Differential Profile of Hemostasis in Dengue Fever Before and After COVID-19
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Detection of Molecular and Serological Markers for Laboratory Confirmation of Dengue
2.3. Serological Markers of COVID-19
2.4. Detection of Nitric Oxide (NO) by Griess Reaction
2.5. Enzyme-Linked Immunosorbent Assay for Quantification of Soluble Mediators TF and TFPI
2.6. Quantification of Fibrinolysis and Coagulation Regulatory Proteins by Multiplex Assay
2.7. Production of Viral Stock
2.8. Platelet Purification
2.9. Platelet Stimulation and Activation
2.10. Extracellular Labeling by Flow Cytometry
2.11. Statistical Analysis
3. Results
3.1. Clinical and Laboratory Profile of Dengue Before and After COVID-19
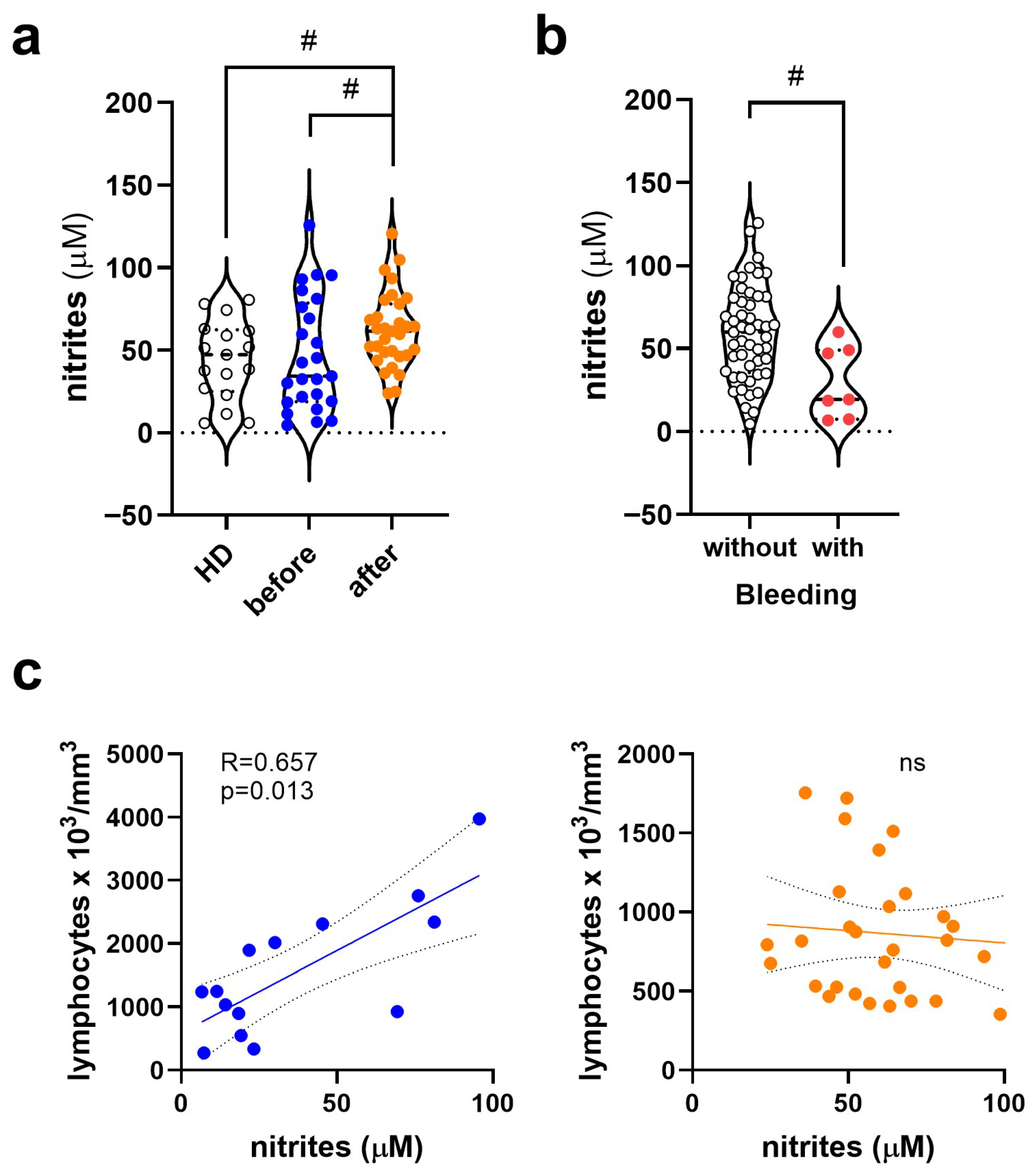
3.2. Change in Nitrite (NO2−) Levels in Dengue Before and After COVID-19
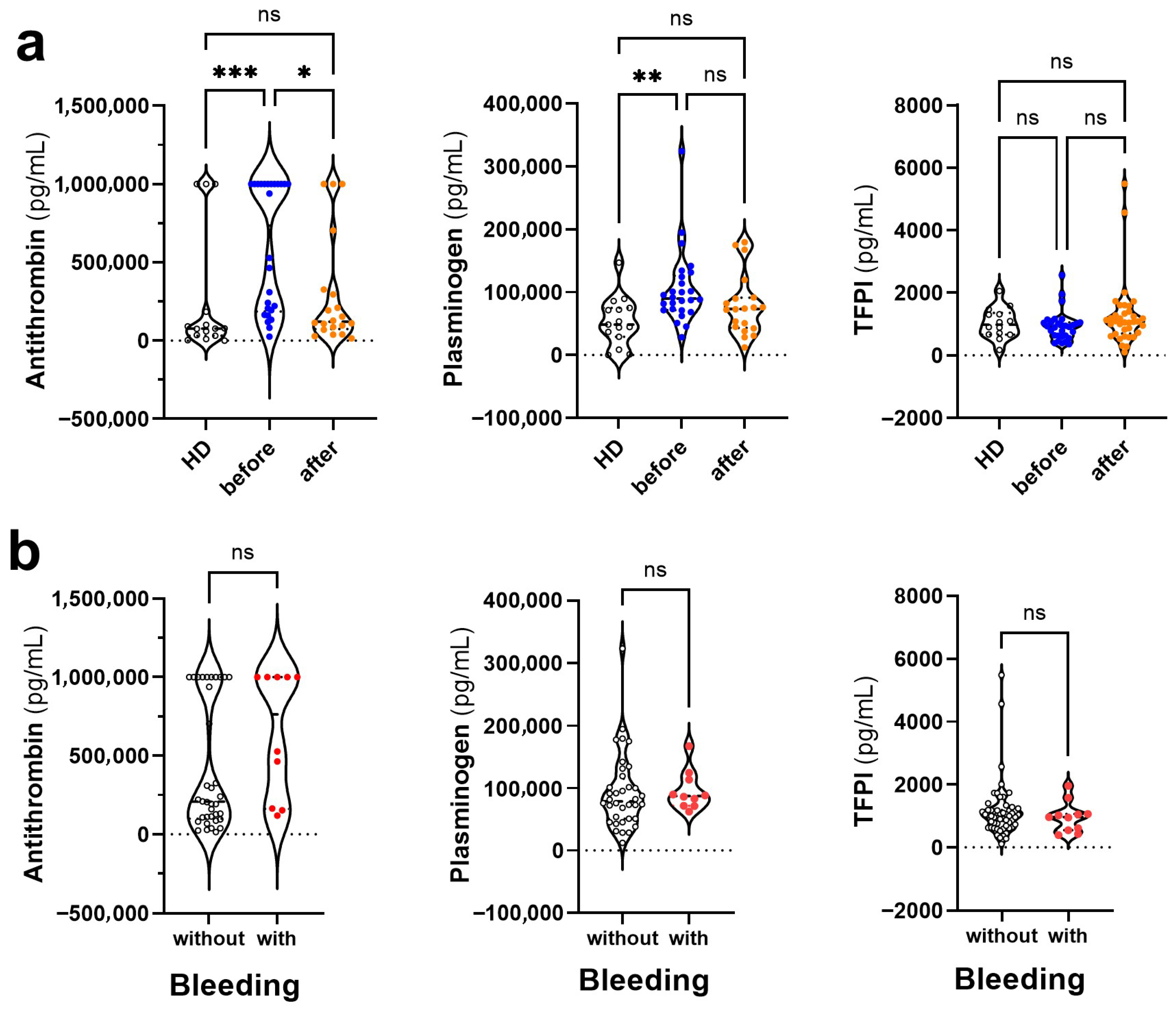
3.3. Changes in the Levels of Anticoagulant and Fibrinolytic Mediators in Dengue Before and After COVID-19
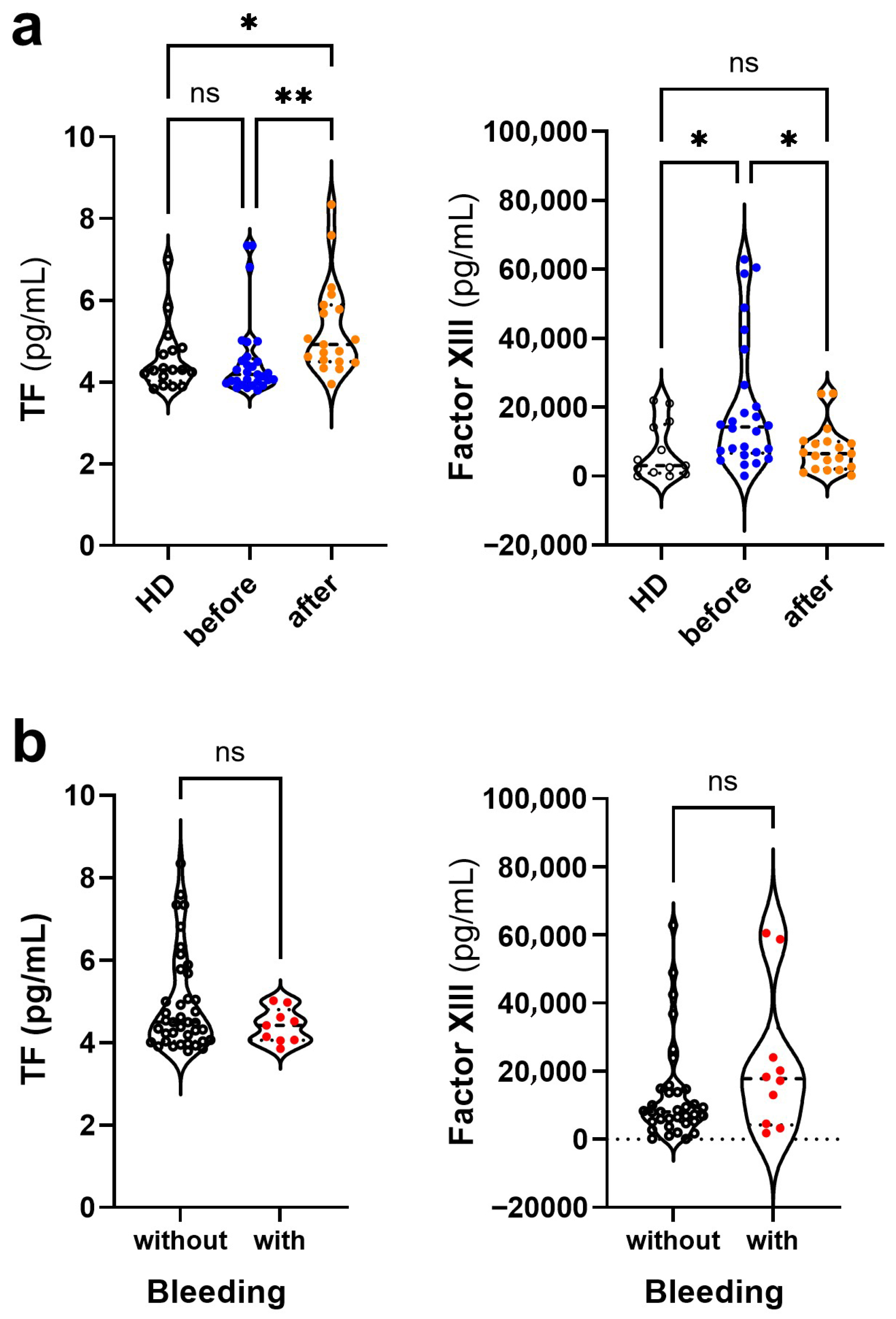
3.4. Changes in the Levels of Coagulation Mediators in Dengue Before and After COVID-19
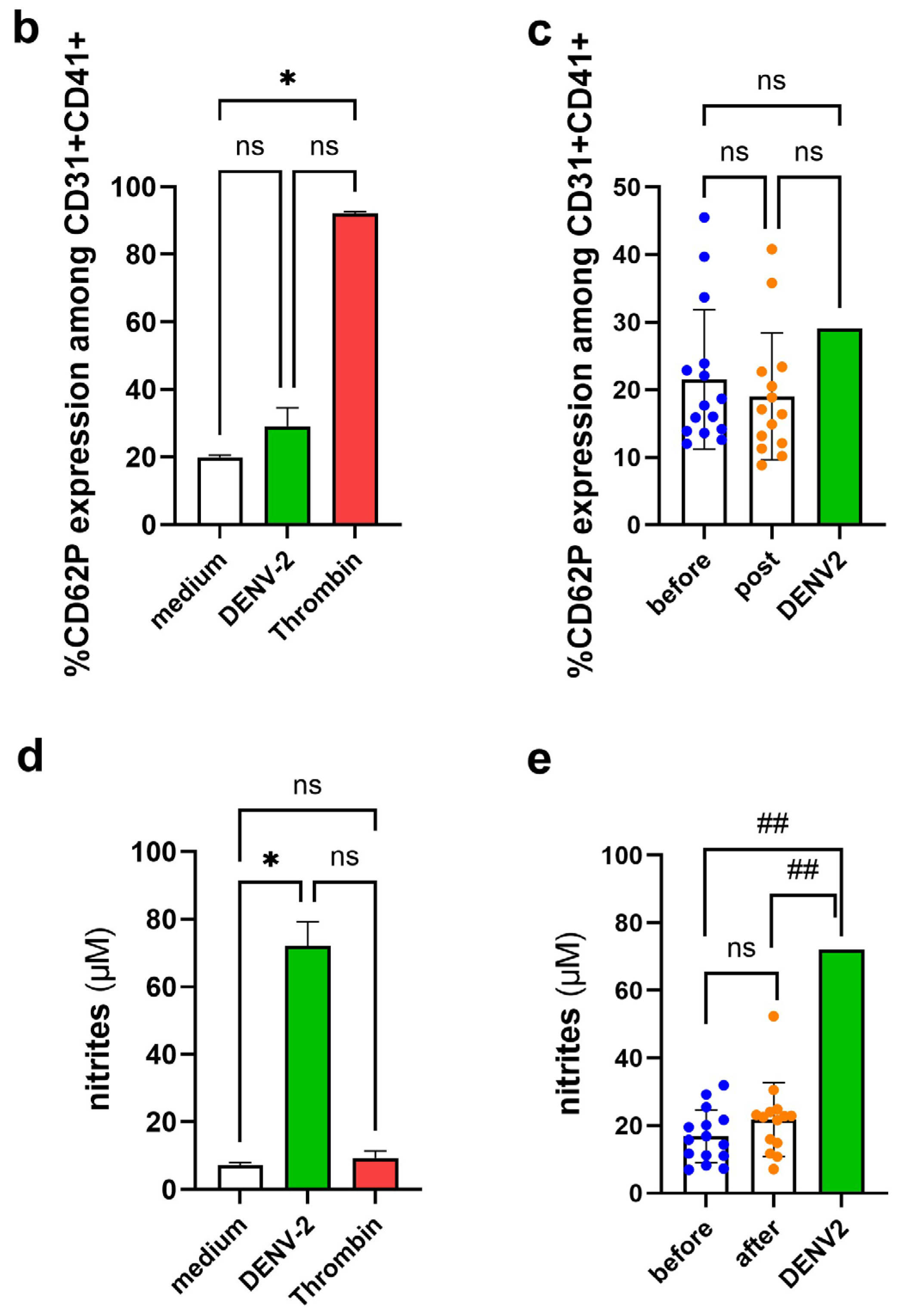
3.5. Platelet Activation Observed by CD62P Expression and NO Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ridwan, R. COVID-19 and dengue: A deadly duo. Trop. Dr. 2020, 50, 270–272. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Sharawat, I.K. COVID-19 and/with dengue infection: A curse in an overburdened healthcare system. Trop. Dr. 2021, 51, 106–108. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, N.; Bishoyi, A.K. Dengue: Epidemiology, diagnosis methods, treatment options, and prevention strategies. Arch. Virol. 2025, 170, 48. [Google Scholar] [CrossRef]
- Oliveira Roster, K.I.; Kissler, S.M.; Omoregie, E.; Wang, J.C.; Amin, H.; Di Lonardo, S.; Hughes, S.; Grad, Y.H. Surveillance strategies for the detection of new pathogen variants across epidemiological contexts. PLoS Comput. Biol. 2024, 20, e1012416. [Google Scholar] [CrossRef]
- Sansone, N.M.S.; Boschiero, M.N.; Marson, F.A.L. Dengue outbreaks in Brazil and Latin America: The new and continuing challenges. Int. J. Infect. Dis. 2024, 147, 107192. [Google Scholar] [CrossRef]
- Rabiu, A.T.; Mohan, A.; Çavdaroğlu, S.; Xenophontos, E.; Costa, A.C.S.; Tsagkaris, C.; Hashim, H.T.; Ahmad, S.; Essar, M.Y. Dengue and COVID-19: A double burden to Brazil. J. Med. Virol. 2021, 93, 4092–4093. [Google Scholar] [CrossRef]
- Carvalho, T.A.; Boschiero, M.N.; Marson, F.A.L. COVID-19 in Brazil: 150,000 deaths and the Brazilian underreporting. Diagn. Microbiol. Infect. Dis. 2021, 99, 115258. [Google Scholar] [CrossRef] [PubMed]
- Conceição, G.M.S.; Barbosa, G.L.; Lorenz, C.; Bocewicz, A.C.D.; Santana, L.M.R.; Marques, C.C.A.; Chiaravalloti-Neto, F. Effect of social isolation in dengue cases in the state of Sao Paulo, Brazil: An analysis during the COVID-19 pandemic. Travel Med. Infect. Dis. 2021, 44, 102149. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, N.; Lourenço, J.; Wang, L.; Cazelles, B.; Dong, L.; Li, B.; Liu, Y.; Jit, M.; Bosse, N.I.; et al. Measuring the effects of COVID-19-related disruption on dengue transmission in southeast Asia and Latin America: A statistical modelling study. Lancet Infect. Dis. 2022, 22, 657–667. [Google Scholar] [CrossRef]
- Liyanage, P.; Rocklöv, J.; Tissera, H.A. The impact of COVID-19 lockdown on dengue transmission in Sri Lanka; A natural experiment for understanding the influence of human mobility. PLoS Neglected Trop. Dis. 2021, 15, e0009420. [Google Scholar] [CrossRef]
- Khairunisa, S.Q.; Amarullah, I.H.; Churrotin, S.; Fitria, A.L.; Amin, M.; Lusida, M.I.; Soegijanto, S. Potential Misdiagnosis between COVID-19 and Dengue Infection Using Rapid Serological Test. Infect. Dis. Rep. 2021, 13, 540–551. [Google Scholar] [CrossRef]
- Yan, G.; Lee, C.K.; Lam, L.T.M.; Yan, B.; Chua, Y.X.; Lim, A.Y.N.; Phang, K.F.; Kew, G.S.; Teng, H.; Ngai, C.H.; et al. Covert COVID-19 and false-positive dengue serology in Singapore. Lancet Infect. Dis. 2020, 20, 536. [Google Scholar] [CrossRef]
- Lokida, D.; Lukman, N.; Salim, G.; Butar-Butar, D.P.; Kosasih, H.; Wulan, W.N.; Naysilla, A.M.; Djajady, Y.; Sari, R.A.; Arlinda, D.; et al. Diagnosis of COVID-19 in a Dengue-Endemic Area. Am. J. Trop. Med. Hyg. 2020, 103, 1220–1222. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Familiar-Macedo, D.; Medeiros, T.; Carvalho, F.R.; Almeida, J.R.; Silva, A.A.; dos Santos, F.B.; de Souza, L.J.; Damasco, P.V.; Azeredo, E.L.; et al. Assessment of threat of concurrent SARS-CoV-2 and DENV infection in the COVID-19 pandemic in Brazil in 2020: Diagnostic and immunological findings. Front. Trop. Dis. 2023, 4, 1249574. [Google Scholar] [CrossRef]
- Cheng, Y.L.; Chao, C.H.; Lai, Y.C.; Hsieh, K.H.; Wang, J.R.; Wan, S.W.; Huang, H.J.; Chuang, Y.C.; Chuang, W.J.; Yeh, T.M. Antibodies against the SARS-CoV-2 S1-RBD cross-react with dengue virus and hinder dengue pathogenesis. Front. Immunol. 2022, 13, 941923. [Google Scholar] [CrossRef] [PubMed]
- Nath, H.; Mallick, A.; Roy, S.; Sukla, S.; Biswas, S. Computational modelling supports that dengue virus envelope antibodies can bind to SARS-CoV-2 receptor binding sites: Is pre-exposure to dengue virus protective against COVID-19 severity? Comput. Struct. Biotechnol. J. 2021, 19, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Korishetty, V.; Rao, P.; Shenoy, S.; Jeppu, U.; Keerthiraj, B. Analysis of Dengue and SARS-CoV-2 Coinfection in a Tertiary Care Hospital: A Retrospective Study. J. Trop. Med. 2024, 2024, 6788850. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lin, Y.S.; Liu, H.S.; Yeh, T.M. Molecular mimicry between dengue virus and coagulation factors induces antibodies to inhibit thrombin activity and enhance fibrinolysis. J. Virol. 2014, 88, 13759–13768. [Google Scholar] [CrossRef]
- Vincent, J.L.; Levi, M.; Hunt, B.J. Prevention and management of thrombosis in hospitalised patients with COVID-19 pneumonia. Lancet Respir. Med. 2022, 10, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wajih, N.; Alipour, E.; Rigal, F.; Zhu, J.; Perlegas, A.; Caudell, D.L.; Kim-Shapiro, D. Effects of nitrite and far-red light on coagulation. Nitric Oxide 2021, 107, 11–18. [Google Scholar] [CrossRef]
- Thein, T.L.; Wong, J.; Leo, Y.S.; Ooi, E.E.; Lye, D.; Yeo, T.W. Association Between Increased Vascular Nitric Oxide Bioavailability and Progression to Dengue Hemorrhagic Fever in Adults. J. Infect. Dis. 2015, 212, 711–714. [Google Scholar] [CrossRef]
- Kasthuri, R.S.; Glover, S.L.; Boles, J.; Mackman, N. Tissue factor and tissue factor pathway inhibitor as key regulators of global hemostasis: Measurement of their levels in coagulation assays. Semin. Thromb. Hemost. 2010, 36, 764–771. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lin, Y.S.; Liu, H.S.; Wang, J.R.; Yeh, T.M. Antibodies against thrombin in Dengue patients contain both anti-thrombotic and pro-fibrinolytic activities. Thromb. Haemost. 2013, 110, 358–365. [Google Scholar] [CrossRef]
- Santiago, G.A.; Vergne, E.; Quiles, Y.; Cosme, J.; Vazquez, J.; Medina, J.F.; Medina, F.; Colón, C.; Margolis, H.; Muñoz-Jordán, J.L. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl. Trop. Dis. 2013, 7, e2311. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A Simple Method of Estimating Fifty Percent Endpoints. Am. J. Hyg. 1938, 27, 493–495. [Google Scholar]
- Gubler, D.J.; Kuno, G.; Sather, G.E.; Velez, M.; Oliver, A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am. J. Trop. Med. Hyg. 1984, 33, 158–165. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, J.; Wang, C.; Chen, X.; Zhao, X.; Jing, H.; Liu, H.; Li, Z.; Wang, L.; Shi, J. COVID-19 and ischemic stroke: Mechanisms of hypercoagulability (Review). Int. J. Mol. Med. 2021, 47, 21. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Ribeiro, A.C.; Moss, M.B.; Siqueira, M.A.; Moraes, T.L.; Ellory, J.C.; Mann, G.E.; Brunini, T.M. Dengue fever activates the L-arginine-nitric oxide pathway: An explanation for reduced aggregation of human platelets. Clin. Exp. Pharmacol. Physiol. 2008, 35, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Kaur, U.; Jethwani, P.; Mishra, S.; Dehade, A.; Yadav, A.K.; Chakrabarti, S.; Chakrabarti, S.S. Did COVID-19 or COVID-19 Vaccines Influence the Patterns of Dengue in 2021? An Exploratory Analysis of Two Observational Studies from North India. Am. J. Trop. Med. Hyg. 2023, 109, 1290–1297. [Google Scholar] [CrossRef]
- Islam, A.; Cockcroft, C.; Elshazly, S.; Ahmed, J.; Joyce, K.; Mahfuz, H.; Islam, T.; Rashid, H.; Laher, I. Coagulopathy of Dengue and COVID-19: Clinical Considerations. Trop. Med. Infect. Dis. 2022, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, R.; Nasri, F.; Kalantari, T. Coagulation and Inflammation in COVID-19: Reciprocal Relationship between Inflammatory and Coagulation Markers. Ann. Hematol. 2024, 103, 1819–1831. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chaturvedi, U.C.; Misra, A.; Mukerjee, R.; Kapoor, S.; Nagar, R.; Tandon, R.; Mathur, A. Production of cytotoxic factor by peripheral blood mononuclear cells (PBMC) in patients with dengue haemorrhagic fever. Clin. Exp. Immunol. 1998, 112, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Nagar, R. Nitric oxide in dengue and dengue haemorrhagic fever: Necessity or nuisance? FEMS Immunol. Med. Microbiol. 2009, 56, 9–24. [Google Scholar] [CrossRef]
- Petito, E.; Guglielmini, G.; De Robertis, E.; Becattini, C.; Franco, L.; Falcinelli, E.; Conti, C.; Gori, F.; Vaudo, G.; Cerotto, V.; et al. Platelets from COVID-19 Patients Show an Altered Nitric Oxide/Reactive Oxygen Species Production Balance. Thromb. Haemost. 2025, 125, 660–672. [Google Scholar] [CrossRef]
- Pinheiro, M.B.M.; Rozini, S.V.; Quirino-Teixeira, A.C.; Barbosa-Lima, G.; Lopes, J.F.; Sacramento, C.Q.; Bozza, F.A.; Bozza, P.T.; Hottz, E.D. Dengue induces iNOS expression and nitric oxide synthesis in platelets through IL-1R. Front. Immunol. 2022, 13, 1029213. [Google Scholar] [CrossRef]
- Mandal, S.M. Nitric oxide mediated hypoxia dynamics in COVID-19. Nitric Oxide 2023, 133, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, J.A.; Stojanovic, A.; Brovkovych, V.M.; Skidgel, R.A.; Du, X. Signaling-mediated functional activation of inducible nitric-oxide synthase and its role in stimulating platelet activation. J. Biol. Chem. 2008, 283, 28827–28834. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef]
- Marongiu, F.; Grandone, E.; Scano, A.; Orrù, G.; Marongiu, S.; Gerosa, C.; Fanni, D.; Faa, G.; Barcellona, D. Infectious agents including COVID-19 and the involvement of blood coagulation and fibrinolysis. A narrative review. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3886–3897. [Google Scholar] [CrossRef]
- Antoniak, S.; Mackman, N. Multiple roles of the coagulation protease cascade during virus infection. Blood 2014, 123, 2605–2613. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lin, Y.S.; Liu, C.C.; Liu, H.S.; Liao, S.H.; Shi, M.D.; Lei, H.Y.; Yeh, T.M. Factors contributing to the disturbance of coagulation and fibrinolysis in dengue virus infection. J. Formos. Med. Assoc. 2013, 112, 12–17. [Google Scholar] [CrossRef]
- da Costa Faria, N.R.; Solorzano, V.E.F.; de Souza, L.J.; Nogueira, R.M.R.; de Bruycker-Nogueira, F.; Chouin-Carneiro, T.; Santos Simões, J.B.; da Rocha Queiroz Lima, M.; de Oliveira Pinto, L.M.; Kubelka, C.F.; et al. Analysis of Clinical and Laboratory Alterations Related to Dengue Case Severity: Comparison between Serotypes 2 and 4 in Brazil. Am. J. Trop. Med. Hyg. 2017, 97, 137–145. [Google Scholar] [CrossRef]
- Eslamifar, Z.; Behzadifard, M.; Soleimani, M.; Behzadifard, S. Coagulation abnormalities in SARS-CoV-2 infection: Overexpression tissue factor. Thromb. J. 2020, 18, 38. [Google Scholar] [CrossRef]
- von Meijenfeldt, F.A.; Havervall, S.; Adelmeijer, J.; Lundström, A.; Magnusson, M.; Mackman, N.; Thalin, C.; Lisman, T. COVID-19 is Associated with an Acquired Factor XIII Deficiency. Thromb. Haemost. 2021, 121, 1668–1669. [Google Scholar] [CrossRef]
- Perumal, R.; Shunmugam, L.; Naidoo, K.; Abdool Karim, S.S.; Wilkins, D.; Garzino-Demo, A.; Brechot, C.; Parthasarathy, S.; Vahlne, A.; Nikolich, J.Ž. Long COVID: A review and proposed visualization of the complexity of long COVID. Front. Immunol. 2023, 14, 1117464. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, H.; Townsend, L.; Morrin, H.; Ahmad, A.; Comerford, C.; Karampini, E.; Englert, H.; Byrne, M.; Bergin, C.; O’Sullivan, J.M.; et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J. Thromb. Haemost. 2021, 19, 2546–2553. [Google Scholar] [CrossRef] [PubMed]

| HD Post-COVID-19 (n = 17) | Dengue Before COVID-19 (n = 32) | Dengue Post-COVID-19 (n = 36) | p Value | |
|---|---|---|---|---|
| Gender (female: male) a | 14:3 | 17:15 | 23:13 | ns |
| Age, years b | 31 (24–60) | 39 (21–88) | 38.5 (18–86) | ns |
| days after the onset of symptoms c | NA | 3 (1–8) | 5 (2–7) | 0.01 |
| Signs and symptoms (%) d | ||||
| fever | NA | 26 (81) | 26 (72) | ns |
| headache | NA | 27 (84) | 22 (61) | ns |
| myalgia/arthralgia/low back pain | NA | 30 (94) | 28 (78) | ns |
| retroorbital pain | NA | 19 (59) | 17 (47) | ns |
| vomiting/nausea/abdominal pain | NA | 21 (66) | 18 (50) | ns |
| Fluid accumulation | NA | 0 | 0 | ns |
| Bleeding | NA | 9 (28) | 2 (6) | 0.012 |
| Dengue Fever | NA | 23 (72) | 26 (72) | ns |
| Dengue with Warning Signs | NA | 9 (28) | 10 (27) | |
| Serological and molecular diagnosis (%) d | ||||
| IgG anti-DENV | 13 (76) | 23 (70) | 31 (86) | ns |
| IgM anti-DENV | 0 | 17 (53) | 19 (54) | ns |
| NS1 DENV Ag | 0 | 21 (66) | 29 (81) | ns |
| RT-qPCR DENV | NA | 17 (53) | 7 (19) | 0.02 |
| Blood Count (×103/mm3) b | ||||
| Platelet | 292 (200–367) | 199 (59–356) | 170 (55–358) | HD vs. Dengue not-exposed p = 0.003 HD vs. Dengue exposed p < 0.001 |
| Leucocyte | 6600 (4680–9690) | 5000 (2100–19,700) | 2850 (1500–5100) | HD vs. Dengue exposed p < 0.001 Dengue not-exposed vs. exposed p < 0.001 |
| Lymphocyte | 2150 (1002–32,000) | 1452 (272–3975) | 816 (354–1754) | HD vs. Dengue not-exposed p < 0.001 Dengue not-exposed vs. exposed p = 0.004 |
| Monocyte | 390 (243–3000) | 366 (22–985) | 355 (71–612) | ns |
| ALT (IU/L) b | 17 (6–54) | 27 (10–76) | 33 (13–1152) | HD vs. Dengue exposed p = 0.031 |
| AST (IU/L) b | 21 (13–52) | 26 (11–111) | 51 (21–438) | HD vs. Dengue exposed p = 0.006 Dengue not-exposed vs. exposed p = 0.001 |
| APRI b | 0.06 (0.04–0.20) | 0.10 (0.03–1.88) | 0.32 (0.07–6.60) | HD vs. Dengue exposed p = 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.C.; Carneiro, J.F.; de Souza e Silva Sales, A.P.; Gandini, M.; dos Santos, F.B.; Damasco, P.V.; de Souza, L.J.; de Azeredo, E.L.; de-Oliveira-Pinto, L.M. Differential Profile of Hemostasis in Dengue Fever Before and After COVID-19. Viruses 2025, 17, 1431. https://doi.org/10.3390/v17111431
Santos AC, Carneiro JF, de Souza e Silva Sales AP, Gandini M, dos Santos FB, Damasco PV, de Souza LJ, de Azeredo EL, de-Oliveira-Pinto LM. Differential Profile of Hemostasis in Dengue Fever Before and After COVID-19. Viruses. 2025; 17(11):1431. https://doi.org/10.3390/v17111431
Chicago/Turabian StyleSantos, Alanna Calheiros, Julya Farias Carneiro, Anna Paula de Souza e Silva Sales, Mariana Gandini, Flávia Barreto dos Santos, Paulo Vieira Damasco, Luis José de Souza, Elzinandes Leal de Azeredo, and Luzia Maria de-Oliveira-Pinto. 2025. "Differential Profile of Hemostasis in Dengue Fever Before and After COVID-19" Viruses 17, no. 11: 1431. https://doi.org/10.3390/v17111431
APA StyleSantos, A. C., Carneiro, J. F., de Souza e Silva Sales, A. P., Gandini, M., dos Santos, F. B., Damasco, P. V., de Souza, L. J., de Azeredo, E. L., & de-Oliveira-Pinto, L. M. (2025). Differential Profile of Hemostasis in Dengue Fever Before and After COVID-19. Viruses, 17(11), 1431. https://doi.org/10.3390/v17111431








