Diagnostic Performance of a Silver-Amplified vs. a Non-Amplified Lateral Flow Kit for Adenoviral Conjunctivitis: A Multicenter Prospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blinding and Operational Details
2.3. Clinical Specimens
2.4. LF Testing
2.5. AdV DNA Extraction
2.6. PCR Systems and Sequencing
2.7. AdV Typing
2.8. Statistical Analysis
3. Results
3.1. Diagnostic Performance of Lateral Flow Kits Compared with qPCR
3.2. LF Kit Positivity by Adenovirus Copy Number
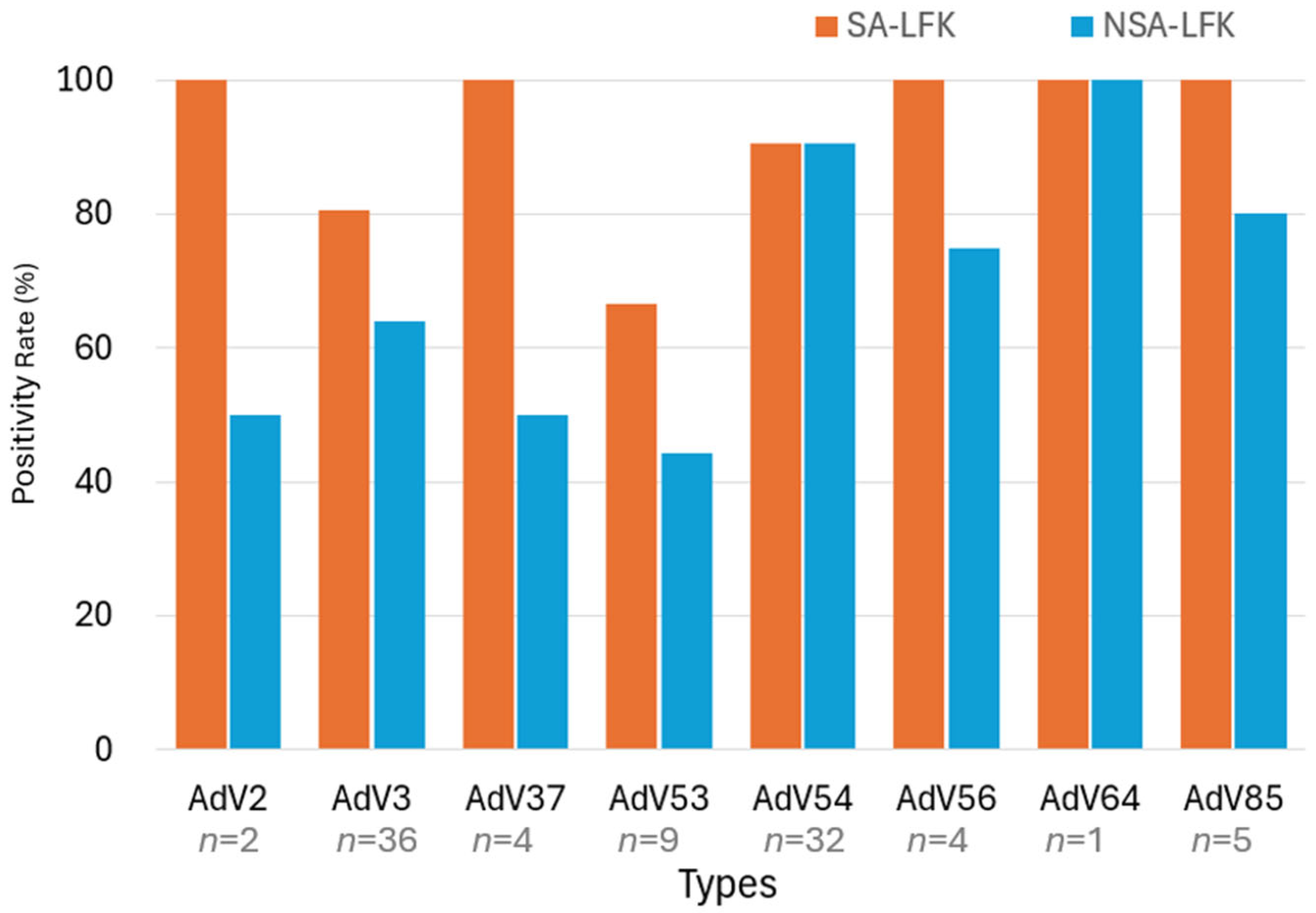
3.3. Genotyping by Hexon Nested PCR and LF Kit Positivity
3.4. Additional PCR Typing Results
3.5. Diagnostic Performance of Lateral Flow Kits by Clinical Severity
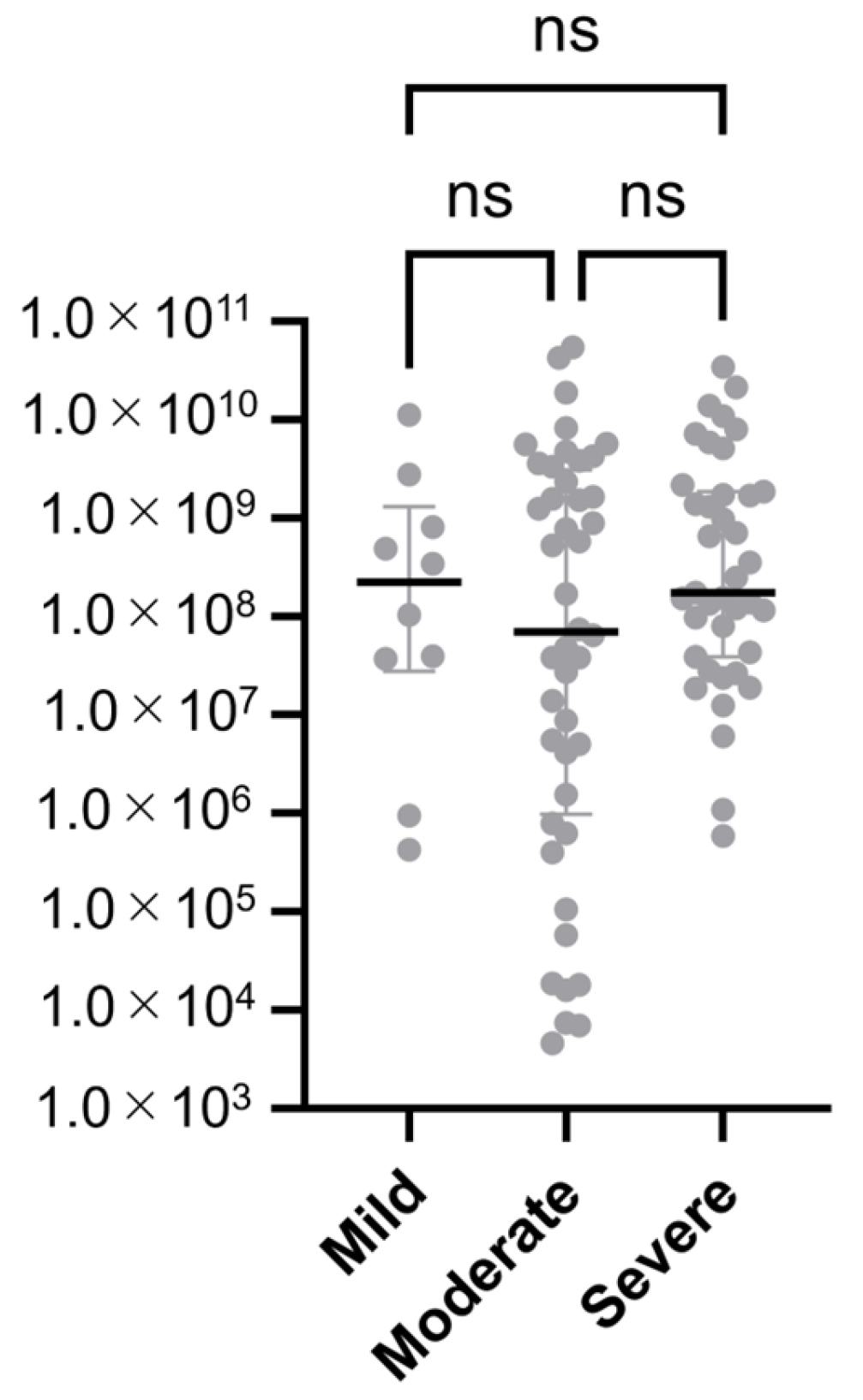
3.6. Adenoviral Load by Clinical Severity
4. Discussion
4.1. Overview of Study Findings
4.2. Interpretation and Broader Context
4.3. Clinical and Public Health Implications
4.4. Future Research Directions
4.5. Limitations
4.6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AdV | Adenovirus |
| BLAST | Basic Local Alignment Search Tool |
| EKC | Epidemic Keratoconjunctivitis |
| LF | Lateral Flow |
| SA-LFK | Silver-Amplified Lateral Flow Kits |
| NSA-LFKs | Non-Silver-Amplified Lateral Flow Kits |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
| qPCR | Quantitative Real-Time PCR |
References
- Aoki, K.; Gonzalez, G.; Hinokuma, R.; Yawata, N.; Tsutsumi, M.; Ohno, S.; Kitaichi, N. Assessment of clinical signs associated with adenoviral epidemic keratoconjunctivitis cases in southern Japan between 2011 and 2014. Diagn. Microbiol. Infect. Dis. 2019, 95, 114885. [Google Scholar] [CrossRef] [PubMed]
- Ghebremedhin, B. Human adenovirus: Viral pathogen with increasing importance. Eur. J. Microbiol. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Hage, E.; Espelage, W.; Eckmanns, T.; Lamson, D.M.; Panto, L.; Ganzenmueller, T.; Heim, A. Molecular phylogeny of a novel human adenovirus type 8 strain causing a prolonged, multi-state keratoconjunctivitis epidemic in Germany. Sci. Rep. 2017, 7, 40680. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Maruko, I.; Iida, T.; Ohguchi, T.; Aoki, K.; Ohno, S.; Suzutani, T. The possibility of human adenovirus detection from the conjunctiva in asymptomatic cases during nosocomial infection. Cornea 2008, 27, 527–530. [Google Scholar] [CrossRef]
- Fujimoto, T.; Hanaoka, N.; Konagaya, M.; Kobayashi, M.; Nakagawa, H.; Hatano, H.; Tsukahara-Kawamura, T.; Uchio, E.; Kaneko, H. Evaluation of a silver-amplified immunochromatography kit for adenoviral conjunctivitis. J. Med. Virol. 2019, 91, 1030–1035. [Google Scholar] [CrossRef]
- Migita, H.; Ueno, T.; Tsukahara-Kawamura, T.; Saeki, Y.; Hanaoka, N.; Fujimoto, T.; Uchio, E. Evaluation of adenovirus amplified detection of immunochromatographic test using tears including conjunctival exudate in patients with adenoviral keratoconjunctivitis. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 815–820. [Google Scholar] [CrossRef]
- Matsushima, Y.; Nakajima, E.; Ishikawa, M.; Kano, A.; Komane, A.; Fujimoto, T.; Hanaoka, N.; Okabe, N.; Shimizu, H. Construction of new primer sets for corresponding to genetic evolution of human adenoviruses in major capsid genes through frequent recombination. Jpn. J. Infect. Dis. 2014, 67, 495–502. [Google Scholar] [CrossRef]
- Walsh, M.P.; Chintakuntlawar, A.; Robinson, C.M.; Madisch, I.; Harrach, B.; Hudson, N.R.; Schnurr, D.; Heim, A.; Chodosh, J.; Seto, D.; et al. Evidence of molecular evolution driven by recombination events influencing tropism in a novel human adenovirus that causes epidemic keratoconjunctivitis. PLoS ONE 2009, 4, e5635. [Google Scholar] [CrossRef]
- Park, A.; Lee, C.; Lee, J.Y. Genomic Evolution and Recombination Dynamics of Human Adenovirus D Species: Insights from Comprehensive Bioinformatic Analysis. J. Microbiol. 2024, 62, 393–407. [Google Scholar] [CrossRef]
- Okada, M.; Ogawa, T.; Kubonoya, H.; Yoshizumi, H.; Shinozaki, K. Detection and sequence-based typing of human adenoviruses using sensitive universal primer sets for the hexon gene. Arch. Virol. 2007, 152, 1–9. [Google Scholar] [CrossRef]
- Fujimoto, T.; Matsushima, Y.; Shimizu, H.; Ishimaru, Y.; Kano, A.; Nakajima, E.; Adhikary, A.K.; Hanaoka, N.; Okabe, N. A molecular epidemiologic study of human adenovirus type 8 isolates causing epidemic keratoconjunctivitis in Kawasaki City, Japan in 2011. Jpn. J. Infect. Dis. 2012, 65, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Banik, U.; Adhikary, A.K.; Suzuki, E.; Inada, T.; Okabe, N. Multiplex PCR assay for rapid identification of oculopathogenic adenoviruses by amplification of the fiber and hexon genes. J. Clin. Microbiol. 2005, 43, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Hanaoka, N.; Konagaya, M.; Kobayashi, M.; Nakagawa, H.; Hatano, H.; Ikuta, K.; Sekiryu, T.; Fujimoto, T. Five Cases of Epidemic Keratoconjunctivitis Due to Human Adenovirus Type 85 in Fukushima, Japan. Jpn. J. Infect. Dis. 2020, 73, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Darougar, S.; Grey, R.H.; Thaker, U.; McSwiggan, D.A. Clinical and epidemiological features of adenovirus keratoconjunctivitis in London. Br. J. Ophthalmol. 1983, 67, 1–7. [Google Scholar] [CrossRef]
- Aoki, K.; Kaneko, H.; Kitaichi, N.; Ohguchi, T.; Tagawa, Y.; Ohno, S. Clinical features of adenoviral conjunctivitis at the early stage of infection. Jpn. J. Ophthalmol. 2011, 55, 11–15. [Google Scholar] [CrossRef]
- Fujimoto, T.; Okafuji, T.; Okafuji, T.; Ito, M.; Nukuzuma, S.; Chikahira, M.; Nishio, O. Evaluation of a bedside immunochromatographic test for detection of adenovirus in respiratory samples, by comparison to virus isolation, PCR, and real-time PCR. J. Clin. Microbiol. 2004, 42, 5489–5492. [Google Scholar] [CrossRef]
- Kaneko, H.; Hanaoka, N.; Konagaya, M.; Tsukahara-Kawamura, T.; Kobayashi, M.; Nakagawa, H.; Hatano, H.; Ikuta, K.; Fujimoto, T. Conjunctivitis Due to the Human Adenovirus Type 2 Variant Identified during Epidemic Keratoconjunctivitis Surveillance in Japan. Jpn. J. Infect. Dis. 2019, 72, 353–355. [Google Scholar] [CrossRef]
- Dumitrache, M. Ophthalmological Pathology of the Eye: Conjunctiva. In Clinical Ophthalmology, A Guide to Diagnosis and Treatment; Dumitrache, M., Ed.; Springer Nature: Bucharest, Romania, 2024; pp. 93–123. [Google Scholar]
- Tsukahara-Kawamura, T.; Hanaoka, N.; Uchio, E. Evaluation of anti-adenoviral effects of the polyvinyl alcohol iodine ophthalmic solution. Jpn. J. Ophthalmol. 2024, 68, 64–69. [Google Scholar] [CrossRef]
- Labib, B.A.; Minhas, B.K.; Chigbu, D.I. Management of Adenoviral Keratoconjunctivitis: Challenges and Solutions. Clin. Ophthalmol. 2020, 14, 837–852. [Google Scholar] [CrossRef]
- Mitamura, K.; Shimizu, H.; Yamazaki, M.; Ichikawa, M.; Nagai, K.; Katada, J.; Wada, A.; Kawakami, C.; Sugaya, N. Clinical evaluation of highly sensitive silver amplification immunochromatography systems for rapid diagnosis of influenza. J. Virol. Methods 2013, 194, 123–128. [Google Scholar] [CrossRef]
- Kuo, I.C.; Gower, E.W. Cost Savings From a Policy to Diagnose and Prevent Transmission of Adenoviral Conjunctivitis in Employees of a Large Academic Medical Center. JAMA Ophthalmol. 2021, 139, 518–524. [Google Scholar] [CrossRef]
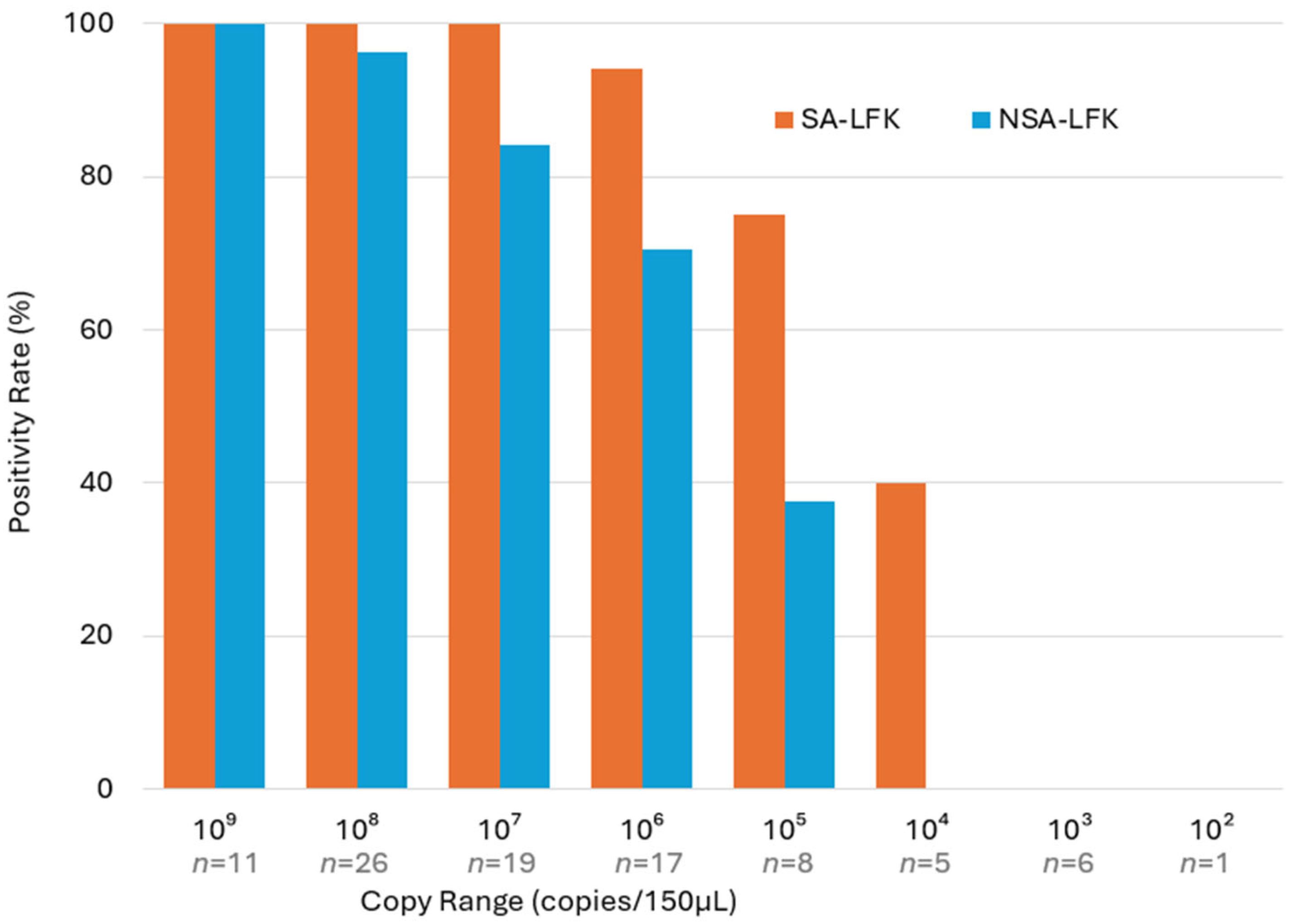
| SA-LFK Kit | NSA-LFK Kit | ||||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Positive | Negative | Total | |||
| qPCR-Positive | 80 | 13 | 67 | 26 | 93 | ||
| qPCR-Negative | 0 | 107 | 2 | 105 | 107 | ||
| Total | 80 | 120 | 69 | 131 | |||
| Kit | Sensitivity (%) | Sensitivity 95% CI | Specificity (%) | Specificity 95% CI | PPV (%) | PPV 95% CI | NPV (%) | NPV 95% CI | False Negative Rate (%) | False Positive Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| SA-LFK | 86.0 | 77.5–91.7 | 100.0 | 96.5–100.0 | 100.0 | 95.4–100.0 | 89.2 | 82.3–93.6 | 14.0 | 0.0 |
| NSA-LFK | 72.0 | 62.2–80.2 | 98.1 | 93.4–99.7 | 97.1 | 90.0–99.5 | 80.2 | 72.5–86.1 | 28.0 | 1.9 |
| Severity | Kit | Sensitivity (%) | Sensitivity 95% CI | Specificity (%) | Specificity 95% CI | PPV (%) | PPV 95% CI | NPV (%) | NPV 95% CI | False Negative Rate (%) | False Positive Rate (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Severe | SA-LFK | 97.4 | 86.8–99.9 | 100 | 90.8–100.0 | 100 | 90.8–100.0 | 97.4 | 86.8–99.9 | 2.6 | 0 |
| Severe | NSA-LFK | 89.7 | 76.4–95.9 | 100 | 90.8–100.0 | 100 | 90.1–100.0 | 90.5 | 77.9–96.2 | 10.3 | 0 |
| Mild + Moderate | SA-LFK | 77.8 | 65.1–86.8 | 100 | 94.7–100.0 | 100 | 91.6–100.0 | 85.2 | 75.9–91.3 | 22.2 | 0 |
| Mild + Moderate | NSA-LFK | 59.3 | 46.0–71.3 | 97.1 | 90.0–99.5 | 94.1 | 80.9–99.0 | 75.3 | 65.4–83.1 | 40.7 | 2.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujimoto, T.; Hanaoka, N.; Takahashi, K.; Kaneko, H.; Kobayashi, M.; Nakagawa, H.; Hatano, H.; Tsukahara-Kawamura, T.; Migita, H.; Nakamura, K.; et al. Diagnostic Performance of a Silver-Amplified vs. a Non-Amplified Lateral Flow Kit for Adenoviral Conjunctivitis: A Multicenter Prospective Study. Viruses 2025, 17, 1442. https://doi.org/10.3390/v17111442
Fujimoto T, Hanaoka N, Takahashi K, Kaneko H, Kobayashi M, Nakagawa H, Hatano H, Tsukahara-Kawamura T, Migita H, Nakamura K, et al. Diagnostic Performance of a Silver-Amplified vs. a Non-Amplified Lateral Flow Kit for Adenoviral Conjunctivitis: A Multicenter Prospective Study. Viruses. 2025; 17(11):1442. https://doi.org/10.3390/v17111442
Chicago/Turabian StyleFujimoto, Tsuguto, Nozomu Hanaoka, Kenichiro Takahashi, Hisatoshi Kaneko, Masaaki Kobayashi, Hisashi Nakagawa, Hiroshi Hatano, Tomoko Tsukahara-Kawamura, Hironori Migita, Kentaro Nakamura, and et al. 2025. "Diagnostic Performance of a Silver-Amplified vs. a Non-Amplified Lateral Flow Kit for Adenoviral Conjunctivitis: A Multicenter Prospective Study" Viruses 17, no. 11: 1442. https://doi.org/10.3390/v17111442
APA StyleFujimoto, T., Hanaoka, N., Takahashi, K., Kaneko, H., Kobayashi, M., Nakagawa, H., Hatano, H., Tsukahara-Kawamura, T., Migita, H., Nakamura, K., Kuramoto, K., & Uchio, E. (2025). Diagnostic Performance of a Silver-Amplified vs. a Non-Amplified Lateral Flow Kit for Adenoviral Conjunctivitis: A Multicenter Prospective Study. Viruses, 17(11), 1442. https://doi.org/10.3390/v17111442






