1. Introduction
Dengue, an arboviral disease caused by dengue viruses and transmitted by
Aedes mosquitoes, can lead to mild or severe sickness. The symptoms include fever, aches (such as eye pain, typically behind the eyes, muscle, joint, or bone pain), nausea or rashes. Approximately 5% of individuals who develop dengue will progress to severe dengue, which may result in shock, internal bleeding, and even death [
1,
2]. The dengue virus comprises four distinct serotypes (DENV-1 to DENV-4), contributing to its complex epidemiology. In 2024, the World Health Organization’s (WHO) reported a record 14.4 million dengue cases globally (
https://worldhealthorg.shinyapps.io/dengue_global/, accessed on 15 October 2025), which is more than double the 7 million cases recorded in 2023 [
3]. The real situation might be more serious. One modeling estimate indicates that dengue fever affects more than 400 million people annually worldwide and causes around 22,000 deaths across over 100 countries. Given the lack of specific antiviral treatment, early diagnosis continues to play a critical role in enabling timely treatment and minimizing the risk of severe dengue [
2,
4].
Reverse transcription-polymerase chain reaction (RT-PCR) remains the benchmark method for the early and accurate detection of dengue virus infection [
5,
6]. To ensure the reliability and validity of RT-PCR, the inclusion of positive controls or reference materials in each run is essential. Genomic RNA has become a widely adopted reference standard in molecular diagnostics and assay calibration due to its full-length genomic sequence, ease of production, and suitability for scalable manufacturing [
7,
8]. However, RNA molecules are inherently unstable and highly susceptible to enzymatic (e.g., ribonuclease-mediated) and chemical degradation (e.g., hydrolysis, oxidation), necessitating immediate stabilization via storage at ultra-low temperatures or under desiccated conditions [
7,
9,
10,
11]. This requirement presents significant challenges for resource-limited settings.
To circumvent these constraints, various ambient-temperature stabilization strategies have been investigated. Lyophilization (freeze-drying) [
12], encapsulation within metallic capsules [
10,
11], or entrapment in silica-based microparticles [
13] offer enhanced stability but are often prohibitively expensive, labor-intensive, or ill-suited for deployment in resource-limited or field settings.
Silk fibroin is a natural protein polymer derived from the cocoons of the domesticated silkworm
Bombyx mori. In its native state, silk fibroin adopts a semicrystalline architecture comprising approximately 65% crystalline domains and 35% amorphous regions, which synergistically contribute to the mechanical resilience and structural robustness of the cocoons. Silk fibroin exhibits high solubility in water and can be processed into a regenerated material with a water-stable structure, featuring a predominant β-sheet crystalline conformation. This conformation endows the protein-based matrix with exceptional thermal stability, mechanical strength under tension and resistance to chemical degradation [
14,
15]. Owing to these unique physicochemical properties—particularly its biocompatibility and ability to stabilize labile biomolecules—silk fibroin has emerged as a highly promising matrix for the encapsulation and preservation of thermosensitive biological agents, including whole blood, DNA, and RNA [
14,
16,
17,
18,
19,
20,
21].
In this study, silk fibroin is employed to form films incorporating dengue viral RNA, serving as reference materials to support the development and evaluation of RT-PCR-based dengue virus diagnostic assays. The resulting RNA-silk fibroin films (RNA-SFFs) effectively stabilize dengue viral RNA, with no significant change in RT-qPCR Ct values observed after storage for at least 14 months at 37 °C (longer durations have not yet been evaluated). RNA-SFFs prepared from various concentrations of silk fibroin maintain RNA stability at 25 °C, 37 °C and 45 °C for 16 days. Additionally, the RNA-SFFs exhibit resistance to UV radiation, as evidenced by stable Ct values following 4 h of exposure to UV light at an intensity of over 90 μW/cm2. These results suggest minimal RNA degradation under thermal and environmental stress. Therefore, the developed RNA-SFFs represent a promising cold-chain-free alternative for the long-term storage and transport of reference materials, with potential utility in resource-limited and field-deployable diagnostic settings.
2. Materials and Methods
2.1. Materials
Dengue virus serotypes 1 through 4 were obtained from clinical specimens derived from infected individuals and propagated in
Aedes albopictus C6/36 cells (IVCAS9.087 in National Virus Resource Center, Wuhan Institute of Virology, CAS, Wuhan, China). C6/36 cells were cultured in T75 flasks using MEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. When cells reached 70–80% confluence, they were infected with DENV-1 to DENV-4 at a multiplicity of infection (MOI) ranging from 0.01 to 0.1. The infected cultures were incubated at 37 °C in a 5% CO
2 humidified incubator until pronounced cytopathic effect (CPE) was observed (3–4 days). Viral supernatants were then collected for RNA extraction. Although the initial infectious virus titer was not determined by plaque assay or TCID
50, the viral load in the supernatant was assessed by RT-qPCR, yielding Ct values between 10 and 15, indicating high levels of viral RNA. Viral nucleic acids were isolated utilizing the QIAamp
® Viral RNA Mini Kit (Qiagen, Hilden, Germany), following the manufacturer’s protocol. The HiScript
® II One Step qRT-PCR Probe Kit (Vazyme Biotech Co., Ltd., Nanjing, China) was used for RT-qPCR. Cocoons were purchased from Crystalgen Ningbo Biotech Ltd. (Ningbo, China). Lithium bromide and sodium carbonate were bought from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China) and Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All primers and fluorescently labeled probes were commercially synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). Nuclease-free water, essential for molecular assays to prevent degradation of nucleic acids, was sourced from Source Leaf Biotech Co., Ltd. (Shanghai, China). The primer/probe sets for the four dengue virus serotypes 1 through 4 are listed in
Table 1. Ultrapure water with a resistivity of 18.2 MΩ·cm was employed in all experiments.
2.2. Preparation and Quantification of Silk Fibroin Solution from Silk Cocoons
Silk fibroin solution was prepared following the established protocol described by Rockwood D. et al. [
16], with minor modifications. Approximately 2.5 g of
Bombyx mori silk cocoons were manually cut into fragments of nail-clipping size using sterilized scissors. To facilitate degumming, 2.12 g of sodium carbonate was dissolved in 1 L of ultrapure water, which was heated to boiling to dissolve. The cocoon pieces were introduced into the boiling sodium carbonate solution and stirred vigorously at 110–200 rpm for 50 min to remove sericin. Following cooling, the degummed silk fibers were retrieved, transferred to 1 L of fresh ultrapure water, and subjected to magnetic stirring for 20 min to remove residual salts. This washing step was repeated three times with complete water replacement. The purified fibers were then dried overnight in a fume hood. The dried silk fibroin was weighed (about 1.70 g). For dissolution, 8.1 g of lithium bromide (LiBr) was dissolved in 10 mL of ultrapure water. The dried fibers were solubilized in the LiBr solution at a ratio of 1:4 (for example, 1.7 g silk fibers to 6.8 mL LiBr solution), followed by incubation at 60 °C for 4 h to ensure complete dissolution of the protein matrix. The resulting solution was dialyzed against 1 L of ultrapure water at 4 °C for 72 h, with the dialysate replaced three times daily to eliminate residual LiBr. After dialysis, the aqueous silk fibroin solution was centrifuged at 9000×
g and 4 °C for 20 min to remove insoluble aggregates. The supernatant was collected and subjected to a second centrifugation under identical conditions to ensure maximal clarity. The purified silk fibroin solution was stored at 4 °C for up to one month and used in subsequent experiments as required. The concentration of the prepared silk fibroin solution was determined as follows. A clean Petri dish lid was pre-weighed (mass recorded as m
1 in grams). Exactly 500 μL of the silk fibroin solution was pipetted onto the lid and dried at 60 °C for 1 h to constant weight. The lid with dried fibroin was weighed (mass recorded as m
2 in grams, and the mass difference in mass (m
2 − m
1). The concentration of the prepared silk fibroin solution is calculated as:
The unit of the protein concentration is in % w/v. For example, if the mass difference is 0.035 g, then the concentration is 0.035 divided by 0.5, which is 7.0% w/v.
2.3. Evaluation of Film Formation Efficiency of the Silk Fibroin on Various Substrate Surfaces
The prepared silk fibroin solution in
Section 2.2 was quantified and adjusted to a final concentration of 7.0%
w/
v. To investigate the influence of substrate properties on film formation and ease of harvest, 10 μL of RNase-free water was mixed with 10 μL of the 7.0%
w/
v silk fibroin solution. The resulting blend was cast onto various surfaces to form circular films with a diameter of approximately 1 cm, including a Petri dish (plastic), a Petri dish (glass), aluminum foil, the inner surface of an incised new sealable plastic bag, and the interior of microcentrifuge tubes. Samples were air-dried under ambient conditions to form thin solid films. The time required for complete film formation and the ease of film detachment were assessed for each substrate.
2.4. RT-qPCR System and Protocol
The RT-qPCR assay was carried out in a total reaction volume of 20 μL, comprising 5 μL of template RNA, 10 μL of 2× one-step reaction mix, 1 μL of enzyme mix, 0.4 μL each of 10 μM forward and reverse primers, 0.4 μL of 10 μM fluorescent probe, and nuclease-free water to achieve the final volume. The RT-qPCR reactions were run in duplicate. Amplification and fluorescence detection were performed using the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) or MA-6000 (Yarui Biotech, Suzhou, China) under the following thermal cycling conditions: an initial reverse transcription phase at 50 °C for 15 min, followed by initial denaturation at 95 °C for 30 s. This was succeeded by 45 consecutive cycles of amplification, each consisting of a denaturation step at 95 °C for 10 s and an annealing/extension step at 60 °C for 30 s, with fluorescence acquisition occurring during the latter phase of each cycle. The Ct thresholds for all qPCR runs were set automatically by the instrument’s software (Bio-Rad CFX Manager version 3.1, Hercules, CA, USA) or MA-6000 (Yarui Biotech, Suzhou, China).
2.5. The Effect of Silk Fibroin Films (SFFs) on Ct Values of DENV-1
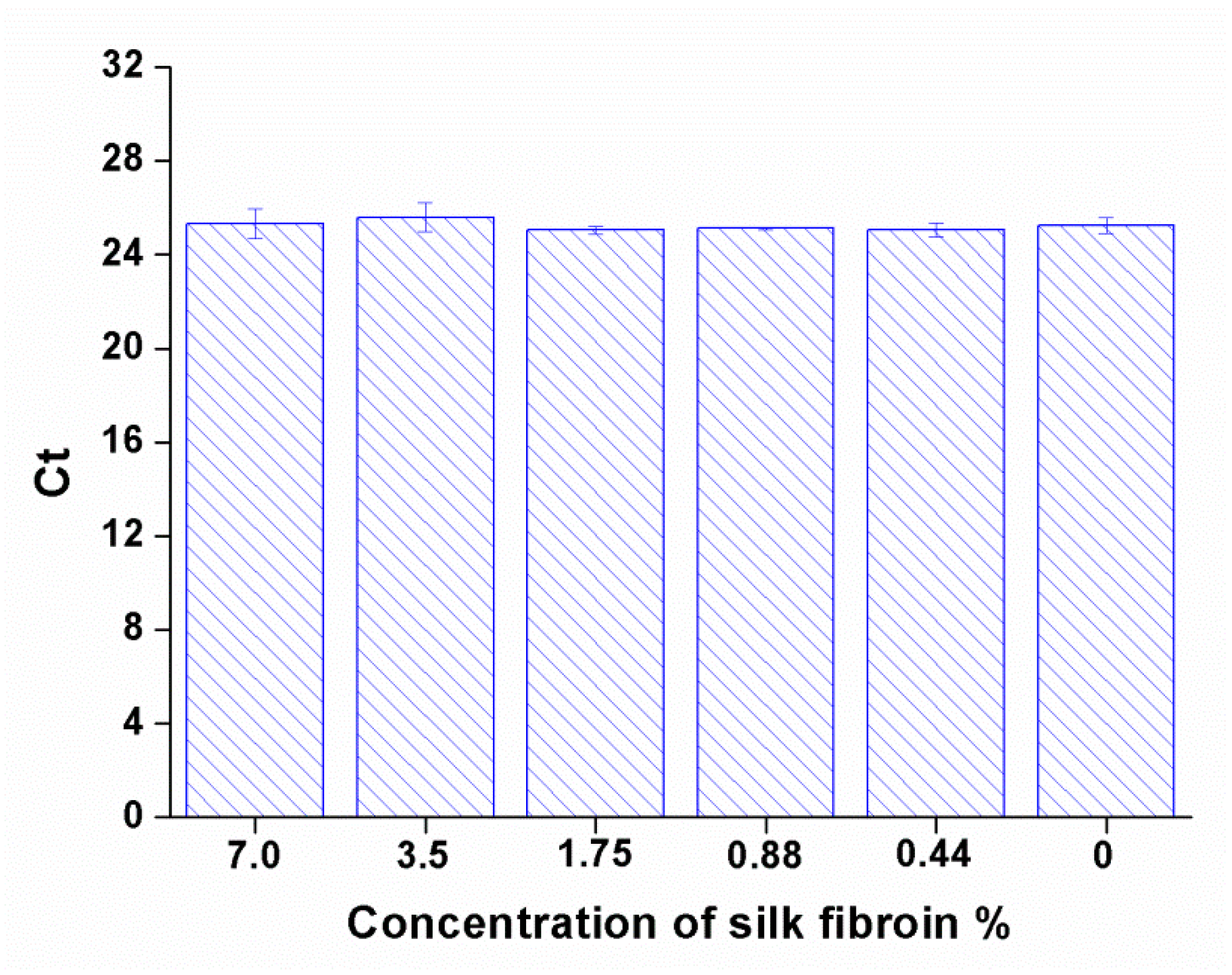
Evaluation of the effect of silk fibroin on Ct values of DENV-1 RNA through RT-qPCR: To assess potential matrix-induced effects of silk fibroin on RT-qPCR quantification, a dilution series of silk fibroin solutions was prepared from 7.0% w/v using a two-fold serial dilution method, yielding concentrations of 7.0%, 3.5%, 1.75%, 0.88%, and 0.44% w/v. Here, a wide range of the silk fibroin concentrations were used to identify the threshold at which silk fibroin might begin to interfere with RT-qPCR. For each concentration, 10 μL of DENV-1 RNA was mixed with 10 μL of the respective silk fibroin solution, resulting in a 1:1 (v/v) RNA-silk matrix. The mixtures were then directly subjected to RT-qPCR analysis without prior processing.
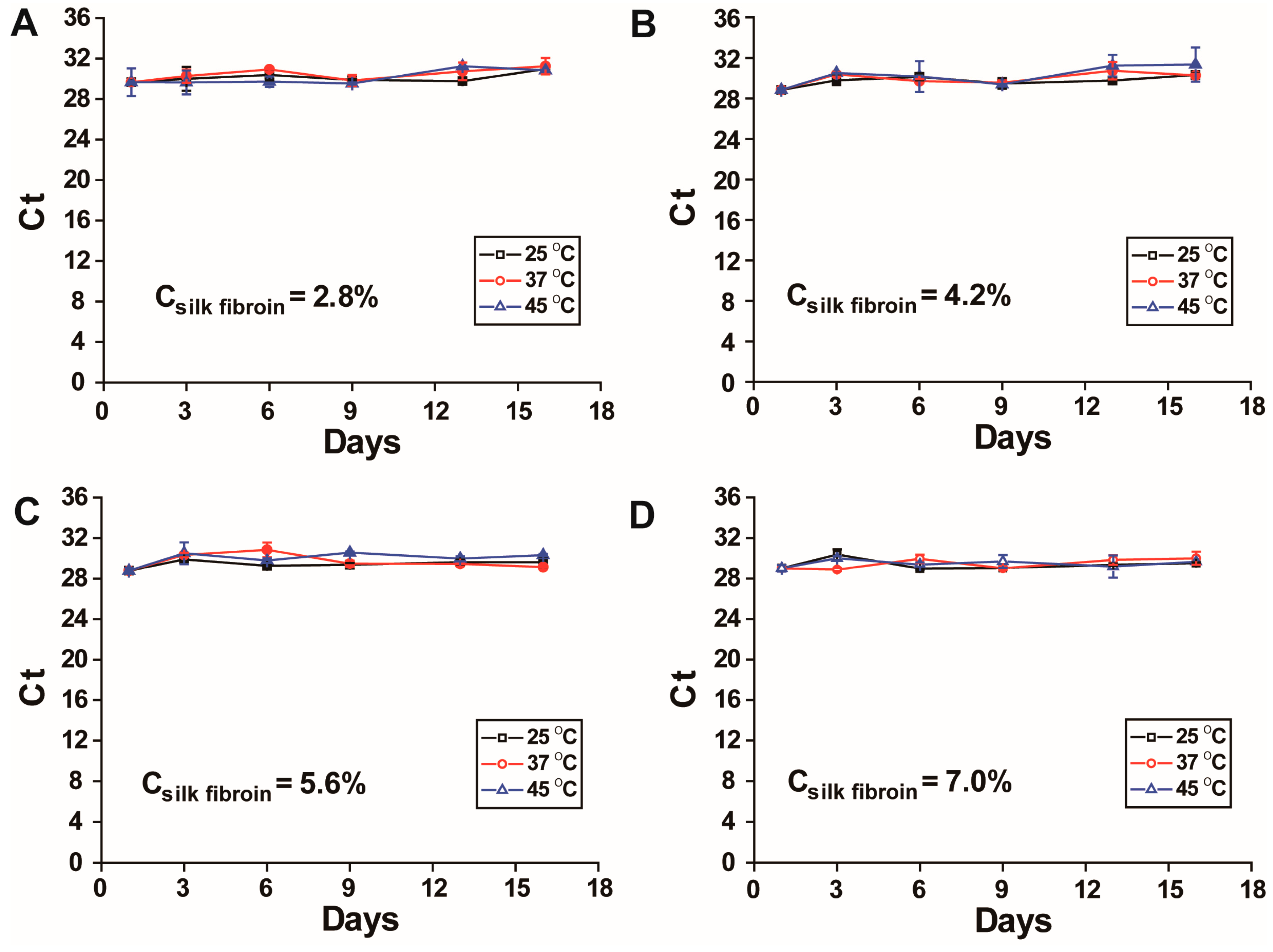
Assessment of DENV-1 RNA stability in the form of RNA-silk fibroin films (RNA-SFFs) prepared from silk fibroin solutions of varying concentrations: Silk fibroin solutions at concentrations of 7.0%, 5.6%, 4.2% and 2.8%
w/
v were prepared by serial dilution of the stock solution with nuclease-free water (Source Leaf Biotech Co., Ltd., Shanghai, China), which are different from the concentrations used above due to that very low concentrations may compromise film integrity. The mixtures of 10 μL of DENV-1 viral RNA and 10 μL of each silk fibroin formulation were spread onto the inner surfaces of sealed polyethylene bags and air-dried under ambient conditions for 30 min to 1 h to yield thin, solid composite films. The resulting RNA-SFFs were transferred into 1.5 mL microcentrifuge tubes and stored under controlled thermal conditions at 25 °C, 37 °C and 45 °C. The preparation process of dengue virus RNA–silk fibroin films (SFFs) is illustrated in
Figure 1. At days 1, 3, 6, 9, 13 and 16, individual samples were retrieved, and each film was rehydrated by adding 500 μL of RNase-free water. Samples were vortexed vigorously to ensure complete dissolution and homogeneous resuspension of RNA. The recovered RNA was then immediately subjected to RT-qPCR, and the cycle threshold (Ct) values were recorded.
2.6. Assessment of DENV-1 RNA Stability in the Form of RNA-SFFs
To evaluate the protective effect of RNA in the form of RNA-SFFs against UV-induced degradation, samples were exposed to UV radiation at an irradiance intensity exceeding 90 μW/cm
2 for durations of 1 h, 2 h, and 4 h. The irradiance level was chosen to reflect typical conditions used for surface decontamination in biological safety cabinets and clean benches. DENV-1 RNA-SFFs were prepared following the protocol outlined in
Section 2.5, and naked DENV-1 RNA in solution served as the control.
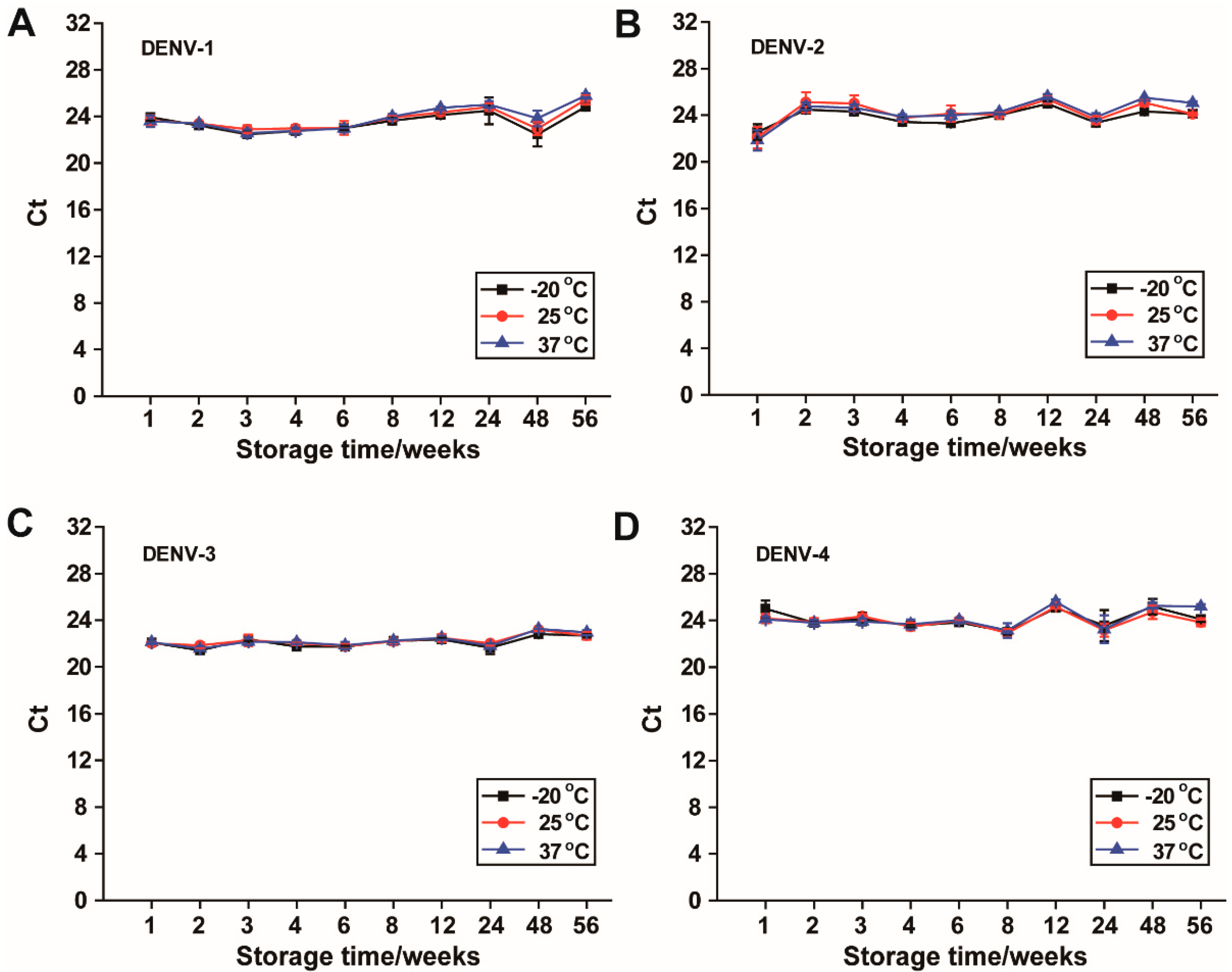
2.7. Long-Term Storage of the Prepared DENV-1–4 RNA-SFFs at −20 °C, 25 °C and 37 °C
Dengue virus serotypes 1–4 RNA were individually encapsulated into SFFs using the protocol described above. The prepared RNA-SFFs were stored at −20 °C, 25 °C, and 37 °C to assess long-term RNA stability under varying thermal conditions. No special precautions against light exposure were taken during storage, and temperature fluctuations were kept to a minimum to ensure consistent experimental conditions. At 1, 2, 3, 4, 6, 8, 12, 24, 48 and 56 weeks, individual films were removed from storage and dissolved in RNase-free water according to the rehydration procedure mentioned above. The dissolved RNA was then subjected to serotype-specific RT-qPCR using corresponding primer-probe sets as listed in
Table 1. Ct values were recorded at each time point to monitor Dengue RNA stability.
2.8. Data Statistics
Data are presented as the mean ± standard deviation (SD) derived from three independent replicates. Statistical significance between experimental groups at different temperature was assessed by one-way ANOVA (and nonparametric or mixed) with Dunn’s multiple comparisons test using GraphPad Prism 9.0.0 (GraphPad Software Inc., San Diego, CA, USA). A probability value of p < 0.05 was designated as statistically significant.
4. Discussion
Positive controls play a pivotal role in RT-PCR-based assays for the diagnosis of viral infections. Dengue viral genomic RNA serves as a critical positive control with advantages of containing all targets of dengue viruses to validate all RT-PCR-based diagnostic kits. However, RNA is inherently labile and highly susceptible to degradation by RNases, chemicals and environmental stressors such as heat and UV radiation. The storage and transport of RNA-based controls depend on cold-chain infrastructure, which limits the practical utility in resource-limited or field-deployable diagnostic settings.
In this study, we demonstrate that dengue RNA-SFFs can effectively stabilize dengue genomic RNA under challenging conditions. Specifically, RNA in SFFs remains stable for up to 14 months at 37 °C, up to 16 days at 45 °C, and for a minimum of 4 h under intense UV irradiation (>90 μW/cm2). These results highlight the robust protective capacity of silk fibroin matrices against both thermal and photodegradation.
Notably, we found that silk fibroin, even at a final concentration of 3.5%
w/
v (derived from the original 7.0%
w/
v solution shown in
Figure 2), did not significantly affect RT-qPCR Ct values. This observation contrasts with a previous report [
21], in which silk concentrations exceeding 1%
w/
v were shown to interfere with Ct values, although this interference was mitigated by RNA purification. The discrepancy may stem from differences in experimental conditions, including variations in silk fibroin preparation methods, PCR master mix composition, or RNA type. Importantly, in our system, RNA can be directly detected from the dissolved films without prior extraction, enabling a simpler and more user-friendly workflow. Consistent with our findings, RNA in SFFs remained stable at elevated temperatures, including up to 45 °C, further supporting the potential for using RNA-SFFs-based reference materials in extreme or resource-limited environments.
To the best of our knowledge, stability for 14 months at 37 °C represents the longest reported storage duration for viral RNA reference materials under non-refrigerated conditions to date. A recent study [
10] demonstrated that RNA encapsulated in dehydrated form within metallic capsules remained stable for up to 3 years at room temperature, as assessed by RT-qPCR. However, that method relies on proprietary technology developed by a commercial entity. Due to intellectual property restrictions and cost considerations, this approach is not readily adaptable for use in independently developed diagnostic assays or in-house testing systems. In contrast our method demonstrated consistent RNA protection across all four dengue virus serotypes, indicating that stability is maintained regardless of genomic sequence variation. This pan-serotypic efficacy underscores the versatility and generalizability of the silk fibroin matrix, making it particularly valuable for multivalent diagnostic kits where balanced detection sensitivity across serotypes is essential.
Several limitations of this study should be acknowledged. First, while this work provides proof-of-concept, the development of RNA-SFFs-based materials into standardized reference materials will require precise quantification of dengue viral RNA copy number in the films, followed by multi-laboratory validation to ensure reproducibility and comparability across testing sites. Second, although the materials exhibited excellent long-term stability, the upper limits of stability—particularly under higher temperatures and more extreme environmental conditions—have not yet been fully defined. Further studies using accelerated aging protocols (e.g., elevated temperatures, variable humidity) are needed to establish shelf life and define optimal storage boundaries. As well, the cost-effectiveness and scalability of large-scale production, and the effects of different batches of silk fibroin preparations, and stability tests by different persons, require further investigation. In addition, the evaluation of RNA stability across a broad dynamic range, particularly at low-copy inputs (e.g., Ct > 30) that are representative of weak-positive or near-limit-of-detection diagnostic controls should be studies further. Moreover, the molecular mechanisms underlying the exceptional thermal stability of silk fibroin-RNA films, especially at temperatures up to 45 °C, remain unclear. A deeper understanding of the interactions between silk fibroin and nucleic acids, as well as the role of water exclusion dynamics during film formation could guide the rational design of more robust biomaterials. Such insights may not only improve RNA preservation but also expand the application of silk fibroin in stabilizing other labile biologics such as vaccines and biopharmaceuticals, thereby contributing to global health.