, H.; Zhang, Y.; Song, Y.; Fan, S.; Wang, P. Identification of the function of the pathogenesis-related protein GmPR1L in the resistance of soybean to Cercospora sojina Hara. Genes 2023, 14, 920. [Google Scholar] [CrossRef]
- Sarowar, S.; Kim, Y.J.; Kim, E.N.; Kim, K.D.; Hwang, B.K.; Islam, R.; Shin, J.S. Overexpression of a pepper basic pathogenesis-related protein 1 gene in tobacco plants enhances resistance to heavy metal and pathogen stresses. Plant Cell Rep. 2005, 24, 216–224. [Google Scholar] [CrossRef]
- Gamir, J.; Darwiche, R.; Van’t Hof, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of pathogenesis-related protein 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef]
- Chen, N.; Shao, Q.; Xiong, Z. Isolation and characterization of a pathogenesis-related protein 1 (SlPR1) gene with induced expression in tomato (Solanum lycopersicum) during Ralstonia solanacearum infection. Gene 2023, 855, 147105. [Google Scholar] [CrossRef]
- Hong, K.J.; Hwang, K.B. Induction of enhanced disease resistance and oxidative stress tolerance by overexpression of pepper basic PR-1 gene in Arabidopsis. Physiol. Plant. 2005, 124, 267–277. [Google Scholar] [CrossRef]
- Elvira, M.I.; Galdeano, M.M.; Gilardi, P.; García-Luque, I.; Serra, M.T. Proteomic analysis of pathogenesis-related proteins (PRs) induced by compatible and incompatible interactions of pepper mild mottle virus (PMMoV) in Capsicum chinense L3 plants. J. Exp. Bot. 2008, 59, 1253–1265. [Google Scholar] [CrossRef]
- Ren, R.; Wei, Y.; Ahmad, S.; Jin, J.; Gao, J.; Lu, C.; Zhu, G.; Yang, F. Identification and characterization of NPR1 and PR1 homologs in Cymbidium orchids in response to multiple hormones salinity and viral stresses. Int. J. Mol. Sci. 2020, 21, 1977. [Google Scholar] [CrossRef]
- Su, W.; Zhao, Z.; Qi, M.; Sun, T.; Wang, D.; Zhang, J. Cloning and functional analysis of pathogenesis-related protein gene GmPR1-6 in soybean resistance to SMV. J. Hebei Agric. Univ. 2023, 46, 8–15. (In Chinese) [Google Scholar] [CrossRef]
- Li, Z.T.; Dhekney, S.A.; Gray, D.J. PR-1 gene family of grapevine: A uniquely puplicated PR-1 gene from a vitis interspecific hybrid confers high level resistance to bacterial disease in transgenic tobacco. Plant Cell Rep. 2011, 30, 1–11. [Google Scholar] [CrossRef]
- Lee, H.R.; An, H.J.; You, Y.G.; Lee, J.; Kim, H.J.; Kang, B.C.; Harn, C.H. Development of a novel codominant molecular marker for Chili veinal mottle virus resistance in Capsicum annuum L. Euphytica 2013, 193, 197–205. [Google Scholar] [CrossRef]
- Lee, J.H.; An, J.T.; Siddique, M.I.; Han, K.; Choi, S.; Kwon, J.K.; Kang, B.C. Identification and molecular genetic mapping of Chili veinal mottle virus (ChiVMV) resistance genes in pepper (Capsicum annuum). Mol. Breed. 2017, 37, 121. [Google Scholar] [CrossRef]
- Huang, C.; Wang, R.; Wu, X.; Zhao, H.; Wang, M.; Wang, L.; Zhao, Z.; Tang, Y.; He, Z.; Li, Z.; et al. Physiological and biochemical changes of different resistant pepper varieties in response to ChiVMV infection. China Veg. 2024, 11, 64–73. (In Chinese) [Google Scholar] [CrossRef]
- AlHudaib, K.A.; Alanazi, N.A.; Ghorbel, M.; El-Ganainy, S.M.; Brini, F. Isolation and characterization of a novel pathogenesis-related protein-1 gene (AvPR-1) with induced expression in oat (Avena sativa L.) during abiotic and hormonal stresses. Plants 2022, 11, 2284. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.C.; Cutt, J.R.; Klessig, D.F. Differential targeting of the tobacco PR-1 pathogenesis-related proteins to the extracellular space and vacuoles of crystal idioblasts. EMBO J. 1991, 10, 1317–1324. [Google Scholar] [CrossRef]
- Kiraga, J.; Mackiewicz, P.; Mackiewicz, D.; Kowalczuk, M.; Biecek, P.; Polak, N.; Smolarczyk, K.; Dudek, M.R.; Cebrat, S. The relationships between the isoelectric point and length of proteins, taxonomy and ecology of organisms. BMC Genom. 2007, 8, 163. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Tang, B.; Zheng, L.; Liu, D.; Cui, X.; Ge, F.; Liu, D. A pathogenesis-related protein-like gene is involved in the Panax notoginseng defense response to the root rot pathogen. Front. Plant Sci. 2021, 11, 610176. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.L.; Yang, H.L.; Zhao, J.P.; Bian, S.J.; Guo, Y.Y.; Chen, X.J.; Li, X.Z. A pathogenesis-related protein 1 of Cucurbita moschata responds to powdery mildew infection. Front. Genet. 2023, 14, 1168138. [Google Scholar] [CrossRef]
- Schaad, C.; Jensen, P.E.; Carrington, J.C. Formation of plant RNA virus replication complexes on membranes: Role of an endoplasmic reticulum-targeted viral protein. EMBO J. 1997, 16, 4049–4059. [Google Scholar] [CrossRef]
- Otulak, K.; Garbaczewska, G. The participation of plant cell organelles in compatible and incompatible potato virus Y-tobacco and -potato plant interaction. Acta Physiol. Plant. 2014, 36, 85–99. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Wei, W.; Lin, Y.; Yin, W.; Luo, C. Development of novel methods for functional evaluation of the signal peptide of secreted protein. Physiol. Mol. Plant P. 2019, 106, 182–186. [Google Scholar] [CrossRef]
- Milne, T.J.; Abbenante, G.; Tyndall, J.D.; Halliday, J.; Lewis, R.J. Isolation and characterization of a cone snail protease with homology to CRISP proteins of the pathogenesis-related protein superfamily. J. Biol. Chem. 2003, 278, 31105–31110. [Google Scholar] [CrossRef] [PubMed]
- Yeats, C.; Bentley, S.; Bateman, A. New knowledge from old: In silico discovery of novel protein domains in Streptomyces coelicolor. BMC Microbiol. 2003, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Xiong, D.; Schneiter, R.; Tian, C. The function of plant PR1 and other members of the CAP protein superfamily in plant-pathogen interactions. Mol. Plant Pathol. 2023, 24, 651–668. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lee, C.Y.; Cheng, K.T.; Chang, W.H.; Huang, R.N.; Nam, H.G.; Chen, Y.R. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell 2014, 26, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shen, S.; Zhao, C.; Cui, Z.; Meng, L.; Wu, W.; Liu, D.; Wang, H. TaPR1 interacts with TaTLP1 via the αIV helix to be involved in wheat defense to Puccinia triticina through the CAPE1 motif. Front. Plant Sci. 2022, 26, 874654. [Google Scholar] [CrossRef]
- Xing, Y.; Wen, Z.; Gao, W.; Lin, Z.; Zhong, J.; Jiu, Y. Multifaceted functions of host cell caveolae/caveolin-1 in virus infections. Viruses 2020, 12, 487. [Google Scholar] [CrossRef]
- Reyes, F.C.; Buono, R.; Otegui, M.S.; Reyes, F.C.; Buono, R.; Oteguim, S. Plant endosomal trafficking pathways. Curr. Opin. Plant Biol. 2011, 14, 666–673. [Google Scholar] [CrossRef]
- Cabanillas, D.G.; Jiang, J.; Movahed, N.; Germain, H.; Yamaji, Y.; Zheng, H.; Laliberté, J.F. Turnip mosaic virus uses the SNARE protein VTI11 in an unconventional route for replication vesicle trafficking. Plant Cell 2018, 30, 2594–2615. [Google Scholar] [CrossRef]
- Wu, G.; Cui, X.; Chen, H.; Renaud, B.J.; Yu, K.; Chen, X.; Wang, A. Dynamin-like proteins of endocytosis in plants are coopted by potyviruses to enhance virus infection. J. Virol. 2018, 92, e01320. [Google Scholar] [CrossRef]
- Roman, S.; Christophe, D.; Kevin, G.; Iulia, A.; Elodie, N.; Nathalie, L.C.; Jan, L.; Francoise, S.P.; Patricia, G.P. Plasma membrane order and fluidity are diversely triggered by elicitors of plant defence. J. Exp. Bot. 2016, 67, 5173–5185. [Google Scholar] [CrossRef]
- Sun, T.; Sun, X.; Li, F.; Ma, N.; Wang, M.; Chen, Y.; Liu, N.; Jin, Y.; Zhang, J.; Hou, C.; et al. H2O2 mediates transcriptome reprogramming during Soybean mosaic virus-induced callose deposition in soybean. Crop J. 2022, 10, 262–272. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, S.Y.; Kang, Z.S.; Han, Q.M.; Bai, Z.Q. Accumulation and distribution of hydrogen peroxide interaction between sugarbeet plant and Sugarbeet necrotic yellow vein virus. Acta Agron. Sin. 2012, 38, 865–870. (In Chinese) [Google Scholar] [CrossRef]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef]
- Zaynab, M.; Peng, J.; Sharif, Y. Expression profiling of pathogenesis—Related protein-1 genes from Solanum tuberosum reveals its critical role in Phytophthora infestans infection. Microb. Pathog. 2021, 161, 105290–105301. [Google Scholar] [CrossRef]
- Sahni, S.; Prasad, B.D.; Abubakar, A.L.; Abarshi, M.M.; Maruthi, M.N.; Ali, E.A.; Mahmoud, A.; Beauchamp, C.; Fridovich, I.; Bradford, M.M.; et al. Salicylic acid-induced resistance against mungbean yellow mosaic virus (MYMV) and enhanced seed yield in resistant and susceptible urdbean [Vigna mungo (L.) heper] genotypes. Legume Res. 2022, 45, 97–103. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Salicylic acid-induced differential resistance to the tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.; Granell, P.; Tárraga, S.; López-Gresa, P.; Conejero, V.; Bellés, J.M.; Rodrigo, I.; Lisón, P. Salicylic acid and gentisic acid induce RNA silencing-related genes and plant resistance to RNA pathogens. Plant Physiol. Bioch. 2014, 77, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Subhash, S.; Geetha, G.A.; Shivashankar, K.S.; Reddy, M.K. Pepper-acquired resistance induced by salicylic acid against Chilli veinal mottle virus. Indian Phytopathol. 2022, 75, 1159–1166. [Google Scholar] [CrossRef]
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123. [Google Scholar] [CrossRef]
- Kinkema, M.; Fan, W.; Dong, X. Nuclear localization of NPR1 is required for activation of PR gene expression. Plant Cell 2000, 12, 2339–2350. [Google Scholar] [CrossRef] [PubMed]
- Despres, C.; DeLong, C.; Glaze, S.; Liu, E.; Fobert, P.R. The arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors. Plant Cell 2000, 12, 279–290. [Google Scholar] [CrossRef]
- Lee, B.J.; Park, C.J.; Kim, S.K.; Kim, K.J.; Paek, K.H. In vivo binding of hot pepper bZIP transcription factor CabZIP1 to the G-box region of pathogenesis-related protein 1 promoter. Biochem. Biophys. Res. Commun. 2006, 344, 55–62. [Google Scholar] [CrossRef]
- Van Verk, M.; Neeleman, L.; Bol, J.; Linthorst, H. Tobacco transcription factor NtWRKY12 interacts with TGA2.2 in vitro and in vivo. Front. Plant Sci. 2011, 2, 32. [Google Scholar] [CrossRef]
- Hussain, R.M.F.; Sheikh, A.H.; Haider, I.; Quareshy, M.; Linthorst, H.J.M. Arabidopsis WRKY50 and TGA transcription factors synergistically activate expression of PR1. Front. Plant Sci. 2018, 9, 930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, Z.; Gao, J.; Zhou, X.; Zhu, S.; Wang, X.Y.; Wang, X.L.; Ren, G.D.; Kuai, B.K. The NPR1-WRKY46-WRKY6 signaling cascade mediates probenazole/salicylic acid-elicited leaf senescence in Arab. thaliana. J. Integr. Plant Biol. 2021, 63, 924–936. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.Q.; Yan, S.; Saleh, A.; Wang, W.; Ruble, J.; Oka, N.; Mohan, R.; Spoel, S.H.; Tada, Y.; Zheng, N.; et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 2012, 486, 228–232. [Google Scholar] [CrossRef]


| Use | Primer Name | Primer Sequence (5′-3′) | Product Length (bp) |
|---|---|---|---|
| Gene cloning | CaPR1-F | CTCCACTAGAACTAAAACAC | 583 |
| CaPR1-R | ATTATCAACCCCCTTAGCTT | ||
| RT-qPCR | Actin-QF | TTGGGATGATATGGAGAAGATATGGCATC | 147 |
| Actin-QR | AACGTCTCAAACATAATCTGGGTCATCT | ||
| CaPR1-QF | GAGCCGAAGTTAGGGTTGGG | 122 | |
| CaPR1-QR | ACCGCTACCCTTAGCAGGAT | ||
| ChiVMV-cp-QF | GGATGTTCGGATTGGACGGT | 97 | |
| ChiVMV-cp-QR | CCCAGCAGGTTGTGCATATTTC | ||
| ORF amplification (without TC) | pBI121-XhoI-F | ctcgagATGAGGTTGTTCAAACATACATTGTTAC | 474 |
| pBI121-SalI-R | gtcgacGTAAGGACGTTGTCCGATGAAGT | ||
| ORF amplification (with TC) | pCAMBI130-BglI-F | agatctATGAGGTTGTTCAAACATACATTGTTAC | 477 |
| pCAMBI1301-BstEII-R | ggtcaccTTAGTAAGGACGTTGTCCGATGAA | ||
| Amplification of target gene fragment | SR1-CaPR1-F | TGACGCACAATCCCACTATC | 440 |
| SR1-CaPR1-R | ATGGTCCACCAACGCATC |
| Disease Index (DI) | Resistance Evaluation |
|---|---|
| DI = 0 | Immune (I) |
| 0 < DI < 10 | Highly resistant (HR) |
| 10 ≤ DI < 20 | Resistant (R) |
| 20 ≤ DI < 40 | Moderately resistant (MR) |
| 40 ≤ DI < 60 | Susceptible (S) |
| 60 ≤ DI | Highly susceptible (HS) |
| Material Type | DI (%) | Disease Resistance Level |
|---|---|---|
| WT | 50.37 ± 1.28 A | Susceptible (S) |
| T2 | 37.78 ± 2.22 B | Moderately resistant (MR) |
| SA Concentration (mM) | DI (%) |
|---|---|
| 0 (CK) | 83.46 ± 2.27 Aa |
| 0.1 | 76.21 ± 2.36 Ab |
| 0.25 | 59.84 ± 3.13 Bc |
| 0.5 | 66.08 ± 2.51 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Zhao, Z.; Wu, X.; Zhao, H.; Wang, M.; He, Z.; Li, Z.; Wang, L.; Tang, Y.; Wang, R.; et al. Functional Analysis of the Pathogenesis-Related Protein 1 (CaPR1) Gene in the Pepper Response to Chilli veinal mottle virus (ChiVMV) Infection. Viruses 2025, 17, 1456. https://doi.org/10.3390/v17111456
Huang C, Zhao Z, Wu X, Zhao H, Wang M, He Z, Li Z, Wang L, Tang Y, Wang R, et al. Functional Analysis of the Pathogenesis-Related Protein 1 (CaPR1) Gene in the Pepper Response to Chilli veinal mottle virus (ChiVMV) Infection. Viruses. 2025; 17(11):1456. https://doi.org/10.3390/v17111456
Chicago/Turabian StyleHuang, Chunzi, Zengjing Zhao, Xing Wu, Hu Zhao, Meng Wang, Zhi He, Zongjun Li, Lihao Wang, Yafei Tang, Risheng Wang, and et al. 2025. "Functional Analysis of the Pathogenesis-Related Protein 1 (CaPR1) Gene in the Pepper Response to Chilli veinal mottle virus (ChiVMV) Infection" Viruses 17, no. 11: 1456. https://doi.org/10.3390/v17111456
APA StyleHuang, C., Zhao, Z., Wu, X., Zhao, H., Wang, M., He, Z., Li, Z., Wang, L., Tang, Y., Wang, R., He, L., & Gong, M. (2025). Functional Analysis of the Pathogenesis-Related Protein 1 (CaPR1) Gene in the Pepper Response to Chilli veinal mottle virus (ChiVMV) Infection. Viruses, 17(11), 1456. https://doi.org/10.3390/v17111456






