Real-World Effectiveness of Antiviral Prophylaxis for Preventing Hepatitis B Virus (HBV) Reactivation in Patients Undergoing Immunosuppressive Therapy
Abstract
1. Introduction
2. Materials and Methods
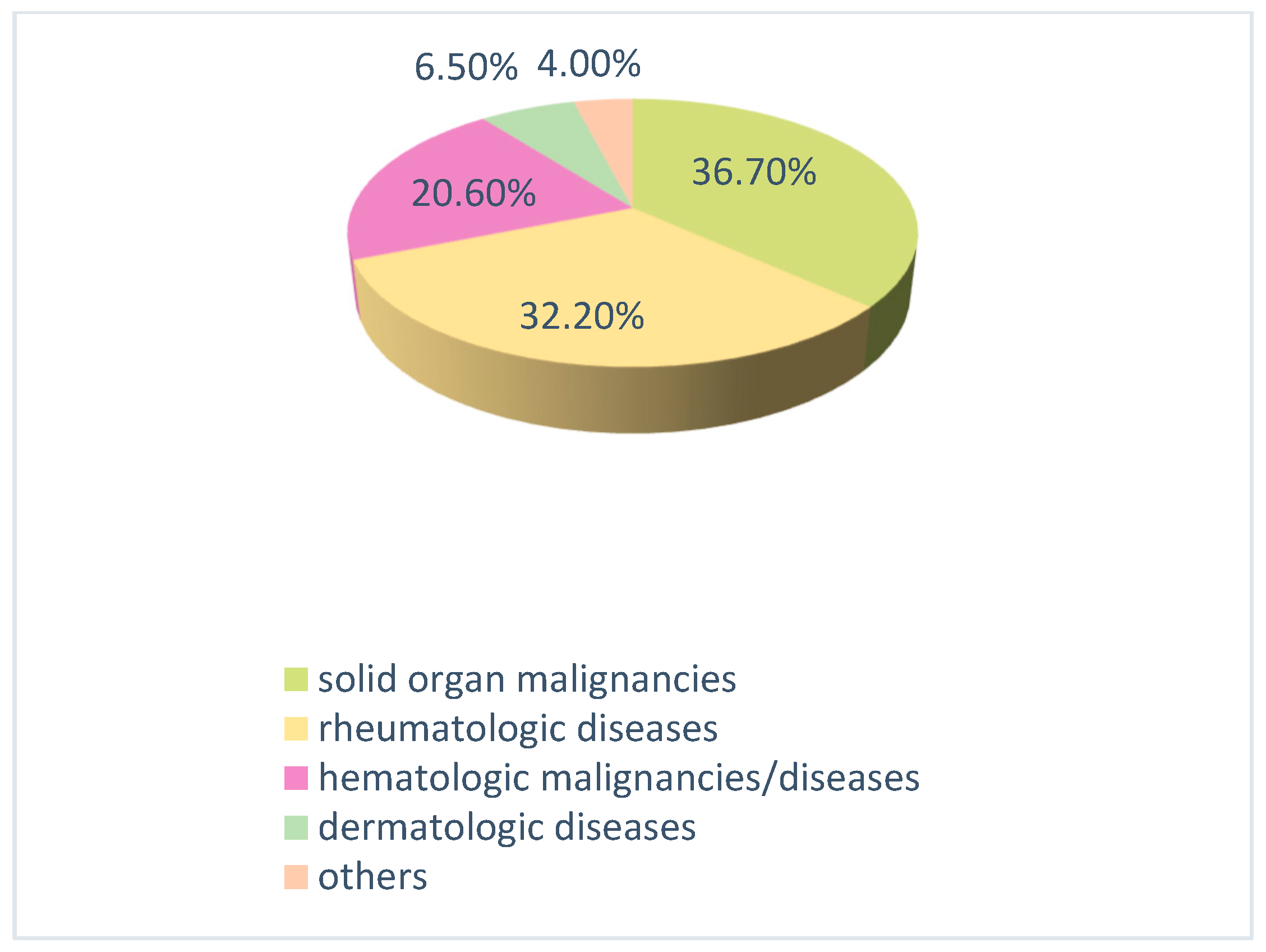
2.1. Immunosuppressive Therapies
- Rheumatologic diseases: corticosteroids, azathioprine, methotrexate, cyclosporine, and rituximab; as well as biological agents such as etanercept and golimumab.
- Hematologic and oncologic diseases: various chemotherapy protocols containing rituximab, cyclophosphamide, doxorubicin, cisplatin, carboplatin, paclitaxel, gemcitabine, bleomycin, and etoposide; as well as targeted therapies including trastuzumab and bortezomib, and immunotherapies such as nivolumab.
- Neurological diseases: ocrelizumab.
- Gastrointestinal and dermatological diseases: a combination of azathioprine and corticosteroids (e.g., methylprednisolone) or biological agents such as ustekinumab, secukinumab, and adalimumab (in some cases in combination with methotrexate).
2.2. Definitions
- A positive HBsAg test result or an increase in HBV DNA levels of ≥1 log in a patient who was previously HBsAg negative.
- The reappearance of detectable HBV DNA (≥100 IU/mL) in a patient who is HBsAg negative but anti-HBc positive.
- The detection of positive HBV DNA along with elevated ALT levels.
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HBV | Hepatitis B virus |
| HBsAg | Hepatitis B surface antigen |
| anti-HBc | Hepatitis B core antibody |
| anti-HBs | Hepatitis B surface antibody |
| ETV | Entecavir |
| TAF | Tenofovir alafenamide |
| TDF | Tenofovir disoproxil fumarate |
| AGA | American Gastroenterological Association |
| ALT | Alanine aminotransferase |
| EASL | European Association for the Study of the Liver |
| AASLD | American Association for the Study of Liver Diseases |
References
- Hsu, Y.C.; Huang, D.Q.; Nguyen, M.H. Global burden of hepatitis B virus: Current status, missed opportunities and a call for action. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 524–537. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 11 April 2024).
- Turkish Association for the Study of the Liver (TASL); Viral Hepatitis Society of Turkey (VHSD). Turkey Hepatitis B Diagnosis and Treatment Guideline. 2023. Available online: https://www.tkad.org.tr/wp-content/uploads/2023/11/TURKIYE-HEPATIT-B-TANI-VE-TEDAVI-KLAVUZU-2023.pdf (accessed on 27 August 2025).
- Şenol Akar, Ş.; Sönmez, U.; Demirdal, T.; Şen, P.; Özer, D.; Atalay, S.; Akyol, D.; Mermutluoğlu, Ç.; Çelen, M.K.; Yamazhan, T.; et al. Tenofovir alafenamide prophylaxis for the prevention of HBV reactivation in immunosuppressed subjects: A multicenter study. Cukurova Med. J. 2022, 47, 446–454. [Google Scholar]
- Anvari, S.; Tsoi, K. Hepatitis B Virus Reactivation with Immunosuppression: A Hidden Threat? J. Clin. Med. 2024, 13, 393. [Google Scholar] [CrossRef]
- Yang, H.C.; Kao, J.H. Persistence of hepatitis B virus covalently closed circular DNA in hepatocytes: Molecular mechanisms and clinical significance. Emerg. Microbes. Infect. 2014, 3, e64. [Google Scholar] [CrossRef]
- Shi, Y.; Zheng, M. Hepatitis B virus persistence and reactivation. BMJ Clin. Res. Ed. 2020, 370, m2200. [Google Scholar] [CrossRef]
- Solay, A.H.; Acar, A.; Eser, F.; Kuşcu, F.; Tütüncü, E.E.; Kul, G.; Şentürk, G.Ç.; Gürbüz, Y. Reactivation rates in patients using biological agents, with resolved HBV infection or isolated anti-HBc IgG positivity. Turk. J. Gastroenterol. 2018, 29, 561–565. [Google Scholar] [CrossRef]
- Ali, F.S.; Nguyen, M.H.; Hernaez, R.; Huang, D.Q.; Wilder, J.; Piscoya, A.; Simon, T.G.; Falck-Ytter, Y. AGA clinical practice guideline on the prevention and treatment of hepatitis B virus reactivation in at-risk individuals. Gastroenterology 2025, 168, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Spera, A.M. Hepatitis B virus infection reactivation in patients under immunosuppressive therapies: Pathogenesis, screening, prevention and treatment. World J. Virol. 2022, 11, 275–282. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver EASL. Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2025, 83, 502–583. [Google Scholar] [CrossRef]
- Seto, W.K. Hepatitis B virus reactivation during immunosuppressive therapy: Appropriate risk stratification. World J. Hepatol. 2015, 7, 825–830. [Google Scholar] [CrossRef]
- Etienne, S.; Vosbeck, J.; Bernsmeier, C.; Osthoff, M. Prevention of hepatitis B reactivation in patients receiving immunosuppressive therapy: A case series and appraisal of society guidelines. J. Gen. Intern. Med. 2023, 38, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Li, X.; Wu, S.; Liu, Y.; Qiao, Y.; Xu, D.; Li, J. Risk of hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA after treatment with rituximab for lymphoma: A meta-analysis. Hepatol. Int. 2017, 11, 429–433. [Google Scholar] [CrossRef]
- Buti, M.; Manzano, M.L.; Morillas, R.M.; García-Retortillo, M.; Martín, L.; Prieto, M.; Gutiérrez, M.L.; Suárez, E.; Gómez Rubio, M.; López, J.; et al. Randomized prospective study evaluating tenofovir disoproxil fumarate prophylaxis against hepatitis B virus reactivation in anti-HBc-positive patients with rituximab-based regimens to treat hematologic malignancies: The Preblin study. PLoS ONE 2017, 12, e0184550. [Google Scholar] [CrossRef]
- Ceylan, M.; Turken, M.; Singil, S.; Adar, P.; Köse, Ş. Evaluation of the risk of hepatitis B reactivation in patients receiving immunosuppressive therapy. Izmir Med. J. 2022, 1, 112–116. [Google Scholar] [CrossRef]
- Kotake, T.; Satake, H.; Okita, Y.; Hatachi, Y.; Hamada, M.; Omiya, M.; Yasui, H.; Hashida, T.; Kaihara, S.; Inokuma, T.; et al. Prevalence and risk factors of hepatitis B virus reactivation in patients with solid tumors with resolved HBV infection. Asia-Pac. J. Clin. Oncol. 2019, 15, 63–68. [Google Scholar] [CrossRef]
- Terrault, N.A.; Lok, A.S.F.; McMahon, B.J.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Brown, R.S., Jr.; Bzowej, N.H.; Wong, J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018, 67, 1560–1599. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Sugiyama, M.; Sato, Y.; Nagashima, K.; Takahashi, S.; Mizokami, M.; Hata, A. Hepatitis B virus reactivation in patients with rheumatoid arthritis: Analysis of the national database of Japan. J. Viral Hepat. 2018, 25, 1312–1320. [Google Scholar] [CrossRef]
- Mustafayev, K.; Torres, H. Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin. Microbiol. Infect. 2022, 28, 1321–1327. [Google Scholar] [CrossRef]
- Jeong, S.; Shin, H.P.; Kim, H.I. Real-world single-center comparison of the safety and efficacy of entecavir, tenofovir disoproxil fumarate, and tenofovir alafenamide in patients with chronic hepatitis B. Intervirology 2022, 65, 94–103. [Google Scholar] [CrossRef]
- Ahmet, Ş.H.; Selda, A.S.A. Evaluation of HBV Reactivation and Antiviral Prophylaxis in Patients Receiving Immunosuppressive Therapy. J. Contemp. Med. 2023, 13, 809–813. [Google Scholar] [CrossRef]
- Chen, M.B.; Wang, H.; Zheng, Q.H.; Zheng, X.W.; Fan, J.N.; Ding, Y.L.; Niu, J.L. Comparative efficacy of tenofovir and entecavir in nucleos(t)ide analogue-naive chronic hepatitis B: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0224773. [Google Scholar] [CrossRef] [PubMed]
| Reactivation Risk | ||||
|---|---|---|---|---|
| Low Risk | Moderate Risk | High Risk | p | |
| (n = 82) | (n = 44) | (n = 73) | ||
| n/(%) | n/(%) | n/(%) | ||
| Age (Mean ± SD) | 61.3 ± 12.1 | 59.4 ± 12.6 | 60 ± 12.6 | 0.582 |
| Sex | 0.442 | |||
| Female | 44 (53.7) | 23 (52.3) | 32 (43.8) | |
| Male | 38 (46.3) | 21 (47.7) | 41 (56.2) | |
| HBsAg (+) | 9 (11) | 14 (31.8) | 30 (41.1) | <0.001 |
| Isolated anti-HBc (+) | 43 (52.4) | 10 (22.7) | 13 (17.8) | <0.001 |
| Anti-HBs (+) and anti-HBc (+) | 30 (36.6) | 20 (45.5) | 30 (41.1) | 0.614 |
| HBV DNA detectable | 6 (7.3) | 10 (22.7) | 21(21.8) | 0.002 |
| Baseline ALT levels | 0.746 | |||
| Mean ± SD | 22.5 ± 16.5 | 22.5 ± 10.8 | 22.3 ± 13.2 | |
| Median | 19.5 | 20.0 | 22.0 | |
| Antiviral treatment | 0.668 | |||
| ETV | 61 (74.4) | 36 (81.8) | 55 (75.3) | |
| TAF | 13 (15.9) | 5 (11.4) | 8 (11.0) | |
| TDF | 8 (9.8) | 3 (6.8) | 10 (13.7) | |
| ETV (n = 152) | TAF + TDF (n = 47) | p | |
|---|---|---|---|
| n/(%) | n/(%) | ||
| Age (Mean ± SD) | 61.5 ± 11.6 | 56.8 ± 14 | 0.025 |
| Sex | 0.382 | ||
| Female | 73 (48) | 26 (55.3) | |
| Male | 79 (52) | 21 (44.7) | |
| HBsAg (+) | 37 (24.3) | 16 (34) | 0.189 |
| Isolated anti-HBc (+) | 52 (34.2) | 14 (29.8) | 0.573 |
| Anti-HBs (+) and anti-HBc (+) | 41 (36.0) | 12 (30.8) | 0.568 |
| HBV DNA detectable | 24 (15.8) | 13 (27.7) | 0.068 |
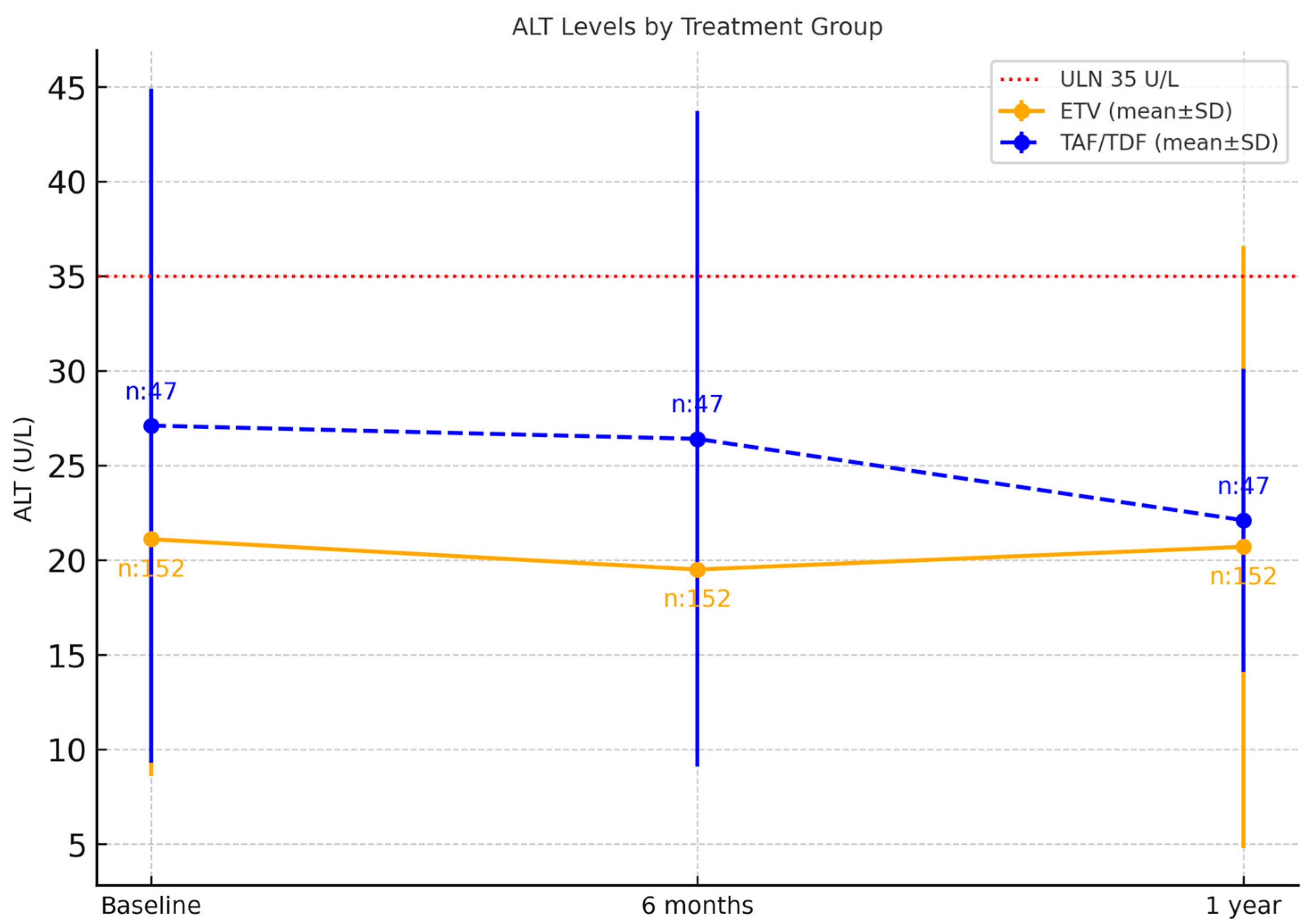
| ALT (Mean ± SD) | |||
| Baseline | 21.1 ± 12.5 | 27.1 ± 17.8 | 0.022 |
| 6rt month | 19.5 ± 9.3 | 26.4 ± 17.3 | 0.002 |
| 12th month | 20.7 ± 15.9 | 22.1 ± 8.0 | 0.033 |
| Baseline ALT | 0.002 | ||
| Normal | 135 (88.8) | 33 (70.2) | |
| High | 17 (11.2) | 14 (29.8) | |
| 6th month ALT | 0.006 | ||
| Normal | 137 (90.1) | 35 (74.5) | |
| High | 15 (9.9) | 12 (25.5) | |
| 12th month ALT | 0.003 | ||
| Normal | 135 (88.8) | 33 (70.2) | |
| High | 17 (11.2) | 14 (29.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz Nakir, İ.; Çağlar, B.; Zerdali, E.; Karaduman, R.G.; Pehlivanoğlu, F. Real-World Effectiveness of Antiviral Prophylaxis for Preventing Hepatitis B Virus (HBV) Reactivation in Patients Undergoing Immunosuppressive Therapy. Viruses 2025, 17, 1436. https://doi.org/10.3390/v17111436
Yılmaz Nakir İ, Çağlar B, Zerdali E, Karaduman RG, Pehlivanoğlu F. Real-World Effectiveness of Antiviral Prophylaxis for Preventing Hepatitis B Virus (HBV) Reactivation in Patients Undergoing Immunosuppressive Therapy. Viruses. 2025; 17(11):1436. https://doi.org/10.3390/v17111436
Chicago/Turabian StyleYılmaz Nakir, İnci, Bilge Çağlar, Esra Zerdali, Rumeysa Gülistan Karaduman, and Filiz Pehlivanoğlu. 2025. "Real-World Effectiveness of Antiviral Prophylaxis for Preventing Hepatitis B Virus (HBV) Reactivation in Patients Undergoing Immunosuppressive Therapy" Viruses 17, no. 11: 1436. https://doi.org/10.3390/v17111436
APA StyleYılmaz Nakir, İ., Çağlar, B., Zerdali, E., Karaduman, R. G., & Pehlivanoğlu, F. (2025). Real-World Effectiveness of Antiviral Prophylaxis for Preventing Hepatitis B Virus (HBV) Reactivation in Patients Undergoing Immunosuppressive Therapy. Viruses, 17(11), 1436. https://doi.org/10.3390/v17111436





