Bayesian Estimation of the True Prevalence of Caprine Arthritis Encephalitis in Hungarian Goat Herds
Abstract
1. Introduction
2. Materials and Methods
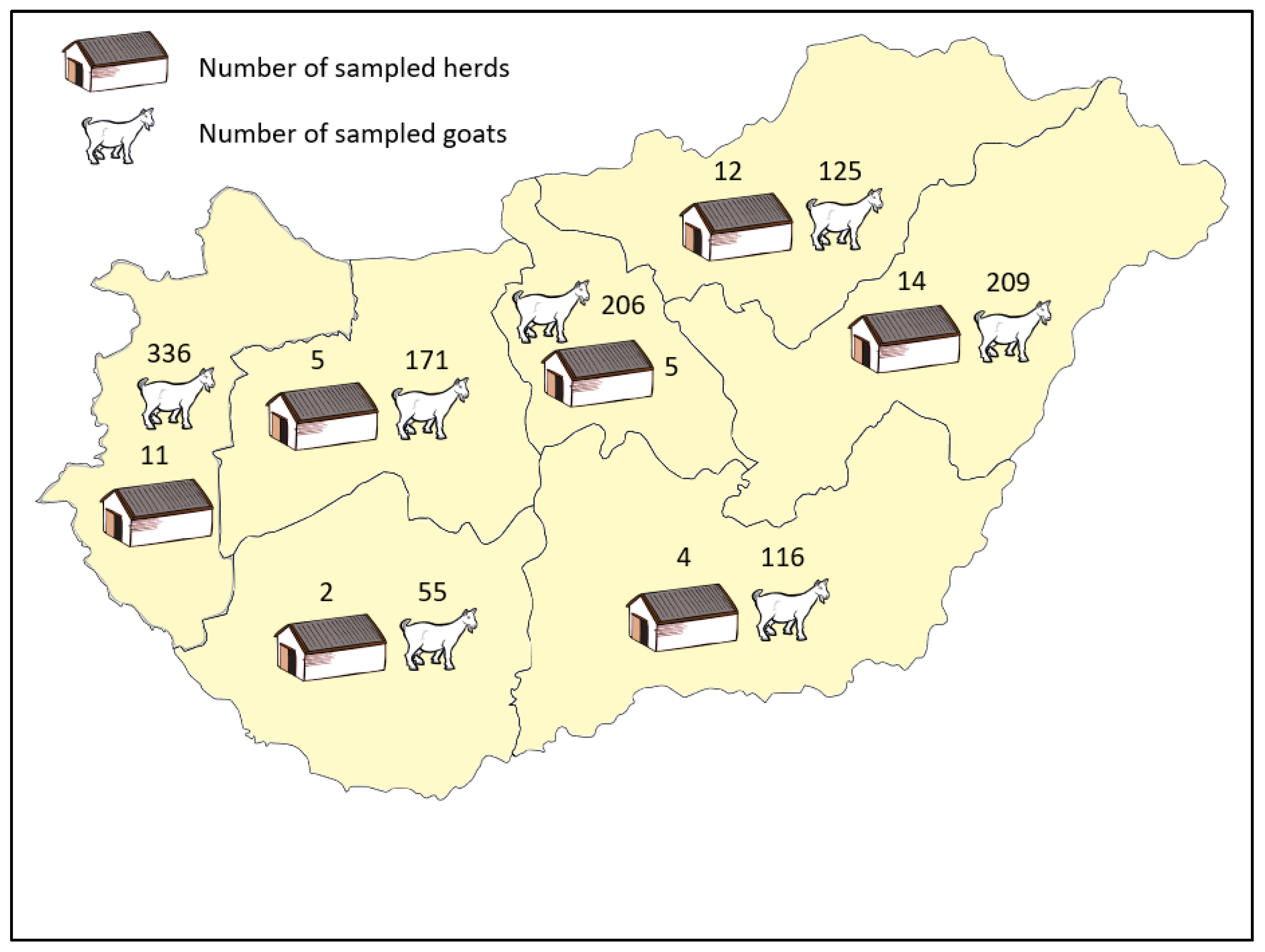
2.1. Sampling and Serological Testing
- S/P ≤ 50%: negative,
- 50% < S/P < 60%: inconclusive,
- S/P ≥ 60%: positive.
2.2. Statistical Model
2.2.1. Description of Model Components
2.2.2. The Choice of Priors
2.2.3. Model Convergence Diagnostics
2.2.4. Posterior Predictive Check of Model Fit
3. Results
3.1. Descriptive Statistics
3.2. Bayesian Model
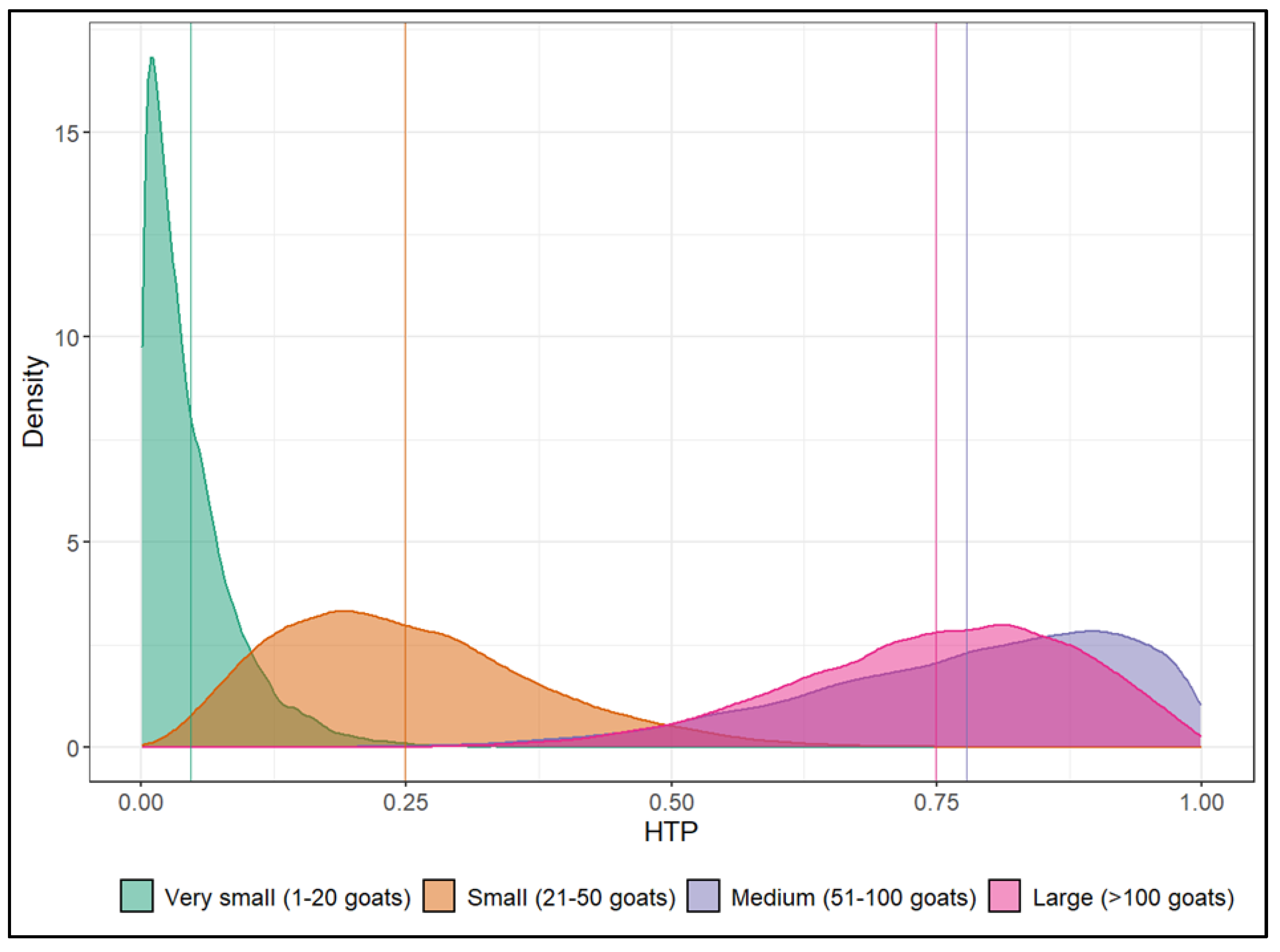
Investigation of the Effects of Herd Size and Regions
4. Discussion
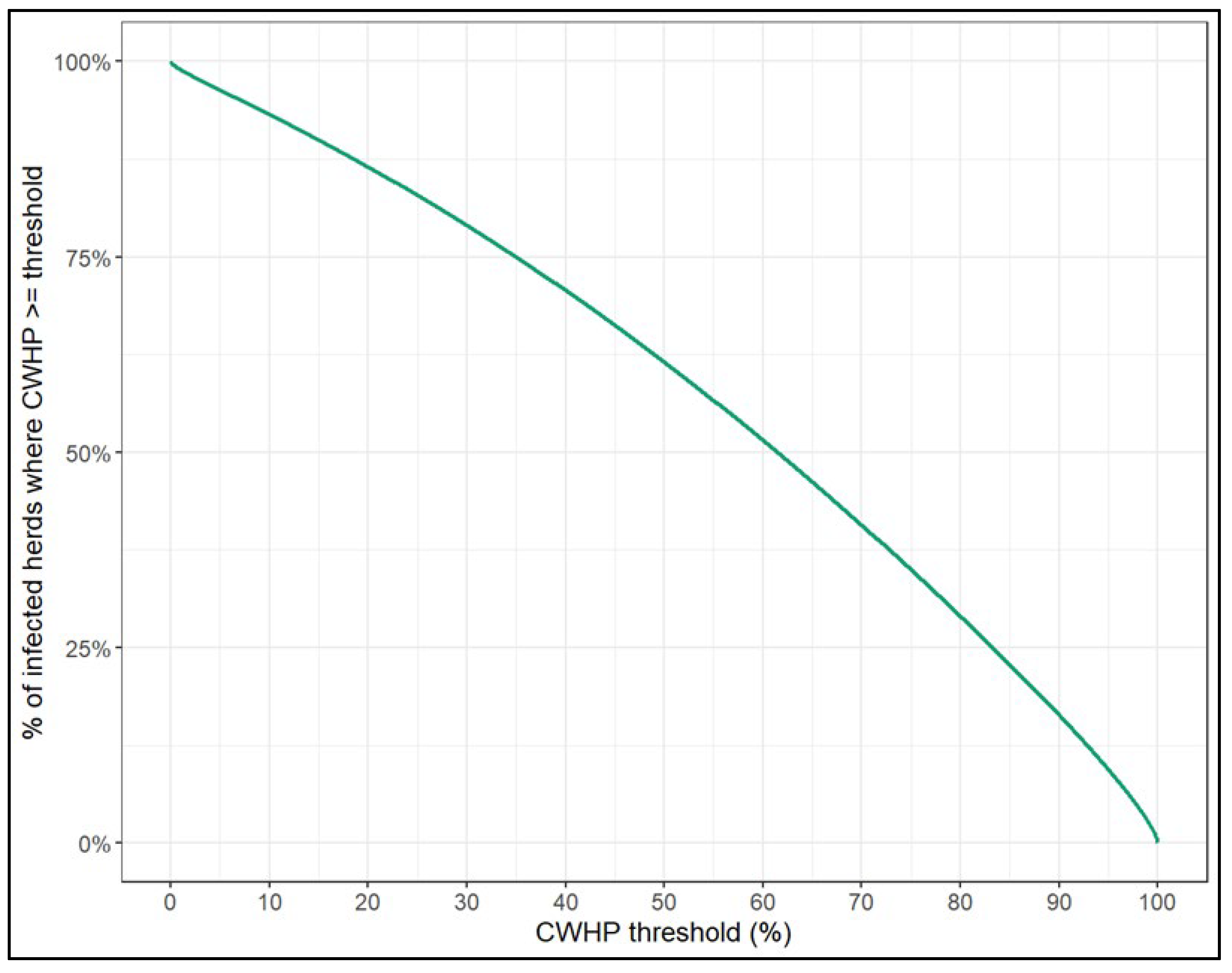
4.1. Prevalence and Within-Herd Dynamics of CAE
4.2. Risk Factors of CAE Infection
4.3. Control of CAE
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SRLV | Small ruminant lentivirus |
| CAEV | Caprine arthritis-encephalitis virus |
| MVV | Maedi-visna virus |
| CAE | Caprine arthritis encephalitis |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PCR | Polymerase chain reaction |
| UVMB | University of Veterinary Medicine Budapest |
| HRP | Horseradish peroxidase |
| TMB | Substrate solution containing 3,3′,5,5′-Tetramethylbenzidine |
| OD | Optical density |
| ODPC | Optical density of the positive control |
| ODNC | Optical density of the negative control |
| S/P% | Sample-to-positive percentage |
| HTP | Herd-level true prevalence |
| CWHP | Conditional within-herd prevalence |
| AP | Apparent prevalence |
| Sp | Specificity |
| Se | Sensitivity |
| MCMC | Markov Chain Monte Carlo |
| CrI | Credible intervals |
| AGID | Agar Gel Immunodiffusion Assay |
| NGS | Next-generation sequencing |
References
- Stonos, N.; Wootton, S.K.; Karrow, N. Immunogenetics of small ruminant lentiviral infections. Viruses 2014, 6, 3311–3333. [Google Scholar] [CrossRef]
- Gómez, Á.; Glaria, I.; Moncayola, I.; Echeverría, I.; Arrizabalaga, J.; Rodríguez-Largo, A.; de Blas, I.; Lacasta, D.; Pérez, E.; Pérez, M.; et al. Characterization of a recombinant Sendai virus vector encoding the small ruminant lentivirus gag-P25: Antiviral properties in vitro and transgene expression in sheep. Vet. Res. 2025, 56, 51. [Google Scholar] [CrossRef]
- Cork, L.C.; Hadlow, W.J.; Crawford, T.B.; Gorham, J.R.; Piper, R.C. Infectious leukoencephalomyelitis of young goats. J. Infect. Dis. 1974, 129, 134–141. [Google Scholar] [CrossRef]
- Crawford, T.; Adams, D.S.; Cheevers, W.P.; Cork, L.C. Chronic arthritis in goats caused by a retrovirus. Science 1980, 207, 997–999. [Google Scholar] [CrossRef]
- Kaba, J.; Strzałkowska, N.; Jóźwik, A.; Krzyżewski, J.; Bagnicka, E. Twelve-year cohort study on the influence of caprine arthritis-encephalitis virus infection on milk yield and composition. J. Dairy Sci. 2012, 95, 1617–1622. [Google Scholar] [CrossRef]
- Martínez-Navalón, B.; Peris, C.; Gómez, E.A.; Peris, B.; Roche, M.L.; Caballero, C.; Goyena, E.; Berriatua, E. Quantitative estimation of the impact of caprine arthritis encephalitis virus infection on milk production by dairy goats. Vet. J. 2013, 197, 311–317. [Google Scholar] [CrossRef]
- Nowicka, D.; Czopowicz, M.; Bagnicka, E.; Rzewuska, M.; Strzałkowska, N.; Kaba, J. Influence of small ruminant lentivirus infection on cheese yield in goats. J. Dairy Res. 2015, 82, 102–106. [Google Scholar] [CrossRef]
- Woodard, J.C.; Gaskin, J.M.; Poulos, P.W.; MacKay, R.J.; Burridge, M.J. Caprine arthritis-encephalitis: Clinicopathologic study. Am. J. Vet. Res. 1982, 43, 2085–2096. [Google Scholar] [CrossRef]
- Angelopoulou, K.; Poutahidis, T.; Brellou, G.; Greenland, T.; Vlemmas, I. A deletion in the R region of long terminal repeats in small ruminant lentiviruses is associated with decreased pathology in the lung. Vet. J. 2008, 175, 346–355. [Google Scholar] [CrossRef]
- Smith, M.C.; Cutlip, R. Effects of infection with caprine arthritis-encephalitis virus on milk production in goats. J. Am. Vet. Med. Assoc. 1988, 193, 63–67. [Google Scholar] [CrossRef]
- Peterhans, E.; Greenland, T.; Badiola, J.; Harkiss, G.; Bertoni, G.; Amorena, B.; Eliaszewicz, M.; Juste, R.A.; Krassnig, R.; Lafont, J.-P.; et al. Routes of transmission and consequences of small ruminant lentiviruses (SRLVs) infection and eradication schemes. Vet. Res. 2004, 35, 257–274. [Google Scholar] [CrossRef]
- Reina, R.; Berriatua, E.; Luján, L.; Juste, R.; Sánchez, A.; de Andrés, D.; Amorena, B. Prevention strategies against small ruminant lentiviruses: An update. Vet. J. 2009, 182, 31–37. [Google Scholar] [CrossRef]
- Leitner, G.; Krifucks, O.; Weisblit, L.; Lavi, Y.; Bernstein, S.; Merin, U. The effect of caprine arthritis encephalitis virus infection on production in goats. Vet. J. 2010, 183, 328–331. [Google Scholar] [CrossRef]
- Shah, C.; Böni, J.; Huder, J.B.; Vogt, H.R.; Mühlherr, J.; Zanoni, R.; Miserez, R.; Lutz, H.; Schüpbach, J. Phylogenetic analysis and reclassification of caprine and ovine lentiviruses based on 104 new isolates: Evidence for regular sheep-to-goat transmission and worldwide propagation through livestock trade. Virology 2004, 319, 12–26. [Google Scholar] [CrossRef]
- Al-Qudah, K.; Al-Majali, A.M.; Ismail, Z.B. Epidemiological studies on caprine arthritis-encephalitis virus infection in Jordan. Small Rumin. Res. 2006, 66, 181–186. [Google Scholar] [CrossRef]
- Crawford, T.B.; Adams, D.S. Caprine arthritis-encephalitis: Clinical features and presence of antibody in selected goat populations. J. Am. Vet. Med. Assoc. 1981, 178, 713–719. [Google Scholar] [CrossRef]
- Herrmann-Hoesing, L.M. Diagnostic assays used to control small ruminant lentiviruses. J. Vet. Diagn. Investig. 2010, 22, 843–855. [Google Scholar] [CrossRef]
- Schultz, E.B.; Santana, T.E.Z.; Silva, F.F.; Garcia, A.O.; Oliveira, H.R.; Rodrigues, M.T.; Brito, L.F. Genetic parameter estimates for caprine arthritis encephalitis in dairy goats. J. Dairy Sci. 2020, 103, 6407–6411. [Google Scholar] [CrossRef]
- Mohammed, Q.A. Protective role of vitamin-TPGS to overcome oxidative stress induced by dipping of sheep with cypermethrin. Plant Arch. 2020, 20, 1105–1109. [Google Scholar]
- Reina, R.; Mora, M.; Glaria, I.; Garcia, I.; Solano, C.; Lujan, L.; Badiola, J.; Contreras, A.; Berriatua, E.; Juste, R.; et al. Molecular characterization and phylogenetic study of Maedi Visna and caprine arthritis encephalitis viral sequences in sheep and goats from Spain. Virus Res. 2006, 121, 189–198. [Google Scholar] [CrossRef]
- Hamzah, K.J.; Hasso, S.A. Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq. Open Vet. J. 2019, 9, 238–245. [Google Scholar] [CrossRef]
- Ramírez, H.; Reina, R.; Amorena, B.; de Andrés, D.; Martínez, H.A. Small ruminant lentiviruses: Genetic variability, tropism and diagnosis. Viruses 2013, 5, 1175–1207. [Google Scholar] [CrossRef]
- Sanjosé, L.; Pinczowski, P.; Crespo, H.; Pérez, M.; Glaria, I.; Gimeno, M.; de Andrés, D.; Amorena, B.; Luján, L.; Reina, R. Diagnosing infection with small ruminant lentiviruses of genotypes A and B by combining synthetic peptides in ELISA. Vet. J. 2015, 204, 88–93. [Google Scholar] [CrossRef]
- Kaba, J.; Czopowicz, M.; Kuźmak, J.; Olech, M.; Witkowski, L.; Moroz-Fik, A.; Mickiewicz, M.; Biernacka, K.; Nalbert, T.; Bereznowski, A.; et al. A large-scale study on the seroprevalence of small ruminant lentiviral infection in the Polish goat population. Prev. Vet. Med. 2023, 213, 105885. [Google Scholar] [CrossRef]
- Konishi, M.; Hayama, Y.; Shirafuji, H.; Kameyama, K.I.; Murakami, K.; Tsutsui, T.; Akashi, H. Serological survey of caprine arthritis-encephalitis virus infection in Japan. J. Vet. Med. Sci. 2016, 78, 447–450. [Google Scholar] [CrossRef]
- Nyi Lin, T.; Ngarmkum, S.; Oraveerakul, K.; Virakul, P.; Techakumphu, M. Seroprevalence and risk factors associated with caprine arthritis-encephalitis virus infection in goats in the western part of Thailand. Thai J. Vet. Med. 2011, 41, 353–360. [Google Scholar] [CrossRef]
- Kaba, J.; Czopowicz, M.; Ganter, M.; Nowicki, M.; Witkowski, L.; Nowicka, D.; Szaluś-Jordanow, O. Risk factors associated with seropositivity to small ruminant lentiviruses in goat herds. Res. Vet. Sci. 2013, 94, 225–227. [Google Scholar] [CrossRef]
- Mosa, A.H.; Aljabory, H.A.H.; Abady, N. Serological detection of small ruminant lentivirus infection in Babylon Governorate, Iraq. Bulg. J. Vet. Med. 2025, 28, 221–227. [Google Scholar] [CrossRef]
- Kalaycı, G.; Kaplan, M.; Özkan, B.; Pekmez, K.; Çağırgan, A.A.; Türkmen, R.; Tunalıgil, S. Epizootiological serosurvey on small ruminant lentiviruses: Prevalence, age and breed effects. Acta Vet. Hung. 2023, 71, 128–135. [Google Scholar] [CrossRef]
- Jacob-Ferreira, J.; Coelho, A.C.; Grau Vila, A.; Lacasta, D.; Quintas, H. Small ruminant lentivirus infection in sheep and goats in North Portugal: Seroprevalence and risk factors. Pathogens 2023, 12, 829. [Google Scholar] [CrossRef]
- Teles, J.A.A.; Nascimento, S.A.; Melo, E.X.; Almeida, E.C.; Marvulo, M.F.V.; Rizzo, H.; Batista Nogueira, D.; Santos de Azevedo, S.; Ramos Silva, J.C.; Castro, R.S. Factors associated with small ruminant lentivirus infection in goat herds from Pernambuco state, Northeast region of Brazil. Prev. Vet. Med. 2023, 211, 105814. [Google Scholar] [CrossRef]
- Bandeira, D.A.; de Castro, R.S.; Azevedo, E.O.; Melo, L.D.S.S.; de Melo, C.B. Seroprevalence of caprine arthritis–encephalitis virus in goats in the Cariri region, Paraiba state, Brazil. Vet. J. 2009, 180, 399–401. [Google Scholar] [CrossRef]
- De Miguel, R.; Arrieta, M.; Rodríguez-Largo, A.; Echeverría, I.; Resendiz, R.; Pérez, E.; Ruiz, H.; Pérez, M.; de Andrés, D.; Reina, R.; et al. Worldwide prevalence of small ruminant lentiviruses in sheep: A systematic review and meta-analysis. Animals 2021, 11, 784. [Google Scholar] [CrossRef]
- Dijkstra, E.; van der Heijden, M.; Holstege, M.; Gonggrijp, M.; van den Brom, R.; Vellema, P. Data analysis supports monitoring and surveillance of goat health and welfare in the Netherlands. Prev. Vet. Med. 2023, 213, 105865. [Google Scholar] [CrossRef]
- Thomann, B.; Falzon, L.C.; Bertoni, G.; Vogt, H.R.; Schüpbach-Regula, G.; Magouras, I. A census to determine the prevalence and risk factors for caprine arthritis-encephalitis virus and visna/maedi virus in the Swiss goat population. Prev. Vet. Med. 2017, 137, 52–58. [Google Scholar] [CrossRef]
- Vincenz, F.; Samoilenko, M.; Abril, C.E.; Zanolari, P.; Bertoni, G.; Thomann, B. A look under the carpet of a successful eradication campaign against small ruminant lentiviruses. Pathogens 2025, 14, 719. [Google Scholar] [CrossRef]
- Kampen, A.H.; Åkerstedt, J.; Klevar, S. The Surveillance Programme for Small Ruminant Lentivirus Infections in Sheep and Goats in Norway 2020; Norwegian Veterinary Institute: Oslo, Norway, 2021. [Google Scholar]
- Nagel-Alne, G.E.; Asheim, L.J.; Hardaker, J.B.; Sølverød, L.; Lindheim, D.; Valle, P.S. The Norwegian Healthier Goats programme—A financial cost-benefit analysis. Prev. Vet. Med. 2014, 114, 96–105. [Google Scholar] [CrossRef]
- Nagel-Alne, G.E.; Valle, P.S.; Krontveit, R.; Sølverød, L.S. Caprine arthritis encephalitis and caseous lymphadenitis in goats: Use of bulk tank milk ELISAs for herd-level surveillance. Vet. Rec. 2015, 176, 173. [Google Scholar] [CrossRef]
- Nardelli, S.; Bettini, A.; Capello, K.; Bertoni, G.; Tavella, A. Eradication of caprine arthritis encephalitis virus in the goat population of South Tyrol, Italy: Analysis of the tailing phenomenon during the 2016–2017 campaign. J. Vet. Diagn. Investig. 2020, 32, 589–593. [Google Scholar] [CrossRef]
- Tavella, A.; Capello, K.; Bertoni, G.; Bettini, A. Risk factors associated with the alpine multispecies farming system in the eradication of CAEV in South Tyrol, Italy. Viruses 2021, 13, 1959. [Google Scholar] [CrossRef]
- Ózsvári, L.; Bárdos, K.; Moroz-Fik, A.; Biernacka, K.; Mickiewicz, M.; Nowek, Z.; Abril, C.E.; Bertoni, G.; Stuen, S.; Petkevičius, S.; et al. First molecular characterization of small ruminant lentiviruses in Hungarian goat population. Pathogens 2024, 13, 939. [Google Scholar] [CrossRef]
- Kusza, S.; Bösze, Z.; Kukovics, S.; Jávor, A. Genetic assay of caprine arthritis encephalitis in the Hungarian goat herd. S. Afr. J. Anim. Sci. 2004, 34, 13–16. [Google Scholar]
- Mocskonyi, M.; Gulyás, L.; Szakács, M.; Malik, P. Experiences of a CAEV (caprine arthritis encephalitis virus) eradication programme in an Alpine goat farm [In Hungarian]. Magy. Állatorvosok Lapja 2024, 146, 259–271. [Google Scholar] [CrossRef]
- Act CXXVII of 2012 on the Hungarian Veterinary Chamber and the Provision of Veterinary Services. Available online: https://njt.hu/jogszabaly/2012-127-00-00 (accessed on 1 October 2024).
- Nowicka, D.; Czopowicz, M.; Mickiewicz, M.; Szaluś-Jordanow, O.; Witkowski, L.; Bagnicka, E.; Kaba, J. Diagnostic performance of ID Screen® MVV-CAEV Indirect Screening ELISA test in identifying SRLV-infected goats. Pol. J. Vet. Sci. 2014, 17, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Michiels, R.; Van Mael, E.; Quinet, C.; Adjadj, N.R.; Cay, A.B.; De Regge, N. Comparative analysis of different serological and molecular tests for the detection of small ruminant lentiviruses (SRLVs) in Belgian sheep and goats. Viruses 2018, 10, 696. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Cribari-Neto, F. Beta regression for modelling rates and proportions. J. Appl. Stat. 2004, 31, 799–815. [Google Scholar] [CrossRef]
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; Chapman and Hall–CRC: Boca Raton, FL, USA, 2013; 676p. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 11 March 2023).
- Stan Development Team. RStan: The R interface to Stan; R Package Version 2.17.3; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://mc-stan.org/rstan/ (accessed on 6 January 2024).
- Gabry, J.; Mahr, T. Plotting for Bayesian Models. 2019. Available online: https://mc-stan.org/bayesplot/ (accessed on 6 January 2022).
- Enache, D.A.; Baraitareanu, S.; Dan, M.; Gurau, M.R.; Danes, D. Methods of lentiviral infection surveillance and diagnosis in sheep and goats farms. Agric. Food 2019, 7, 143–159. [Google Scholar]
- Petkevičius, S.; Klibavičė, P.; Šalomskas, A.; Kupčinskas, T.; Moroz-Fik, A.; Biernacka, K.; Mickiewicz, M.; Nowek, Z.; Ózsvári, L.; Bárdos, K.; et al. The herd-level prevalence of caprine arthritis-encephalitis and genetic characteristics of small ruminant lentivirus in the Lithuanian goat population. Prev. Vet. Med. 2024, 233, 106363. [Google Scholar] [CrossRef] [PubMed]
- Kaba, J.; Ganter, M.; Czopowicz, M. Humoral immune response to caprine arthritis-encephalitis virus in goat herds. Cent. Eur. J. Immunol. 2010, 35, 196–198. [Google Scholar]
- Rezart, P.; Enkeleida, O.; Igor, D.; Anita, K.; Sonila, Ç.; Kristi, M.; Vera, V.; Xhelil, K. Serological evidence of Maedi-Visna and caprine arthritis encephalitis in sheep and goats in the Korçë region in Albania. Ger. J. Vet. Res. 2023, 3, 33–38. [Google Scholar] [CrossRef]
- Yang, W.C.; Chen, H.Y.; Wang, C.Y.; Pan, H.Y.; Wu, C.W.; Hsu, Y.H.; Su, J.C.; Chan, K.W. High prevalence of caprine arthritis encephalitis virus (CAEV) in Taiwan revealed by large-scale serological survey. J. Vet. Med. Sci. 2017, 79, 273–276. [Google Scholar] [CrossRef] [PubMed]
- De Andrés, D.; Klein, D.; Watt, N.J.; Berriatua, E.; Torsteinsdottir, S.; Blacklaws, B.A.; Harkiss, G.D. Diagnostic tests for small ruminant lentiviruses. Vet. Microbiol. 2005, 107, 49–62. [Google Scholar] [CrossRef]
- Olech, M.; Osiński, Z.; Kuźmak, J. Bayesian estimation of seroprevalence of small ruminant lentiviruses in sheep from Poland. Prev. Vet. Med. 2017, 147, 66–78. [Google Scholar] [CrossRef]
- Tubalinal, G.A.S.; Belotindos, L.P.; Mingala, C.N. High positivity rate of caprine arthritis encephalitis virus in Luzon, the Philippines revealed by nested-polymerase chain reaction assay. Virus Dis. 2024, 35, 11–16. [Google Scholar] [CrossRef]
- Tariba, B.; Kostelić, A.; Šalamon, D.; Roić, B.; Benić, M.; Prvanović Babić, N.; Salajpal, K. Prevalence of caprine arthritis encephalitis virus in association with clinical arthritis in six production farms of French Alpine goats in north-western Croatia. Poljoprivreda 2015, 21, 135–137. [Google Scholar] [CrossRef]
- De la Luz-Armendáriz, J.; Alberti-Navarro, A.B.; Hernández-Rojas, E.G.; Ducoing-Watty, A.E.; Galindo-Barboza, A.J.; Rivera-Benítez, J.F. Distribution of small ruminant lentivirus genotypes A and B in goat and sheep production units in Mexico. Vet. Sci. 2025, 12, 204. [Google Scholar] [CrossRef]
- Ghanem, Y.M.; El-Khodery, S.A.; Saad, A.A.; Elragaby, S.A.; Abdelkader, A.H.; Heybe, A. Prevalence and risk factors of caprine arthritis encephalitis virus infection (CAEV) in Northern Somalia. Small Rumin. Res. 2009, 85, 142–148. [Google Scholar] [CrossRef]
- Oem, J.K.; Chung, J.Y.; Byun, J.W.; Kim, H.Y.; Kwak, D.; Jung, B.Y. Large-scale serological survey of caprine arthritis-encephalitis virus (CAEV) in Korean black goats (Capra hircus aegagrus). J. Vet. Med. Sci. 2012, 74, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.N.; Jesse, F.F.A.; Paul, B.T.; Charles, S.S.C.; Noor Zaman, U.R.N.; Teik Chung, E.L.; Azhar, N.A.; Barre, A.; Mikail, M.; Mohd Lila, M.A. Seroprevalence and associated risk factors of caprine arthritis encephalitis (CAE) and peste des petits ruminants (PPR) among deer in an institutional farm in Malaysia. Thai J. Vet. Med. 2025, 55, 1–7. [Google Scholar] [CrossRef]
- Nord, K.; Løken, T.; Orten, Å. Control of caprine arthritis–encephalitis virus infection in three Norwegian goat herds. Small Rumin. Res. 1998, 28, 109–114. [Google Scholar] [CrossRef]
- Panneum, S.; Rukkwamsuk, T. Diagnosis of caprine arthritis encephalitis virus infection in dairy goats by ELISA, PCR and viral culture. Pol. J. Vet. Sci. 2017, 20, 347–353. [Google Scholar] [CrossRef]
- Czopowicz, M.; Szaluś-Jordanow, O.; Mickiewicz, M.; Moroz, A.; Witkowski, L.; Bereznowski, A.; Markowska-Daniel, B.; Bagnicka, E.; Kaba, J. Relationship between the dissemination of small ruminant lentivirus infection in goat herds and opinion of farmers on the occurrence of arthritis. PLoS ONE 2018, 13, e0204134. [Google Scholar] [CrossRef] [PubMed]
- Bouzalas, I.; Apostolidi, E.D.; Scalas, D.; Davidopoulou, E.; Chassalevris, T.; Rosati, S.; Colitti, B. A combined approach for the characterization of small ruminant lentivirus strains circulating in the islands and mainland of Greece. Animals 2024, 14, 1119. [Google Scholar] [CrossRef] [PubMed]
- Colitti, B.; Daif, S.; Choukri, I.; Scalas, D.; Jerre, A.; El Berbri, I.; Fassi Fihri, Q.; Rosati, S. Serological and molecular characterization of small ruminant lentiviruses in Morocco. Animals 2024, 14, 550. [Google Scholar] [CrossRef] [PubMed]
- Peterson, K.; van den Brom, R.; Aalberts, M.; Bogt-Kappert, C.T.; Vellema, P. Loss of caprine arthritis encephalitis virus (CAEV) herd accreditation: Characteristics, diagnostic approach, and specific follow-up scenarios on large dairy goat farms. Pathogens 2022, 11, 1541. [Google Scholar] [CrossRef]
- Shuralev, E.A.; Khammadov, N.I.; Osyanin, K.A.; Elizarova, I.A.; Salmanova, G.R.; Shamaev, N.D.; Petrov, S.V.; Whelan, C.; Saushkin, N.Y.; Samsonova, J.V.; et al. Initial multi-target approach shows importance of improved caprine arthritis-encephalitis virus control program in Russia for hobbyist goat farms. Vet. World 2021, 14, 1718–1726. [Google Scholar] [CrossRef]

| Very Small (1–20 Goats) | Small (21–50 Goats) | Medium (51–100 Goats) | Large (>100 Goats) | Overall | |
|---|---|---|---|---|---|
| Number of tests | 231 | 273 | 181 | 533 | 1218 |
| Negative | 225 | 227 | 115 | 278 | 845 |
| Positive | 6 | 46 | 66 | 255 | 373 |
| Apparent prevalence (%) | 2.6% | 16.9% | 36.5% | 47.8% | 30.6% |
| Herd Size | Mean | 2.5% | 97.5% |
|---|---|---|---|
| HTP—Very small herds (≤20) | 4.7 | 0.2 | 16.5 |
| HTP—Small herds (21–50) | 24.9 | 6.0 | 52.8 |
| HTP—Medium herds (51–100) | 77.8 | 43.7 | 98.6 |
| HTP—Large herds (>100) | 74.9 | 45.8 | 96.1 |
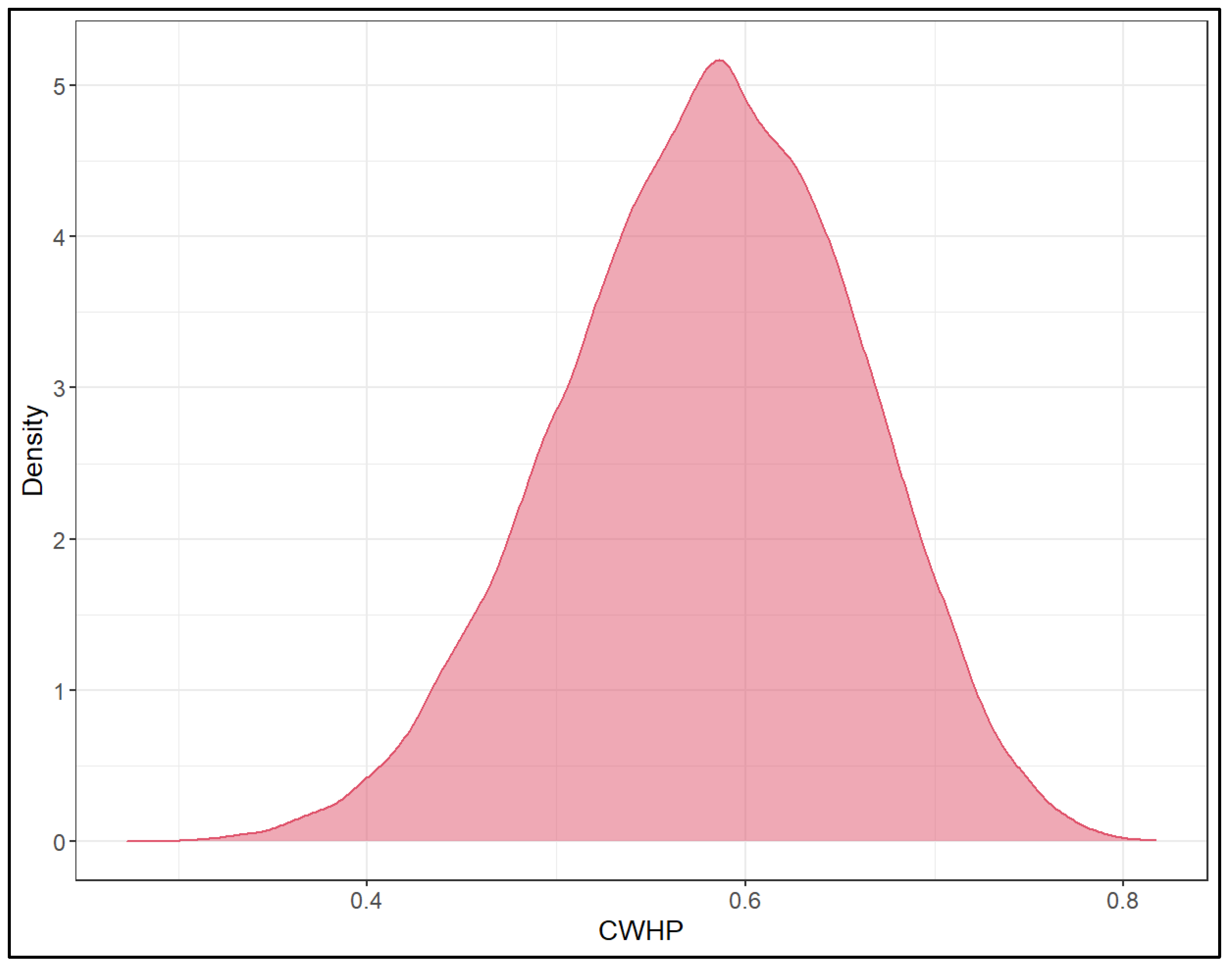
| CWHP | 58.0 | 42.3 | 72.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárdos, K.; Máté, M.; Veres, K.; Lang, Z.; Bertoni, G.; Abril, C.E.; Stuen, S.; Petkevičius, S.; Mickiewicz, M.; Czopowicz, M.; et al. Bayesian Estimation of the True Prevalence of Caprine Arthritis Encephalitis in Hungarian Goat Herds. Viruses 2025, 17, 1455. https://doi.org/10.3390/v17111455
Bárdos K, Máté M, Veres K, Lang Z, Bertoni G, Abril CE, Stuen S, Petkevičius S, Mickiewicz M, Czopowicz M, et al. Bayesian Estimation of the True Prevalence of Caprine Arthritis Encephalitis in Hungarian Goat Herds. Viruses. 2025; 17(11):1455. https://doi.org/10.3390/v17111455
Chicago/Turabian StyleBárdos, Krisztina, Marietta Máté, Katalin Veres, Zsolt Lang, Giuseppe Bertoni, Carlos Eduardo Abril, Snorre Stuen, Saulius Petkevičius, Marcin Mickiewicz, Michał Czopowicz, and et al. 2025. "Bayesian Estimation of the True Prevalence of Caprine Arthritis Encephalitis in Hungarian Goat Herds" Viruses 17, no. 11: 1455. https://doi.org/10.3390/v17111455
APA StyleBárdos, K., Máté, M., Veres, K., Lang, Z., Bertoni, G., Abril, C. E., Stuen, S., Petkevičius, S., Mickiewicz, M., Czopowicz, M., Kaba, J., & Ózsvári, L. (2025). Bayesian Estimation of the True Prevalence of Caprine Arthritis Encephalitis in Hungarian Goat Herds. Viruses, 17(11), 1455. https://doi.org/10.3390/v17111455







