Cryptotanshinone Suppresses BVDV Propagation by Suppressing Cell Apoptosis and Restoring Hormone Secretion in Bovine Granulosa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Culture and Identification of Bovine Ovarian Granulosa Cells
2.2. Virus Amplification
2.3. Virulence Assay
2.4. Cell Viability Assay
2.5. Trials of CRY’s Effect on BVDV
2.6. Immunofluorescence
2.7. Gene Relative Expression and Absolute Quantitation
2.8. Measurement of Proinflammatory Cytokine Secretion Levels
2.9. Estradiol and Progesterone Secretion Level Assay
2.10. Apoptosis Analysis
2.11. Western Blotting
2.12. Data Analysis
3. Results
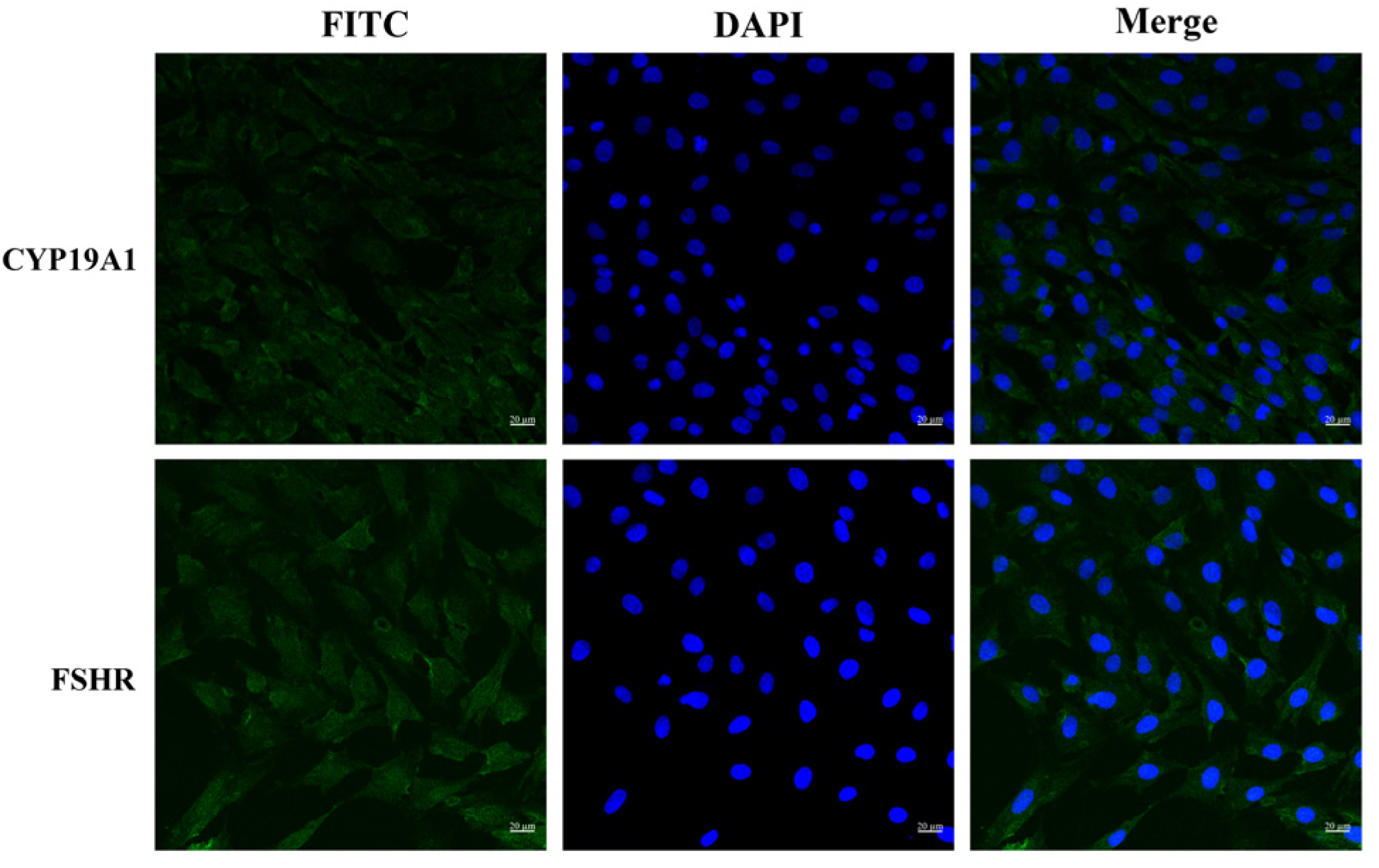
3.1. Bovine Granulosa Cells Identification
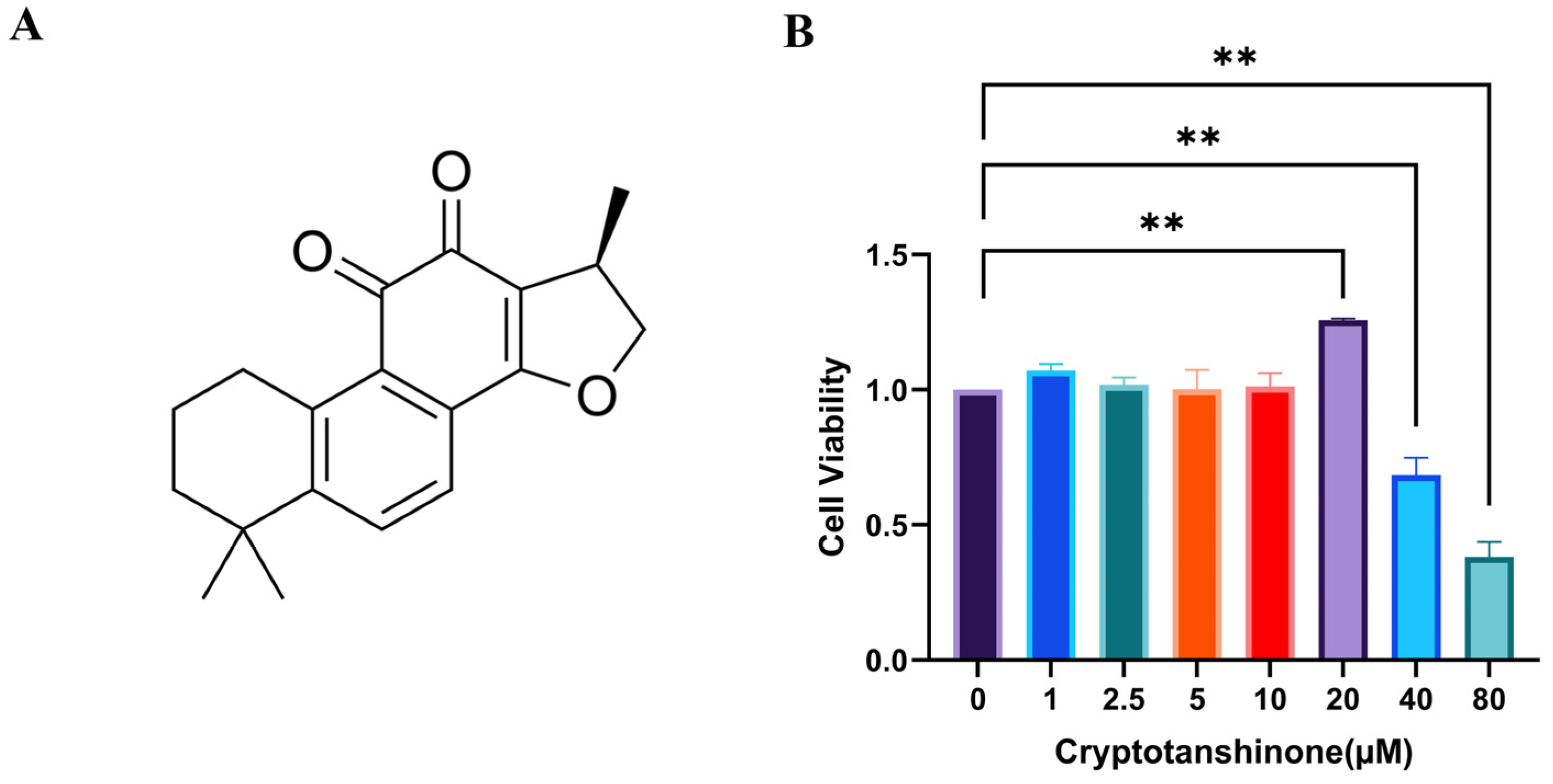
3.2. The Optimal Concentration of CRY
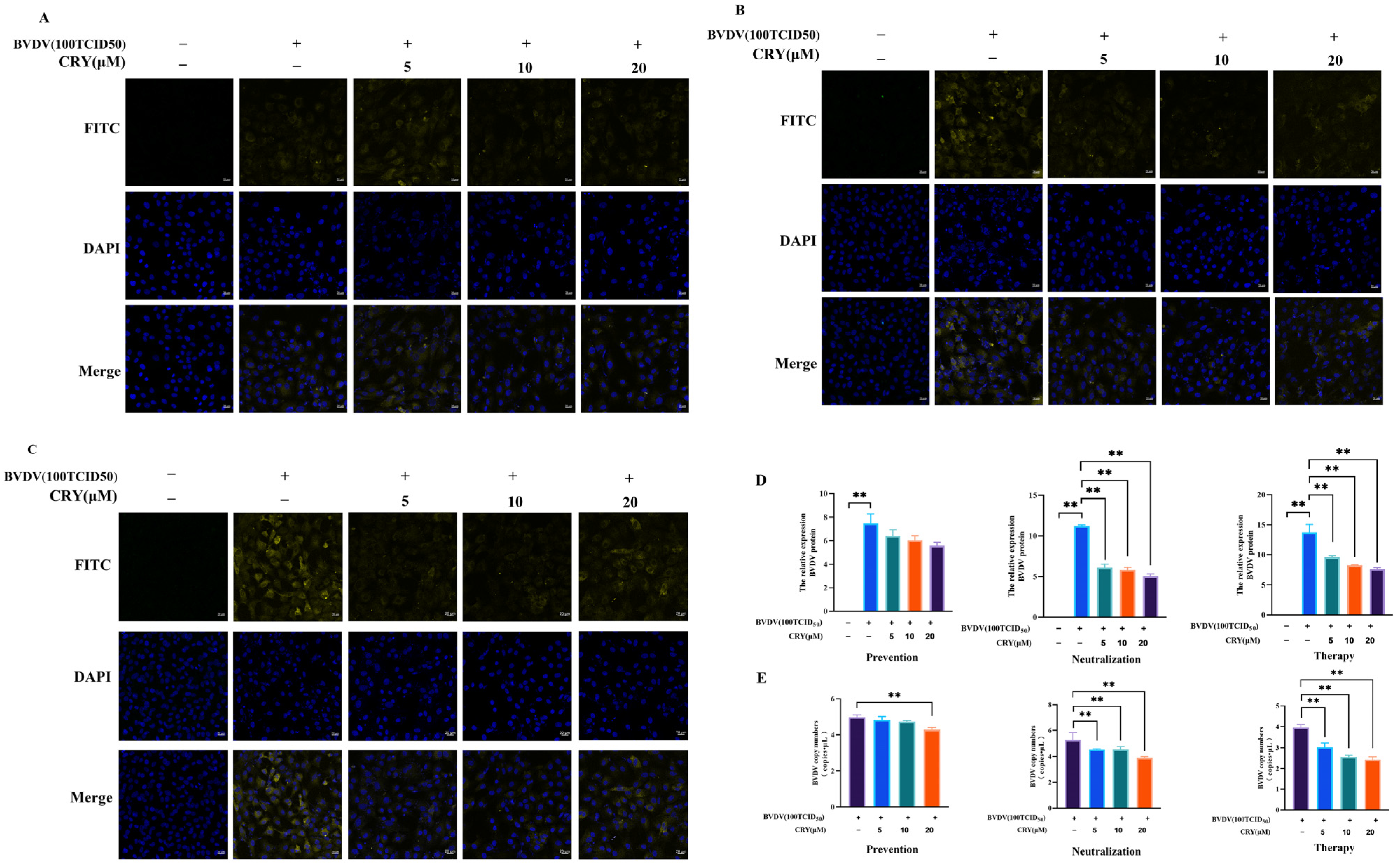
3.3. CRY Inhibits BVDV Replication in Three Treatment Methods
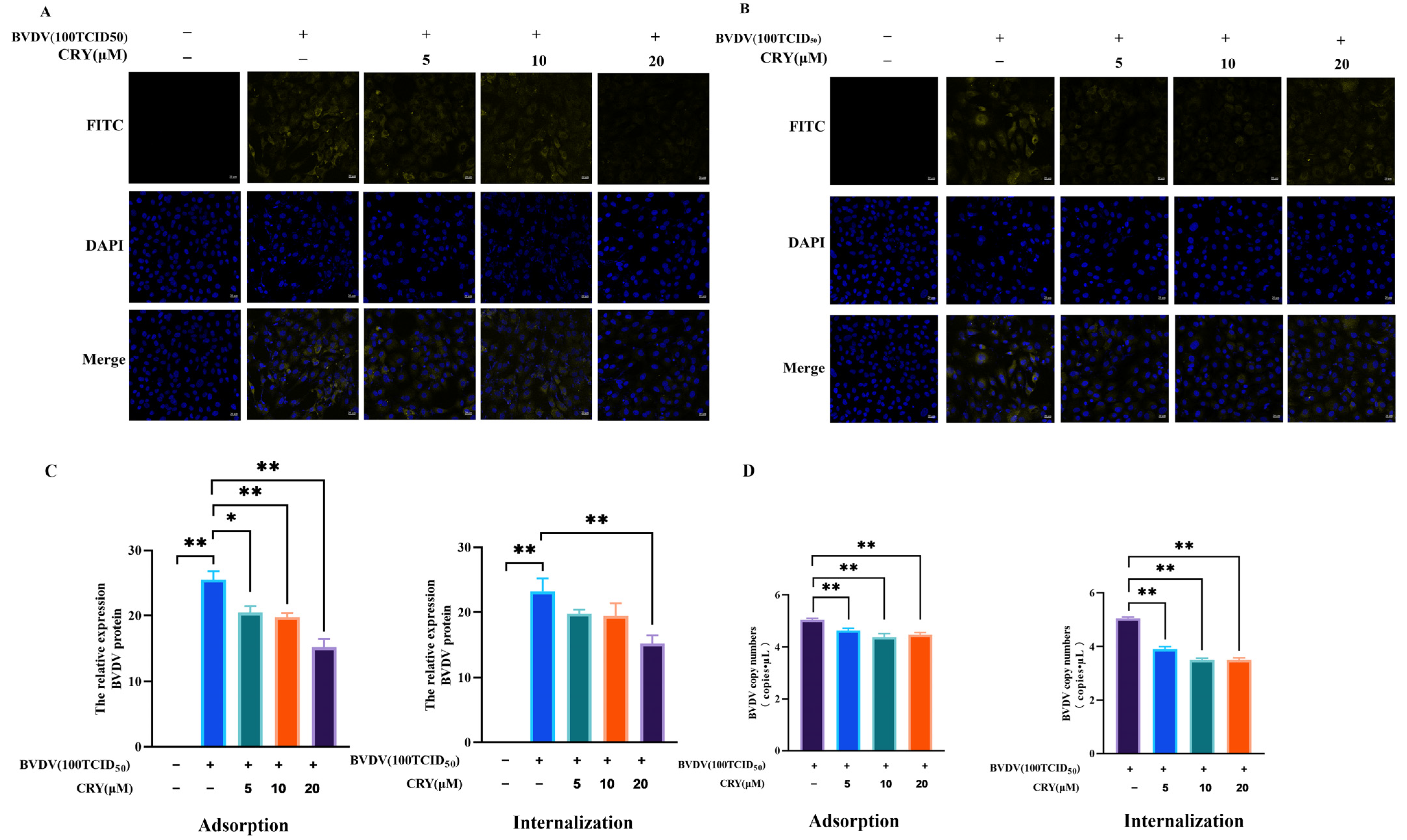
3.4. Effects of CRY on BVDV Adsorption and Internalization in BOGCs
3.5. CRY Inhibits BVDV-Induced Inflammatory Responses
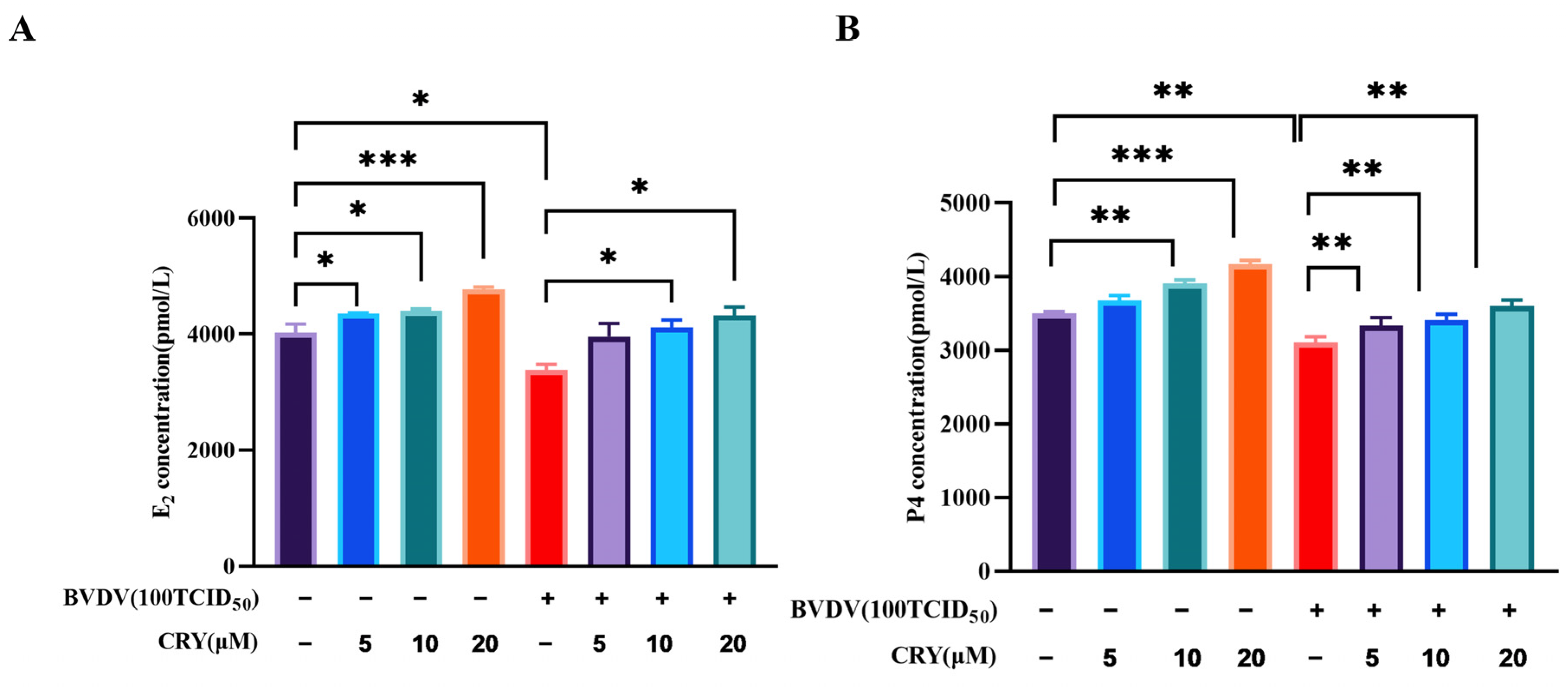
3.6. Effect of CRY on E2 and P4 Levels of BOGCs
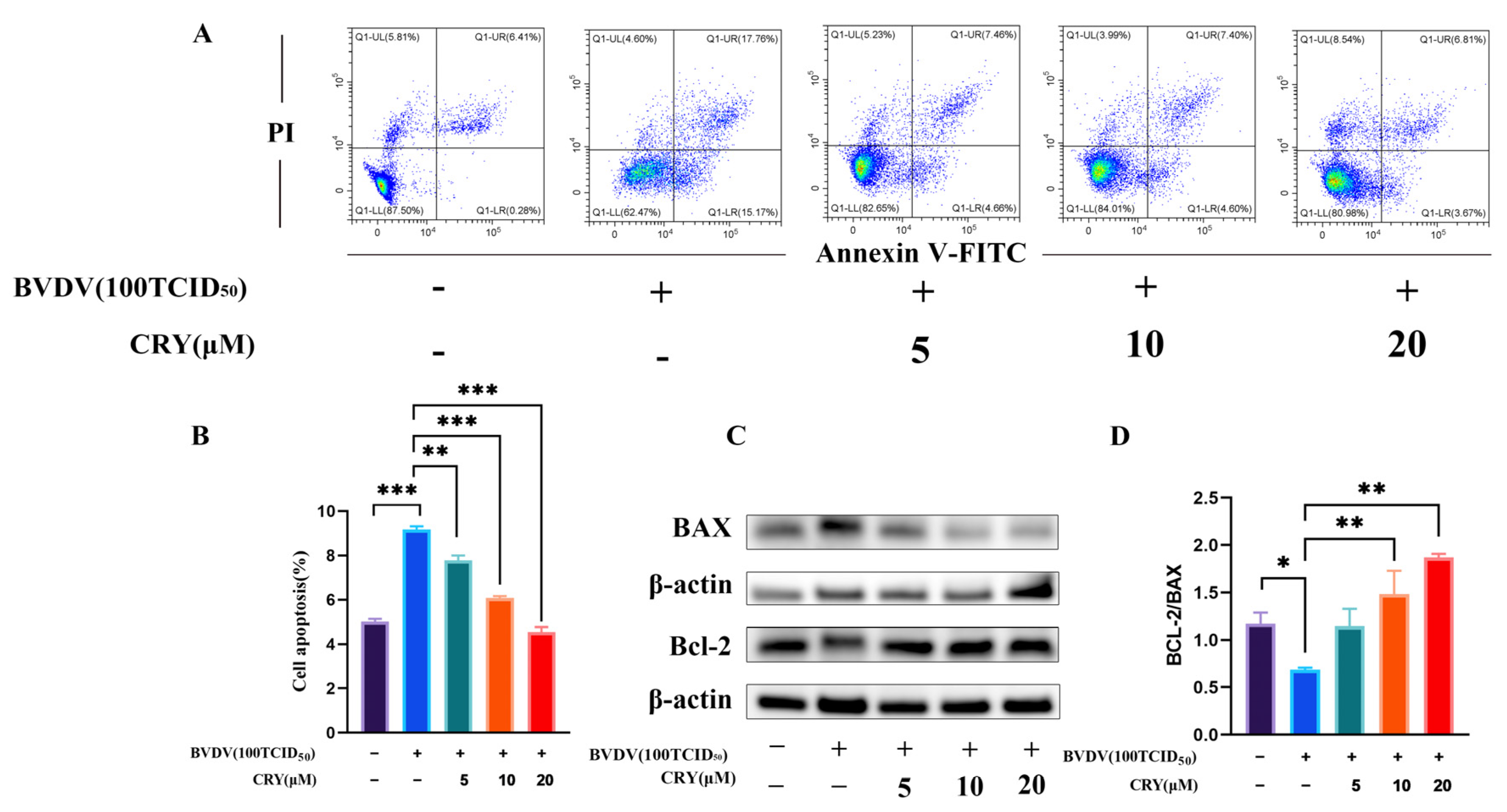
3.7. Effects of CRY on Apoptosis Induced by BVDV in BOGCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BVDV | Bovine viral diarrhea virus |
| BVD | Bovine viral diarrhea-mucosal disease |
| CRY | Cryptotanshinone |
| BOGCs | Bovine ovarian granulosa cells |
| E2 | Estradiol |
| P4 | Progesterone |
| MDBK | Madin-Darby bovine kidney |
| CPE | Cytopathic effect |
| CP | Cytopathic Biotype |
| OD | Optical density |
| IL | Interleukin |
| TNF-α | Tumor necrosis factor-α |
| TCID50 | 50% Tissue culture infective dose |
| FSHR | Follicle-stimulating hormone receptor |
| CYP19A1 | Cytochrome P450 family 19 subfamily A member 1 |
| Bcl-2 | B-cell lymphoma/lewkmia-2 |
| BAX | BCL2-Associated X Protein |
References
- Oguejiofor, C.F.; Thomas, C.; Cheng, Z.; Wathes, D.C. Mechanisms linking bovine viral diarrhea virus (BVDV) infection with infertility in cattle. Anim. Health Res. Rev. 2019, 20, 72–85. [Google Scholar] [CrossRef]
- Adkins, M.; Moisa, S.; Beever, J.; Lear, A. Targeted Transcriptome Analysis of Beef Cattle Persistently Infected with Bovine Viral Diarrhea Virus. Genes 2024, 15, 1500. [Google Scholar] [CrossRef]
- Al-Mubarak, A.I.A.; Al-Kubati, A.A.G.; Skeikh AHussen, J.; Kandeel, M.; Flemban, B.; Hemida, M.G. A longitudinal study of bovine viral diarrhea virus in a semi-closed management dairy cattle herd, 2020–2022. Front. Vet. Sci. 2023, 10, 1221883. [Google Scholar] [CrossRef]
- Wathes, D.C.; Oguejiofor, C.F.; Thomas, C.; Cheng, Z. Importance of Viral Disease in Dairy Cow Fertility. Engineering 2020, 6, 26–33. [Google Scholar] [CrossRef]
- Braun, U.; Hilbe, M.; Peterhans, E.; Schweizer, M. Border disease in cattle. Vet. J. 2019, 246, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Kubati, A.A.G.; Hussen, J.; Kandeel, M.; Al-Mubarak, A.I.A.; Hemida, M.G. Recent Advances on the Bovine Viral Diarrhea Virus Molecular Pathogenesis, Immune Response, and Vaccines Development. Front. Vet. Sci. 2021, 8, 665128. [Google Scholar] [CrossRef]
- Chi, S.; Chen, S.; Jia, W.; He, Y.; Ren, L.; Wang, X. Non-structural proteins of bovine viral diarrhea virus. Virus Genes 2022, 58, 491–500. [Google Scholar] [CrossRef]
- Brownlie, J.; Hooper, L.B.; Thompson, I.; Collins, M.E. Maternal recognition of foetal infection with bovine virus diarrhoea virus (BVDV)—The bovine pestivirus. Clin. Diagn. Virol. 1998, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, C.; Zhang, L.; Zhu, T.; Li, H.; Wang, Y.; Xue, K.; Qi, M.; Peng, Q.; Chen, Y.; et al. Isolation of BVDV-1a, 1m, and 1v strains from diarrheal calf in China and identification of its genome sequence and cattle virulence. Front. Vet. Sci. 2022, 9, 1008107. [Google Scholar] [CrossRef]
- Al-Khaliyfa, M.A.; Abuelzein, E.M.E.; Gameel, A.A. Identification of cattle persistently infected with BVDV by ear-notch testing in Saudi Arabia. Vet. Rec. 2010, 167, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Griebel, P.J. BVDV vaccination in North America: Risks versus benefits. Anim. Health Res. Rev. 2015, 16, 27–32. [Google Scholar] [CrossRef]
- Lanyon, S.R.; Hill, F.I.; Reichel, M.P.; Brownlie, J. Bovine viral diarrhoea: Pathogenesis and diagnosis. Vet. J. 2014, 199, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Buratini, J.; Price, C.A. Follicular somatic cell factors and follicle development. Reprod. Fertil. Dev. 2011, 23, 32–39. [Google Scholar] [CrossRef]
- Vundavilli, H.; Datta, A.; Sima, C.; Hua, J.; Lopes, R.; Bittner, M. Bayesian Inference Identifies Combination Therapeutic Targets in Breast Cancer. IEEE Trans. Biomed. Eng. 2019, 66, 2684–2692. [Google Scholar] [CrossRef]
- Zhang, W.; Suo, M.; Yu, G.; Zhang, M. Antinociceptive and anti-inflammatory effects of cryptotanshinone through PI3K/Akt signaling pathway in a rat model of neuropathic pain. Chem. Biol. Interact. 2019, 305, 127–133. [Google Scholar] [CrossRef]
- Li, H.; Gao, C.; Liu, C.; Liu, L.; Zhuang, J.; Yang, J.; Zhou, C.; Feng, F.; Sun, C.; Wu, J. A review of the biological activity and pharmacology of cryptotanshinone, an important active constituent in Danshen. Biomed. Pharmacother. 2021, 137, 111332. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, P.; Huang, H.; Xie, Y.; Lu, R.; Dong, L. Cryptotanshinone reverses reproductive disturbances in rats with dehydroepiandrosterone-induced polycystic ovary syndrome. Am. J. Transl. Res. 2017, 9, 2447–2456. [Google Scholar] [PubMed]
- Huang, J.; Zeng, F.; Xu, Q.; Ma, J. Cryptotanshinone decreases granulosa cell apoptosis and restores ovarian function in mice with premature ovarian failure. Gen. Physiol. Biophys. 2020, 39, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, L.; Qi, C.; Hu, G.; Wang, L.; Sun, Z.; Ni, X. Cryptotanshinone alleviates polycystic ovary syndrome in rats by regulating the HMGB1/TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2020, 22, 3851–3861. [Google Scholar] [CrossRef]
- Zhao, Y.; Du, X.; Duan, Y.; Pan, X.; Sun, Y.; You, T.; Han, L.; Jin, Z.; Shang, W.; Yu, J.; et al. High-throughput screening identifies established drugs as SARS-CoV-2 PLpro inhibitors. Protein Cell 2021, 12, 877–888. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, J.; Wang, L.; Chu, A.; Yin, Y.; Vali, K.; Garmendia, A.; Tang, Y. Cryptotanshinone protects porcine alveolar macrophages from infection with porcine reproductive and respiratory syndrome virus. Antiviral Res. 2020, 183, 104937. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, L.; Wang, X.; Wang, X.; Yang, Z.; Li, J. IL-6 Promotes FSH-Induced VEGF Expression Through JAK/STAT3 Signaling Pathway in Bovine Granulosa Cells. Cell. Physiol. Biochem. 2017, 44, 293–302. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, K.; Zhang, K.; Zhang, J.; Wang, L.; Wang, X.; Hu, X.; Liang, Z.; Li, J. Toll-like receptors and high mobility group box 1 in granulosa cells during bovine follicle maturation. J. Cell. Physiol. 2020, 235, 3447–3462. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, L.; Zhang, J.; Feng, H.; Chen, X.; Wang, J.; Shi, M.; Zhang, K.; Li, J. Codonopsis pilosula Polysaccharides Exert Antiviral Effect Through Activating Immune Function in a Macrophage Model of Bovine Viral Diarrhea Virus Infection. Vet. Sci. 2025, 12, 415. [Google Scholar] [CrossRef]
- Duan, H.; Wang, F.; Wang, K.; Yang, S.; Zhang, R.; Xue, C.; Zhang, L.; Ma, X.; Du, X.; Kang, J.; et al. Quercetin ameliorates oxidative stress-induced apoptosis of granulosa cells in dairy cow follicular cysts by activating autophagy via the SIRT1/ROS/AMPK signaling pathway. J. Anim. Sci. Biotechnol. 2024, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, F.; Han, X.; Jia, D.; Chen, J.; Wei, Y.; Yu, Z.; He, L.; Liao, C.; Ding, K. Exposure to Bovine Viral Diarrhea Virus Disrupts Intestinal Barrier Function via NLRP3/Caspase-1-Mediated Pyroptosis and Gut Microbiota Dysbiosis. J. Agric. Food Chem. 2025, 73, 22384–22396. [Google Scholar] [CrossRef]
- Madia, V.N.; Toscanelli, W.; De Vita, D.; De Angelis, M.; Messore, A.; Ialongo, D.; Scipione, L.; Tudino, V.; D’Auria, F.D.; Di Santo, R.; et al. Ultrastructural Damages to H1N1 Influenza Virus Caused by Vapor Essential Oils. Molecules 2022, 27, 3718. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, J.; Wang, S.; Wang, J.; Wang, J.; Zhu, Y.; Wang, J. Gypenoside Inhibits Bovine Viral Diarrhea Virus Replication by Interfering with Viral Attachment and Internalization and Activating Apoptosis of Infected Cells. Viruses 2021, 13, 1810. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Zhao, Z.; Yuan, X.; Li, C.; Liu, S.; Cui, Y.; Liu, Y.; Zhou, Y.; Zhu, Z. In Vivo and In Vitro Antiviral Activity of Phlorizin Against Bovine Viral Diarrhea Virus. J. Agric. Food Chem. 2022, 70, 14841–14850. [Google Scholar] [CrossRef]
- de Camargo, L.J.; Picoli, T.; Fischer, G.; de Freitas, A.C.O.; de Almeida, R.B.; da Silva Pinto, L. Antiviral activity of native banana lectin against bovine viral diarrhea virus and bovine alphaherpesvirus type 1. Int. J. Biol. Macromol. 2020, 157, 569–576. [Google Scholar] [CrossRef]
- Cheng, C.; Ma, W.; Li, Q.; Kong, L.; Xie, F.; Jia, X.; Wang, Y. In vitro antiviral function and mechanism of cryptotanshinone. Drug Eval. Res. 2022, 45, 1706–1715. [Google Scholar] [CrossRef]
- Jingyan, L. The Antiviral Activity and Mechanisms of Salvianolic acid A and Cryptotanshinone from Salvia miltiorrhiza on Zika Virus. Master’s Thesis, Southern Medical University, Guangzhou, China, 29 May 2021. [Google Scholar] [CrossRef]
- Araújo Sde Tavares, L.P.; Costa, V.V. Special Issue “Viral-Induced Inflammation”. Viruses 2024, 16, 588. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Jiang, S.; Li, Y.; Yao, X.; Wang, M.; Zhao, J.; Sun, X.; Jiang, X.; Zhong, L.; et al. Identification of key proteins of cytopathic biotype bovine viral diarrhoea virus involved in activating NF-κB pathway in BVDV-induced inflammatory response. Virulence 2022, 13, 1884–1899. [Google Scholar] [CrossRef]
- Ma, M.; Bao, T.; Li, J.; Cao, L.; Yu, B.; Hu, J.; Cheng, H.; Tian, Z. Cryptotanshinone affects HFL-1 cells proliferation by inhibiting cytokines secretion in RAW264.7 cells and ameliorates inflammation and fibrosis in newborn rats with hyperoxia induced lung injury. Front. Pharmacol. 2023, 14, 1192370. [Google Scholar] [CrossRef]
- Xie, L.; Zhong, Y.; Chen, Y.; Wang, Y.; Xian, P.; Liu, S.; Xin, X.; Chen, Y.; Guan, Y.; Li, K. Cryptotanshinone alleviates immunosuppression in endometriosis by targeting MDSCs through JAK2/STAT3 pathway. Phytomedicine 2025, 136, 156227. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Zhang, J.; Xia, X.; Li, H.; Qiu, C.; Liao, Y.; Chen, H.; He, Z.; Song, Z.; et al. Activated STAT3 signaling pathway by ligature-induced periodontitis could contribute to neuroinflammation and cognitive impairment in rats. J. Neuroinflamm. 2021, 18, 80. [Google Scholar] [CrossRef]
- Guo, X.; Ying, S.; Xiao, H.; An, H.; Guo, R.; Dai, Z.; Wu, W. miR-21/SMAD2 Is Involved in the Decrease in Progesterone Synthesis Caused by Lipopolysaccharide Exposure in Follicular Granulosa Cells of Laying Goose. Metabolites 2024, 14, 362. [Google Scholar] [CrossRef]
- Rekawiecki, R.; Wrobel, M.H.; Zajac, P.; Serej, O.; Kowalik, M.K. Luteotropic and Luteolytic Factors Modulate the Expression of Nuclear Receptor Coregulators in Bovine Luteal Cells Independently of Histone Acetyltransferase and Histone Deacetylase Activities. Animals 2023, 13, 2784. [Google Scholar] [CrossRef]
- Su, P.; Wang, X.; Cai, R.; Yan, C.; Sun, Y. Effects of cryptotanshinone in treatment of polycystic ovary syndrome in rats: A systematic review and meta-analysis. Front. Pharmacol. 2025, 16, 1561164. [Google Scholar] [CrossRef]
- Wei, P.; Lin, D.; Zhang, M.; Luo, C.; Wu, X.; Deng, B.; Cui, K.; Chen, Z. Cryptotanshinone modulates proliferation, apoptosis, and fibrosis through inhibiting AR and EGFR/STAT3 axis to ameliorate benign prostatic hyperplasia progression. Eur. J. Pharmacol. 2023, 938, 175434. [Google Scholar] [CrossRef]
- Zhu, L.; Luo, C.; Ma, C.; Kong, L.; Huang, Y.; Yang, W.; Huang, C.; Jiang, W.; Yi, J. Inhibition of the NF-κB pathway and ERK-mediated mitochondrial apoptotic pathway takes part in the mitigative effect of betulinic acid on inflammation and oxidative stress in cyclophosphamide-triggered renal damage of mice. Ecotoxicol. Environ. Saf. 2022, 246, 114150. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X.; Zhang, J.; Wu, Y.; Wang, J.; Wang, J. Melatonin inhibits bovine viral diarrhea virus replication by ER stress-mediated NF-κB signal pathway and autophagy in MDBK cells. Front. Cell. Infect. Microbiol. 2024, 14, 1431836. [Google Scholar] [CrossRef]
- Hou, X.; Li, Y.; Song, J.; Zhang, W.; Liu, R.; Yuan, H.; Feng, T.; Jiang, Z.; Li, W.; Zhu, C. Cryptotanshinone induces apoptosis of activated hepatic stellate cells via modulating endoplasmic reticulum stress. World J. Gastroenterol. 2023, 29, 2616–2627. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, X.; Gu, W.; Ci, X.; Peng, L. Hyperoside relieves particulate matter-induced lung injury by inhibiting AMPK/mTOR-mediated autophagy deregulation. Pharmacol. Res. 2021, 167, 105561. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Zeng, K.; Zhu, J.; Wang, X.; Huang, W. Potential antiviral activity of rhamnocitrin against influenza virus H3N2 by inhibiting cGAS/STING pathway in vitro. Sci. Rep. 2024, 14, 28287. [Google Scholar] [CrossRef]
- Hu, T.; Gao, S.; Yu, Z.; Liu, Y.; Tang, H.; Xu, Z.; Zhu, L.; Zhao, L.; Ye, G.; Shi, F. Rosmarinic Acid inhibits Pseudorabies Virus (PRV) infection by activating the cGAS-STING signaling pathway. BMC Microbiol. 2025, 25, 149. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Wei, G.-Y.; Zhang, R.-P.; Zhu, Y.; Wang, Z.; Wang, S.-M.; Du, G.-H. Cryptotanshinone alleviates chemotherapy-induced colitis in mice with colon cancer via regulating fecal-bacteria-related lipid metabolism. Pharmacol. Res. 2021, 163, 105232. [Google Scholar] [CrossRef]
- Ko, G.; Kim, J.; Jeon, Y.-J.; Lee, D.; Baek, H.-M.; Chang, K.-A. Salvia miltiorrhiza Alleviates Memory Deficit Induced by Ischemic Brain Injury in a Transient MCAO Mouse Model by Inhibiting Ferroptosis. Antioxidants 2023, 12, 785. [Google Scholar] [CrossRef]
- Huang, Y.; Li, W.; Wang, C.C.; Wu, X.; Zheng, J. Cryptotanshinone reverses ovarian insulin resistance in mice through activation of insulin signaling and the regulation of glucose transporters and hormone synthesizing enzymes. Fertil. Steril. 2014, 102, 589–596.e4. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Su, S.; Li, Y.; Chen, N.; He, L.; Dong, M.; Xu, B.; Zhang, Z.; Zhou, Y.; et al. Blockade of BTLA alone or in combination with PD-1 restores the activation and proliferation of CD8+ T cells during in vitro infection with NCP BVDV. Vet Microbiol. 2024, 290, 110004. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′-3′) | Product Length (bp) | Serial Number |
|---|---|---|---|
| IL-6 | F: AGATCCTGAAGCAAAAGATCGC | 101 | NM_173923.2 |
| R: TGCGTTCTTTACCCACTCGT | |||
| IL-1β | F: CCTCCGACGAGTTTCTGTGT | 158 | NM_174093.1 |
| R: GCTCATGCAGAACACCACTTC | |||
| TNF-α | F: CCCACGTTGTAGCCGACAT | 133 | NM_173966.3 |
| R: ATGAGGTAAAGCCCGTCAGC | |||
| β-actin | F: ATCGGCAATGAGCGGTTCC | 143 | NM_173979.3 |
| R: GTGTTGGCGTAGAGGTCCTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Feng, H.; Wang, L.; Zhang, J.; Lu, X.; Xu, G.; Liu, S.; Yang, Q.; Feng, X.; Wang, J.; et al. Cryptotanshinone Suppresses BVDV Propagation by Suppressing Cell Apoptosis and Restoring Hormone Secretion in Bovine Granulosa Cells. Viruses 2025, 17, 1433. https://doi.org/10.3390/v17111433
Chen X, Feng H, Wang L, Zhang J, Lu X, Xu G, Liu S, Yang Q, Feng X, Wang J, et al. Cryptotanshinone Suppresses BVDV Propagation by Suppressing Cell Apoptosis and Restoring Hormone Secretion in Bovine Granulosa Cells. Viruses. 2025; 17(11):1433. https://doi.org/10.3390/v17111433
Chicago/Turabian StyleChen, Xiaoliang, Haipeng Feng, Lei Wang, Jingyan Zhang, Xiaorong Lu, Guowei Xu, Siqi Liu, Qinxin Yang, Xiaowei Feng, Junyan Wang, and et al. 2025. "Cryptotanshinone Suppresses BVDV Propagation by Suppressing Cell Apoptosis and Restoring Hormone Secretion in Bovine Granulosa Cells" Viruses 17, no. 11: 1433. https://doi.org/10.3390/v17111433
APA StyleChen, X., Feng, H., Wang, L., Zhang, J., Lu, X., Xu, G., Liu, S., Yang, Q., Feng, X., Wang, J., Zhang, K., & Li, J. (2025). Cryptotanshinone Suppresses BVDV Propagation by Suppressing Cell Apoptosis and Restoring Hormone Secretion in Bovine Granulosa Cells. Viruses, 17(11), 1433. https://doi.org/10.3390/v17111433






