Rational Design of a Potent Two-Phage Cocktail Against a Contemporary Acinetobacter baumannii Strain Recovered from a Burned Patient at the Lausanne University Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Bacteriophages, and Antibiotics
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Phage Amplification and Titration of Phage Suspensions
2.4. Genome Extraction, Sequencing, and Annotation
2.5. Electron Microscopy
2.6. Turbidity Assay and Virulence Index Determination
2.7. Synogram
2.8. Time–Kill Assay
2.9. Proteomic Analyses
2.10. Bacterial Virulence Testing in Galleria mellonella
2.11. Antibacterial Activity of Phages in the Galleria mellonella Model of Infectious Diseases
3. Results
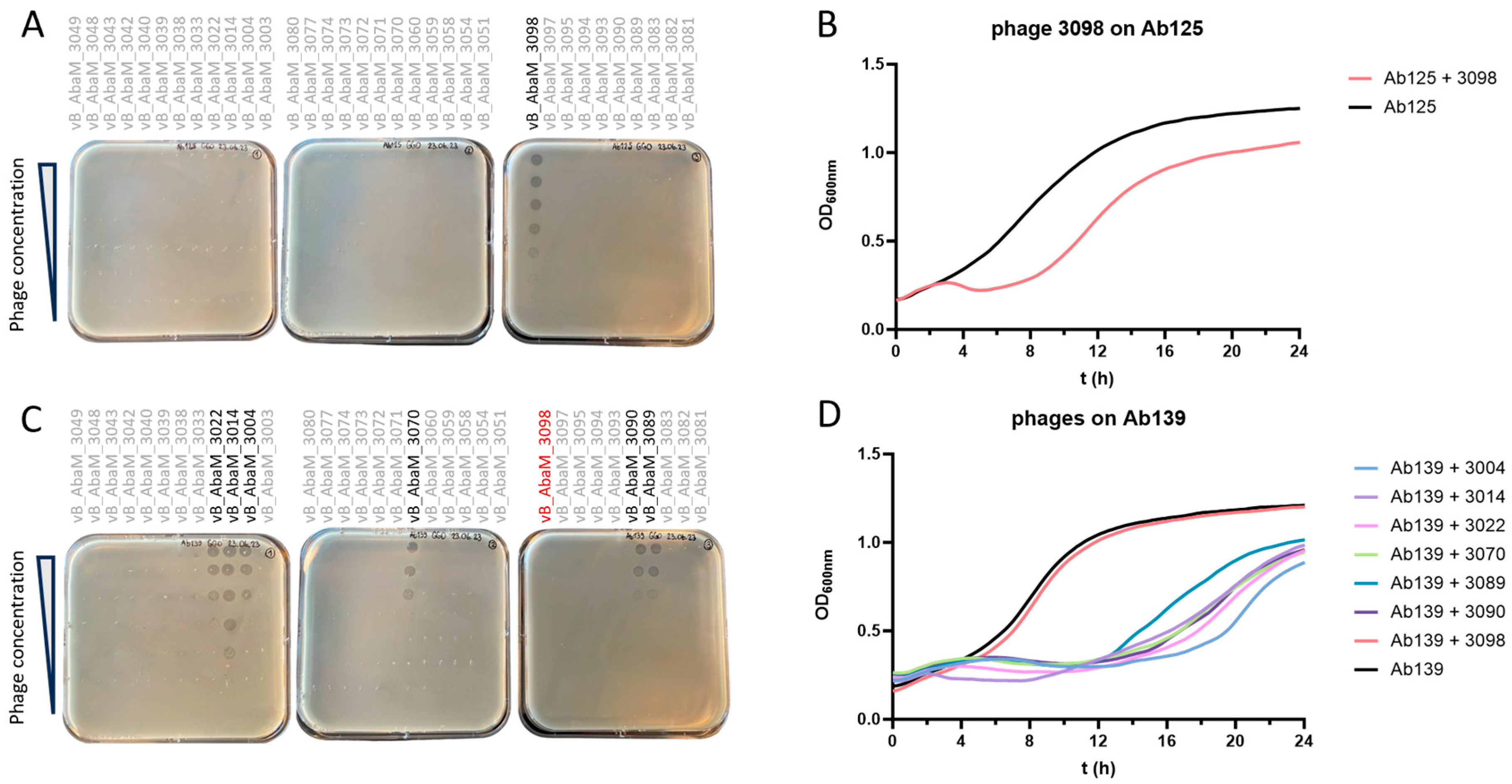
3.1. Ab125 Challenge with Phage 3098 Restored Susceptibility to Different Phages
3.2. Comparative Genomics Between Ab125 and Ab139 Identified Genomic Variants
3.3. Proteome Profiling Does Not Correlate with the Identified Genomic Variants
3.4. Ab139 Showed No Impaired Virulence In Vivo
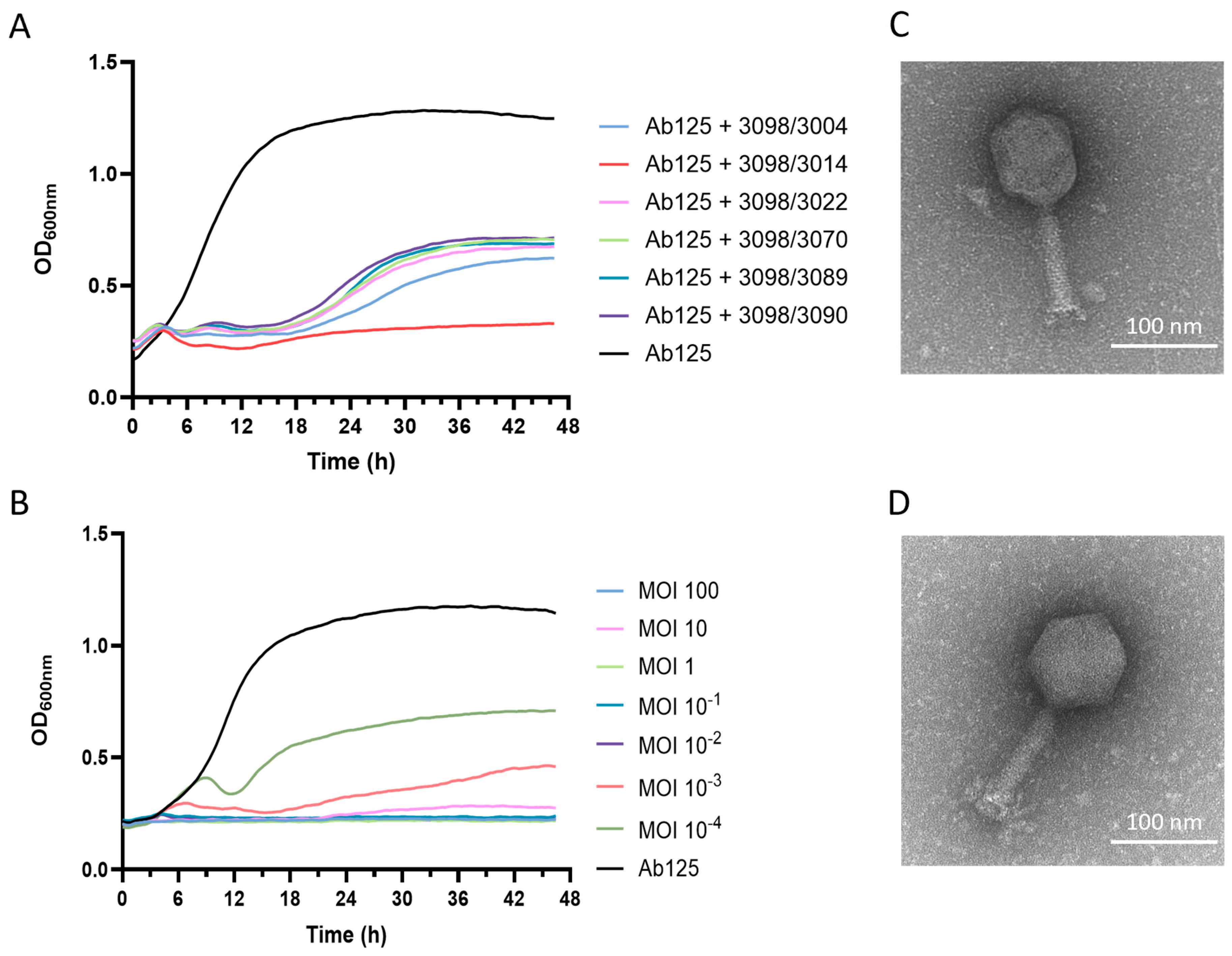
3.5. A Phage Cocktail Composed of Phage 3098 and a “Non-Active” Phage Fully Inhibited the Growth of the Parental Strain Ab125
3.6. Both Phages 3014 and 3098 Were Suited for Phage Therapy
3.7. The High Potency of the 3014/3098 Combination Was Confirmed In Vivo
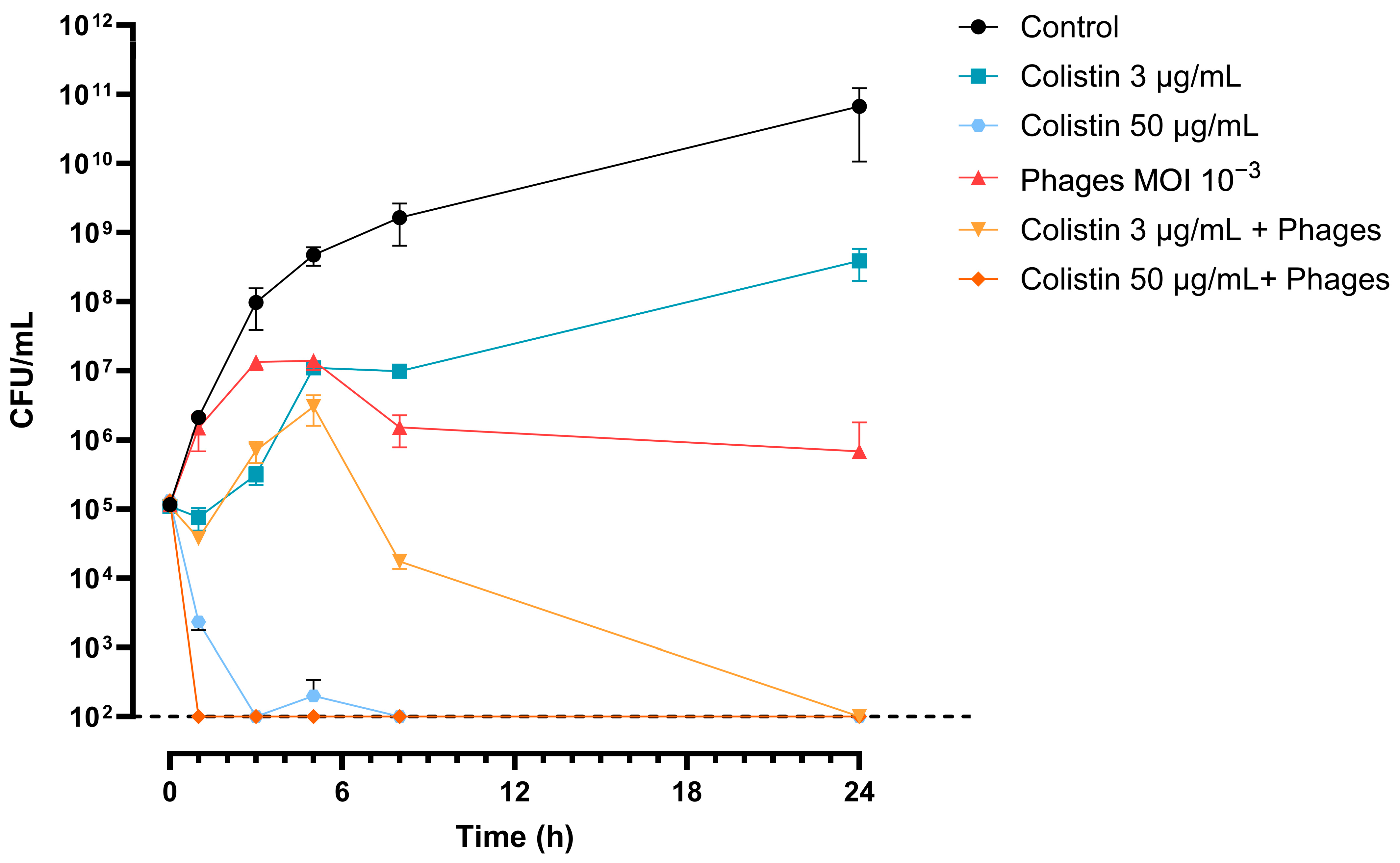
3.8. The 3014/3098 Phage Cocktail Showed Additivity with Colistin In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ibrahim, S.; Al-Saryi, N.; Al-Kadmy, I.M.S.; Aziz, S.N. Multidrug-resistant Acinetobacter baumannii as an emerging concern in hospitals. Mol. Biol. Rep. 2021, 48, 6987–6998. [Google Scholar] [CrossRef]
- Antunes, L.C.; Visca, P.; Towner, K.J. Acinetobacter baumannii: Evolution of a global pathogen. Pathog. Dis. 2014, 71, 292–301. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Weiner-Lastinger, L.M.; Abner, S.; Edwards, J.R.; Kallen, A.J.; Karlsson, M.; Magill, S.S.; Pollock, D.; See, I.; Soe, M.M.; Walters, M.S.; et al. Antimicrobial-resistant pathogens associated with adult healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network, 2015–2017. Infect. Control Hosp. Epidemiol. 2020, 41, 1–18. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses: Unlocking the greatest biodiversity on Earth. Genome 2013, 56, 542–544. [Google Scholar] [CrossRef] [PubMed]
- Vos, M.; Birkett, P.J.; Birch, E.; Griffiths, R.I.; Buckling, A. Local Adaptation of Bacteriophages to Their Bacterial Hosts in Soil. Science 2009, 325, 833. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Young, R. Phage lysis: Do we have the hole story yet? Curr. Opin. Microbiol. 2013, 16, 790–797. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and Limitations of Bacteriophages for the Treatment of Bacterial Infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized bacteriophage therapy outcomes for 100 consecutive cases: A multicentre, multinational, retrospective observational study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Ferry, T.; Resch, G. Recent progress toward the implementation of phage therapy in Western medicine. FEMS Microbiol. Rev. 2022, 46, fuab040. [Google Scholar] [CrossRef]
- Available online: https://www.clinicaltrials.gov/search?intr=Phage%20Therapy (accessed on 26 March 2025).
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standards; CLSI: Malvern, PA, USA, 2015; Volume 26, p. 27. [Google Scholar]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Bioinformatics: Cambridge, UK, 2010. [Google Scholar]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Mikheenko, A.; Saveliev, V.; Hirsch, P.; Gurevich, A. WebQUAST: Online evaluation of genome assemblies. Nucleic Acids Res. 2023, 51, W601–W606. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- DePristo, M.A.; Banks, E.; Poplin, R.; Garimella, K.V.; Maguire, J.R.; Hartl, C.; Philippakis, A.A.; del Angel, G.; Rivas, M.A.; Hanna, M.; et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat. Genet. 2011, 43, 491–498. [Google Scholar] [CrossRef]
- Vasimuddin, M.; Misra, S.; Li, H.; Aluru, S. Efficient Architecture-Aware Acceleration of BWA-MEM for Multicore Systems. In Proceedings of the 2019 IEEE International Parallel and Distributed Processing Symposium (IPDPS), Rio de Janeiro, Brazil, 20–24 May 2019; pp. 314–324. [Google Scholar]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Bouras, G.; Nepal, R.; Houtak, G.; Psaltis, A.J.; Wormald, P.J.; Vreugde, S. Pharokka: A fast scalable bacteriophage annotation tool. Bioinformatics 2023, 39, btac776. [Google Scholar] [CrossRef]
- von Meijenfeldt, F.A.B.; Arkhipova, K.; Cambuy, D.D.; Coutinho, F.H.; Dutilh, B.E. Robust taxonomic classification of uncharted microbial sequences and bins with CAT and BAT. Genome Biol. 2019, 20, 217. [Google Scholar] [CrossRef]
- Hockenberry, A.J.; Wilke, C.O. BACPHLIP: Predicting bacteriophage lifestyle from conserved protein domains. PeerJ 2021, 9, e11396. [Google Scholar] [CrossRef] [PubMed]
- Storms, Z.J.; Teel, M.R.; Mercurio, K.; Sauvageau, D. The Virulence Index: A Metric for Quantitative Analysis of Phage Virulence. PHAGE 2020, 1, 27–36. [Google Scholar] [CrossRef]
- Nikolic, I.; Vukovic, D.; Gavric, D.; Cvetanovic, J.; Aleksic Sabo, V.; Gostimirovic, S.; Narancic, J.; Knezevic, P. An Optimized Checkerboard Method for Phage-Antibiotic Synergy Detection. Viruses 2022, 14, 1542. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Terminology relating to methods for the determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infect. 2000, 6, 503–508. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Muller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-pot, solid-phase-enhanced sample preparation for proteomics experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef]
- Cox, J.; Neuhauser, N.; Michalski, A.; Scheltema, R.A.; Olsen, J.V.; Mann, M. Andromeda: A peptide search engine integrated into the MaxQuant environment. J. Proteome Res. 2011, 10, 1794–1805. [Google Scholar] [CrossRef]
- Iovleva, A.; Fowler, V.G., Jr.; Doi, Y. Treatment Approaches for Carbapenem-Resistant Acinetobacter baumannii Infections. Drugs 2025, 85, 21–40. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.; Trivedi, M.; Dwivedi, M. An insight into MDR Acinetobacter baumannii infection and its pathogenesis: Potential therapeutic targets and challenges. Microb. Pathog. 2024, 192, 106674. [Google Scholar] [CrossRef] [PubMed]
- Manley, R.; Fitch, C.; Francis, V.; Temperton, I.; Turner, D.; Fletcher, J.; Phil, M.; Michell, S.; Temperton, B. Resistance to bacteriophage incurs a cost to virulence in drug-resistant Acinetobacter baumannii. J. Med. Microbiol. 2024, 73, 1829. [Google Scholar] [CrossRef]
- Bai, J.; Raustad, N.; Denoncourt, J.; van Opijnen, T.; Geisinger, E. Genome-wide phage susceptibility analysis in Acinetobacter baumannii reveals capsule modulation strategies that determine phage infectivity. PLoS Pathog. 2023, 19, e1010928. [Google Scholar] [CrossRef]
- Weel-Sneve, R.; Bjoras, M.; Kristiansen, K.I. Overexpression of the LexA-regulated tisAB RNA in E. coli inhibits SOS functions; implications for regulation of the SOS response. Nucleic Acids Res. 2008, 36, 6249–6259. [Google Scholar] [CrossRef][Green Version]
- Butala, M.; Zgur-Bertok, D.; Busby, S.J. The bacterial LexA transcriptional repressor. Cell Mol. Life Sci. 2009, 66, 82–93. [Google Scholar] [CrossRef]
- Fornelos, N.; Butala, M.; Hodnik, V.; Anderluh, G.; Bamford, J.K.; Salas, M. Bacteriophage GIL01 gp7 interacts with host LexA repressor to enhance DNA binding and inhibit RecA-mediated auto-cleavage. Nucleic Acids Res. 2015, 43, 7315–7329. [Google Scholar] [CrossRef]
- Tram, G.; Poole, J.; Adams, F.G.; Jennings, M.P.; Eijkelkamp, B.A.; Atack, J.M. The Acinetobacter baumannii Autotransporter Adhesin Ata Recognizes Host Glycans as High-Affinity Receptors. ACS Infect. Dis. 2021, 7, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Weidensdorfer, M.; Ishikawa, M.; Hori, K.; Linke, D.; Djahanschiri, B.; Iruegas, R.; Ebersberger, I.; Riedel-Christ, S.; Enders, G.; Leukert, L.; et al. The Acinetobacter trimeric autotransporter adhesin Ata controls key virulence traits of Acinetobacter baumannii. Virulence 2019, 10, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Sistrom, M.; Wertz, J.E.; Kortright, K.E.; Narayan, D.; Turner, P.E. Phage selection restores antibiotic sensitivity in MDR Pseudomonas aeruginosa. Sci. Rep. 2016, 6, 26717. [Google Scholar] [CrossRef]
- Wang, R.; You, X.; Liu, X.; Fei, B.; Li, Y.; Wang, D.; Zhu, R.; Li, Y. Characterization of phage HZY2308 against Acinetobacter baumannii and identification of phage-resistant bacteria. Virol. J. 2024, 21, 283. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.; Forsyth, J.H.; Patwa, R.; Kostoulias, X.; Trim, M.; Subedi, D.; Archer, S.K.; Morris, F.C.; Oliveira, C.; Kielty, L.; et al. Bacteriophage-resistant Acinetobacter baumannii are resensitized to antimicrobials. Nat. Microbiol. 2021, 6, 157–161. [Google Scholar] [CrossRef]
- Wang, X.; Loh, B.; Gordillo Altamirano, F.; Yu, Y.; Hua, X.; Leptihn, S. Colistin-phage combinations decrease antibiotic resistance in Acinetobacter baumannii via changes in envelope architecture. Emerg. Microbes Infect. 2021, 10, 2205–2219. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef]
- Weinberg, S.E.; Villedieu, A.; Bagdasarian, N.; Karah, N.; Teare, L.; Elamin, W.F. Control and management of multidrug resistant Acinetobacter baumannii: A review of the evidence and proposal of novel approaches. Infect. Prev. Pract. 2020, 2, 100077. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, W.N.; Concepcion-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and Order Effects of Antibiotics and Phages in Killing Pseudomonas aeruginosa Biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef] [PubMed]
- Save, J.; Que, Y.A.; Entenza, J.; Resch, G. Subtherapeutic Doses of Vancomycin Synergize with Bacteriophages for Treatment of Experimental Methicillin-Resistant Staphylococcus aureus Infective Endocarditis. Viruses 2022, 14, 1792. [Google Scholar] [CrossRef] [PubMed]
- Save, J.; Que, Y.A.; Entenza, J.M.; Kolenda, C.; Laurent, F.; Resch, G. Bacteriophages Combined With Subtherapeutic Doses of Flucloxacillin Act Synergistically Against Staphylococcus aureus Experimental Infective Endocarditis. J. Am. Heart Assoc. 2022, 11, e023080. [Google Scholar] [CrossRef]
- Madison, C.L.; Steinert, A.S.J.; Luedeke, C.E.; Hajjafar, N.; Srivastava, P.; Berti, A.D.; Bayer, A.S.; Kebriaei, R. It takes two to tango: Preserving daptomycin efficacy against daptomycin-resistant MRSA using daptomycin-phage co-therapy. Microbiol. Spectr. 2024, 12, e0067924. [Google Scholar] [CrossRef]
- Kunz Coyne, A.J.; Eshaya, M.; Bleick, C.; Vader, S.; Biswas, B.; Wilson, M.; Deschenes, M.V.; Alexander, J.; Lehman, S.M.; Rybak, M.J. Exploring synergistic and antagonistic interactions in phage-antibiotic combinations against ESKAPE pathogens. Microbiol. Spectr. 2024, 12, e0042724. [Google Scholar] [CrossRef]
- Coulter, L.B.; McLean, R.J.; Rohde, R.E.; Aron, G.M. Effect of bacteriophage infection in combination with tobramycin on the emergence of resistance in Escherichia coli and Pseudomonas aeruginosa biofilms. Viruses 2014, 6, 3778–3786. [Google Scholar] [CrossRef]
- Pons, B.J.; Dimitriu, T.; Westra, E.R.; van Houte, S. Antibiotics that affect translation can antagonize phage infectivity by interfering with the deployment of counter-defenses. Proc. Natl. Acad. Sci. USA 2023, 120, e2216084120. [Google Scholar] [CrossRef]
- CHUV. DM_DAM_Guide_Antibiotherapie. 2022. Available online: https://www.chuv.ch/fileadmin/sites/min/552801_22_DM_DAM_guide_antibiotherapie_version_mai_2022.pdf (accessed on 1 January 2025).
- Leshkasheli, L.; Kutateladze, M.; Balarjishvili, N.; Bolkvadze, D.; Save, J.; Oechslin, F.; Que, Y.A.; Resch, G. Efficacy of newly isolated and highly potent bacteriophages in a mouse model of extensively drug-resistant Acinetobacter baumannii bacteraemia. J. Glob. Antimicrob. Resist. 2019, 19, 255–261. [Google Scholar] [CrossRef]
- Rastegar, S.; Skurnik, M.; Niaz, H.; Tadjrobehkar, O.; Samareh, A.; Hosseini-Nave, H.; Sabouri, S. Isolation, characterization, and potential application of Acinetobacter baumannii phages against extensively drug-resistant strains. Virus Genes 2024, 60, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, B.; Xu, L.; Cong, C.; Murtaza, B.; Wang, L.; Li, X.; Li, J.; Xu, M.; Yin, J.; et al. In vivo efficacy of phage cocktails against carbapenem resistance Acinetobacter baumannii in the rat pneumonia model. J. Virol. 2024, 98, e0046724. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Yong, D. Efficacy of bacteriophage treatment against carbapenem-resistant Acinetobacter baumannii in Galleria mellonella larvae and a mouse model of acute pneumonia. BMC Microbiol. 2019, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zou, J.; Zhang, J.; Qu, J.; Lu, H. Phage therapy for extensively drug resistant Acinetobacter baumannii infection: Case report and in vivo evaluation of the distribution of phage and the impact on gut microbiome. Front. Med. 2024, 11, 1432703. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical Experience of Personalized Phage Therapy Against Carbapenem-Resistant Acinetobacter baumannii Lung Infection in a Patient With Chronic Obstructive Pulmonary Disease. Front. Cell Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef]
- Nir-Paz, R.; Onallah, H.; Dekel, M.; Gellman, Y.N.; Haze, A.; Ben-Ami, R.; Braunstein, R.; Hazan, R.; Dror, D.; Oster, Y.; et al. Randomized double-blind study on safety and tolerability of TP-102 phage cocktail in patients with infected and non-infected diabetic foot ulcers. Med 2024, 6, 100565. [Google Scholar] [CrossRef] [PubMed]
- Eales, B.M.; Tam, V.H. Case Commentary: Novel Therapy for Multidrug-Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2022, 66, e0199621. [Google Scholar] [CrossRef]
- Wu, N.; Dai, J.; Guo, M.; Li, J.; Zhou, X.; Li, F.; Gao, Y.; Qu, H.; Lu, H.; Jin, J.; et al. Pre-optimized phage therapy on secondary Acinetobacter baumannii infection in four critical COVID-19 patients. Emerg. Microbes Infect. 2021, 10, 612–618. [Google Scholar] [CrossRef]
- Nir-Paz, R.; Gelman, D.; Khouri, A.; Sisson, B.M.; Fackler, J.; Alkalay-Oren, S.; Khalifa, L.; Rimon, A.; Yerushalmy, O.; Bader, R.; et al. Successful Treatment of Antibiotic-resistant, Poly-microbial Bone Infection With Bacteriophages and Antibiotics Combination. Clin. Infect. Dis. 2019, 69, 2015–2018. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, X.; Li, Y.; Han, W.; Lei, L.; Yang, Y.; Zhao, H.; Gao, Y.; Song, J.; Lu, R.; et al. A method for generation phage cocktail with great therapeutic potential. PLoS ONE 2012, 7, e31698. [Google Scholar] [CrossRef] [PubMed]


| Ab125 | |||
|---|---|---|---|
| VP | MV50 | ||
| Phage 3014 | 0 | - | |
| Phage 3098 | 0.47 | - | |
| Cocktail 3014/3098 | 0.62 | 1.00 × 10−3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Villiers de la Noue, H.; Golliard, G.; Vuattoux, X.; Resch, G. Rational Design of a Potent Two-Phage Cocktail Against a Contemporary Acinetobacter baumannii Strain Recovered from a Burned Patient at the Lausanne University Hospital. Viruses 2025, 17, 1441. https://doi.org/10.3390/v17111441
de Villiers de la Noue H, Golliard G, Vuattoux X, Resch G. Rational Design of a Potent Two-Phage Cocktail Against a Contemporary Acinetobacter baumannii Strain Recovered from a Burned Patient at the Lausanne University Hospital. Viruses. 2025; 17(11):1441. https://doi.org/10.3390/v17111441
Chicago/Turabian Stylede Villiers de la Noue, Hugues, Gwenaëlle Golliard, Xavier Vuattoux, and Grégory Resch. 2025. "Rational Design of a Potent Two-Phage Cocktail Against a Contemporary Acinetobacter baumannii Strain Recovered from a Burned Patient at the Lausanne University Hospital" Viruses 17, no. 11: 1441. https://doi.org/10.3390/v17111441
APA Stylede Villiers de la Noue, H., Golliard, G., Vuattoux, X., & Resch, G. (2025). Rational Design of a Potent Two-Phage Cocktail Against a Contemporary Acinetobacter baumannii Strain Recovered from a Burned Patient at the Lausanne University Hospital. Viruses, 17(11), 1441. https://doi.org/10.3390/v17111441







