Characterization of Chemically-Induced Endogenous Retroviral Particles in the CHO-K1 Cell Line
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
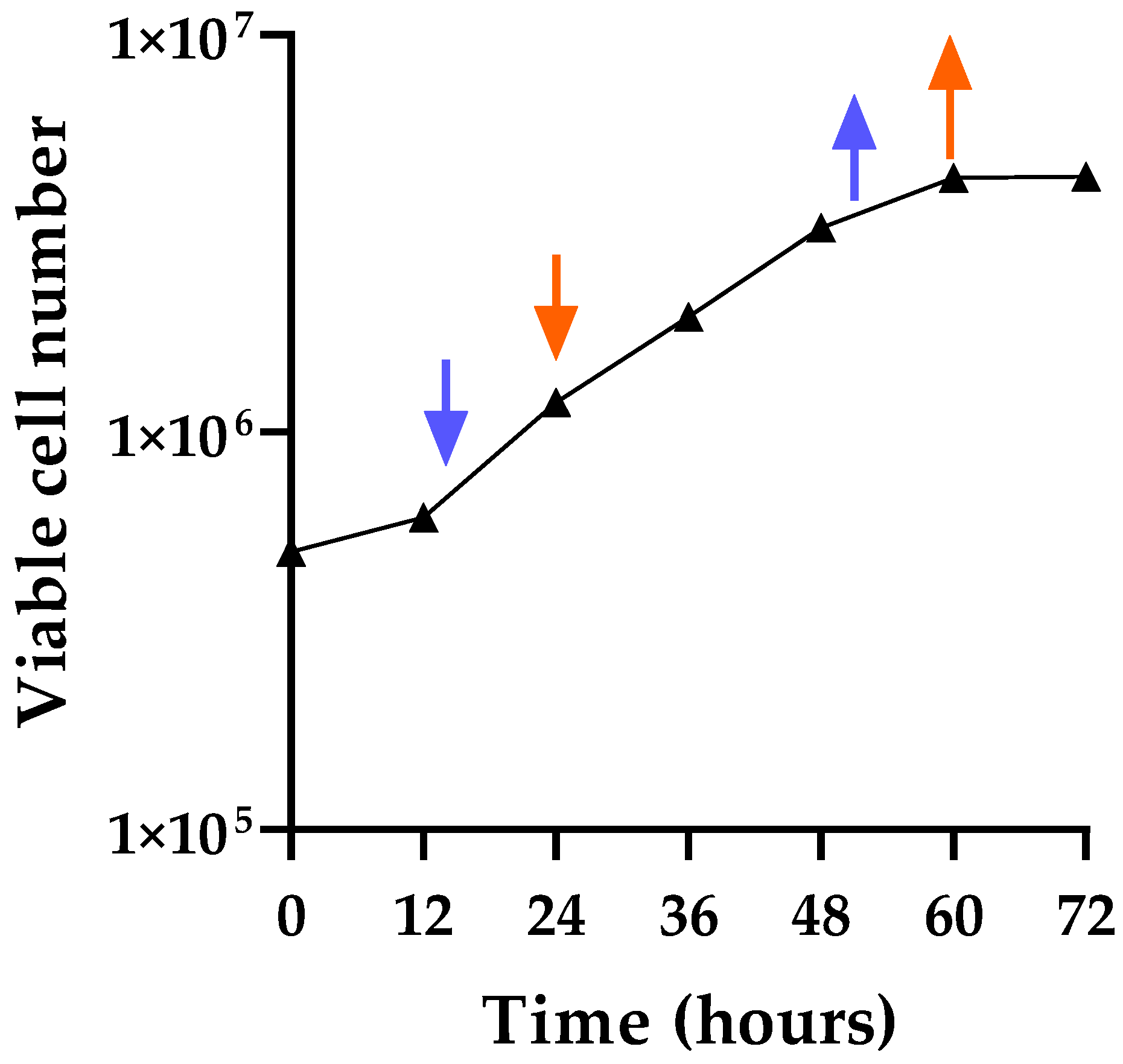
2.2. Growth Curve and Population Doubling Time
2.3. Drug Dose Evaluation and Virus Induction Assays
2.4. PERT Analysis
2.5. TEM Analysis
2.6. Infectivity Analysis
2.7. HTS Analysis
3. Results
3.1. Determination of Cell Growth Characteristics and Optimization of Virus Induction Conditions
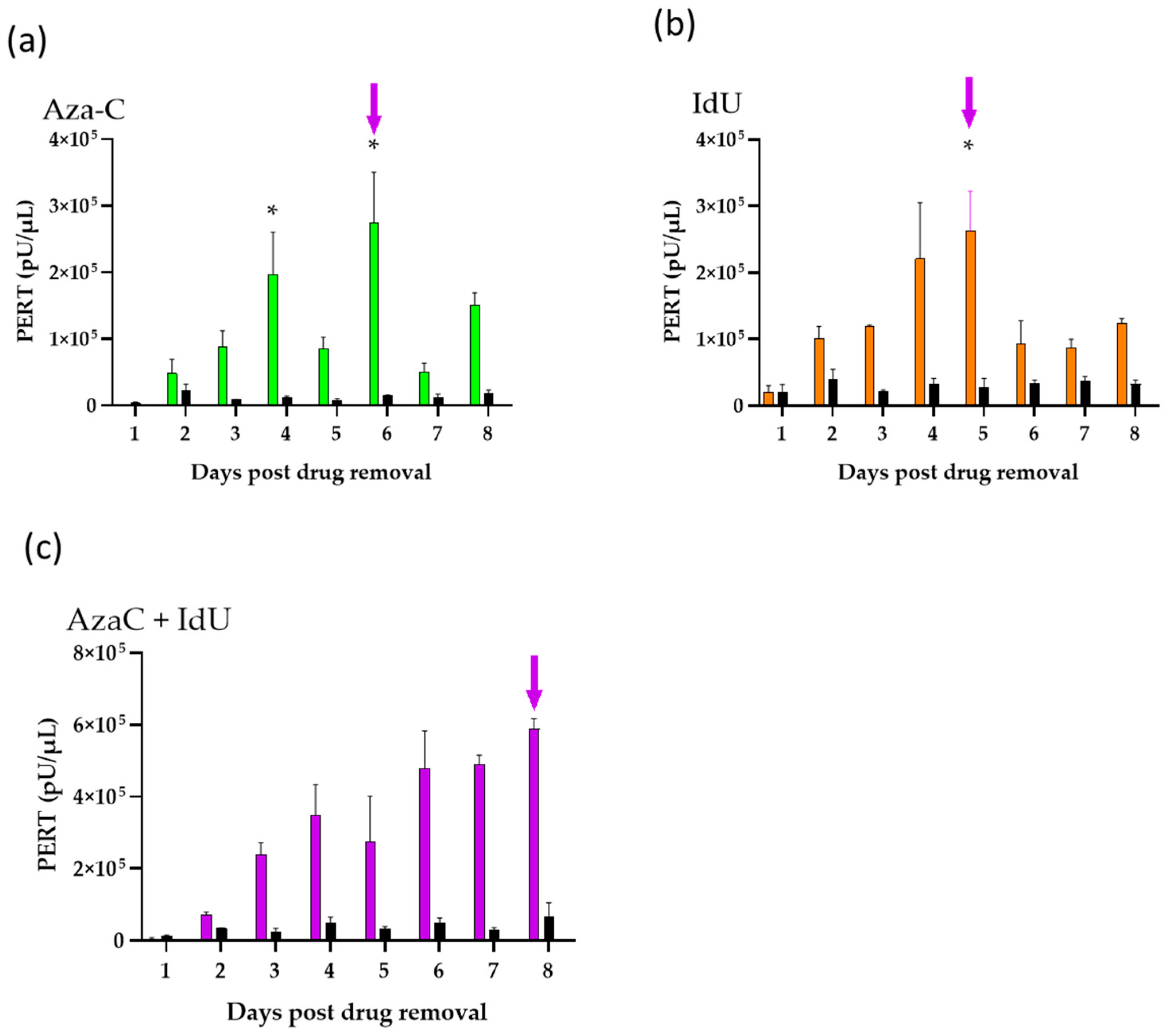
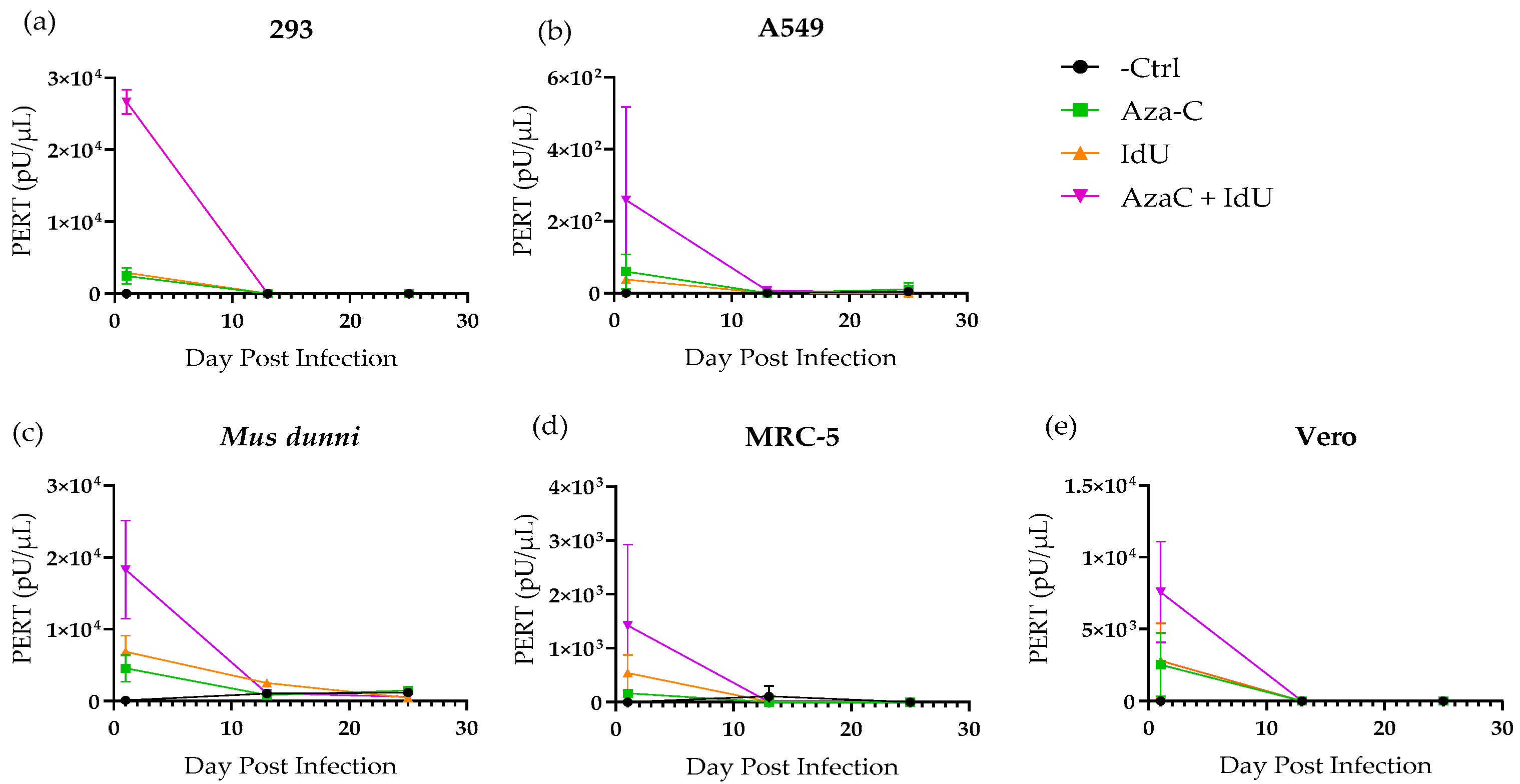
3.2. Evaluation of Endogenous Retrovirus Induction from CHO-K1 Cells
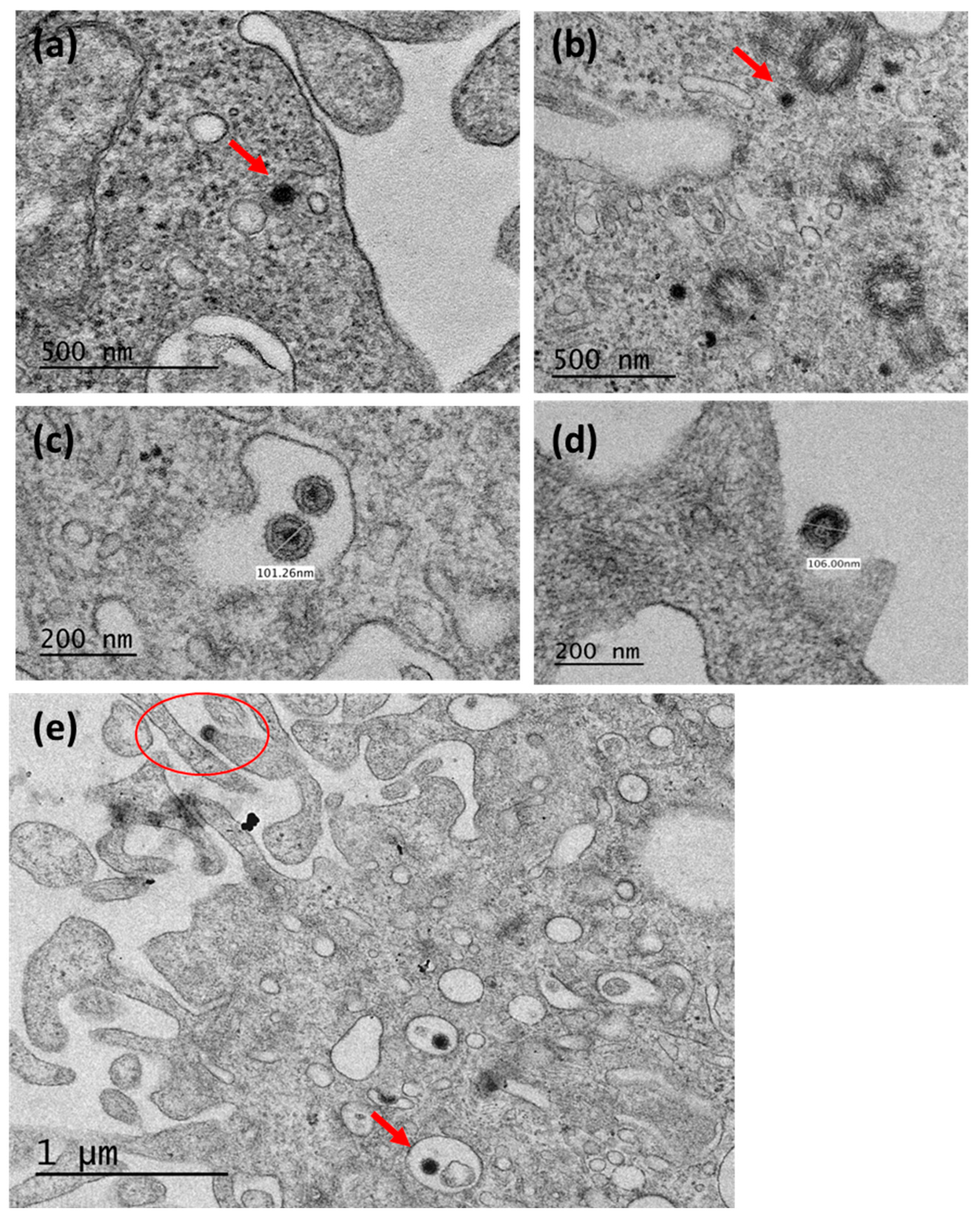
3.3. TEM Determination of Virus Particles
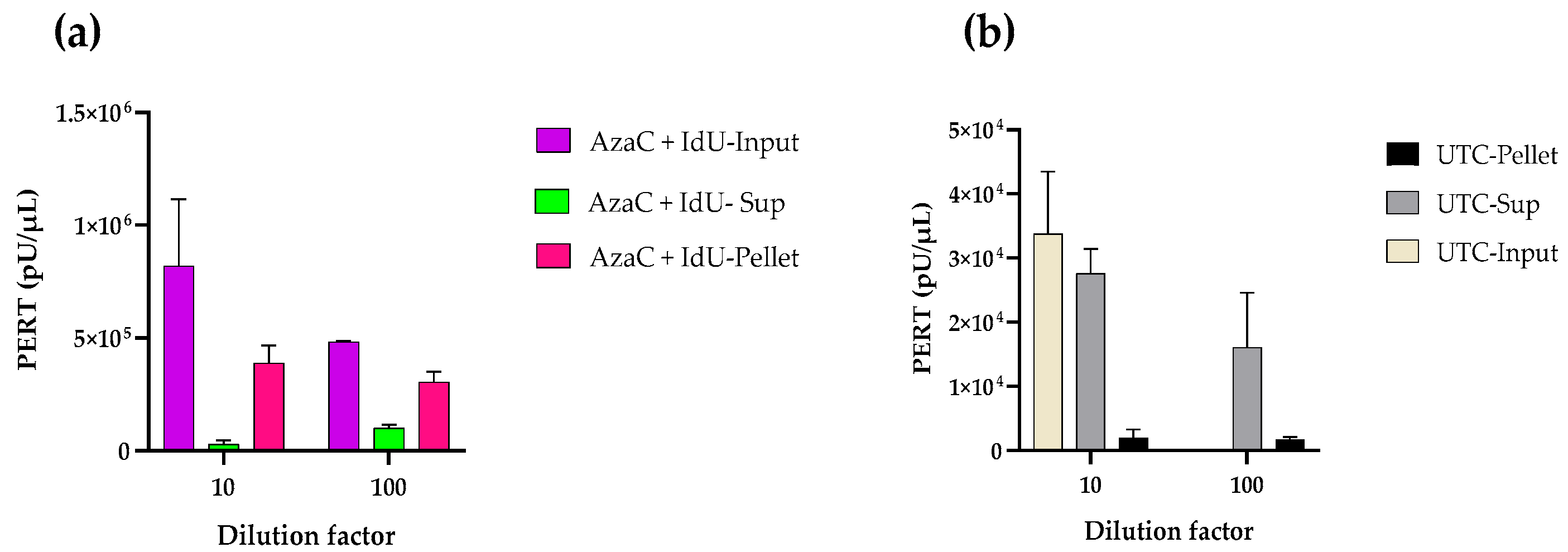
3.4. Infectivity Analysis of CHO RVLPs
3.5. Characterization of Inducible CHO RVLPs Using Long-Read HTS
| Ref. Seq. ID * | Description | Ref. Seq Length (bp) | Minimap Alignment to the 77K Reads vs. Each Ref Independently | |
|---|---|---|---|---|
| Read No. | % Genome Length Covered | |||
| M34949 | C. griseus intracisternal A-particle retrovirus like sequences. | 1953 | 139 | 81.7 |
| M34950 | C. griseus intracisternal A-particle retrovirus like sequences. | 1570 | 112 | 86.6 |
| X07947 | Chinese hamster Intracisternal A-Particle gene (IAP) corresponding to 5′LTR-GAG | 2061 | 58 | 99.2 |
| M73970 | Chinese hamster provirus gene, complete cds (IAP) | 6404 | 261 | 83.0 |
| M34951 | C. griseus intracisternal A-particle retrovirus like sequences. (IAP) | 2186 | 103 | 99.8 |
| MN527960 | Cricetulus griseus endogenous virus CHERV-1b cell line CHO-K1, complete sequence (IAP) | 7924 | 402 | 100 |
| MN527961 | Cricetulus griseus endogenous virus CHERV-2g cell line CHO-K1, complete sequence. (Type C) | 8704 | 2742 | 100 |
| M99691 | Hamster retroviral sequence mRNA (Type C) | 1184 | 1292 | 100 |
| U09104 | Cricetulus griseus 5′ long terminal repeat, complete sequence; gag, pol, and env genes, complete sequence; and 3′ long terminal repeat complete sequence. (Type C) | 9603 | 1151 | 81.9 |
| MN527962 | Cricetulus griseus endogenous virus CHERV-3g cell line CHO-K1, complete sequence (Type C) | 8340 | 874 | 100 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Puck, T.T.; Cieciura, S.J.; Robinson, A. Genetics of somatic mammalian cells. III. Long-term cultivation of euploid cells from human and animal subjects. J. Exp. Med. 1958, 108, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Grillberger, L.; Kreil, T.R.; Nasr, S.; Reiter, M. Emerging trends in plasma-free manufacturing of recombinant protein therapeutics expressed in mammalian cells. Biotechnol. J. 2009, 4, 186–201. [Google Scholar] [CrossRef]
- Tihanyi, B.; Nyitray, L. Recent advances in CHO cell line development for recombinant protein production. Drug Discov. Today Technol. 2020, 38, 25–34. [Google Scholar] [CrossRef]
- Sanchez-Martinez, Z.V.; Alpuche-Lazcano, S.P.; Stuible, M.; Durocher, Y. CHO cells for virus-like particle and subunit vaccine manufacturing. Vaccine 2024, 42, 2530–2542. [Google Scholar] [CrossRef]
- Nagy, A.; Chakrabarti, L.; Kurasawa, J.; Mulagapati, S.H.R.; Devine, P.; Therres, J.; Chen, Z.; Schmelzer, A.E. Engineered CHO cells as a novel AAV production platform for gene therapy delivery. Sci. Rep. 2023, 13, 19210. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, D.N. Pericentriolar virus-like particles in Chinese hamster ovary cells. J. Gen. Virol. 1974, 24, 395–399. [Google Scholar] [CrossRef]
- Heine, U.I.; Todaro, G.J. Unique association of three new type B retrovirus isolates with kinetochores and centrioles of the host cell. In Electron Microscopy; Sturgess, J.M., Ed.; Microscopical Society of Canada: Toronto, ON, Canada, 1978; Volume II, pp. 358–359. [Google Scholar]
- Heine, U.I.; Todaro, G.J. New type B retrovirus isolates associated with kinetochores and centrioles of the host cell. J. Gen. Virol. 1978, 39, 41–52. [Google Scholar] [CrossRef]
- Calafat, J.; Hilkens, J.G. Ultrastructural study of virus-like particles in Chinese hamster lung cells. J. Gen. Virol. 1978, 41, 417–420. [Google Scholar] [CrossRef]
- Heine, U.I.; Kramarsky, B.; Wendel, E.; Suskind, R.G. Enhanced proliferation of endogenous virus in Chinese hamster cells associated with microtubules and the mitotic apparatus of the host cell. J. Gen. Virol. 1979, 44, 45–55. [Google Scholar] [CrossRef]
- Anderson, K.P.; Lie, Y.S.; Low, M.A.; Williams, S.R.; Fennie, E.H.; Nguyen, T.P.; Wurm, F.M. Presence and transcription of intracisternal A-particle-related sequences in CHO cells. J. Virol. 1990, 64, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, A.J.; Wilson, N.J.; Smith, K.T. Characterisation of endogenous retrovirus in rodent cell lines used for production of biologicals. Biologicals 2003, 31, 251–260. [Google Scholar] [CrossRef]
- Lieber, M.M.; Benveniste, R.E.; Livingston, D.M.; Todaro, G.J. Mammalian cells in culture frequently release type C viruses. Science 1973, 182, 56–59. [Google Scholar] [CrossRef]
- Manly, K.F.; Givens, J.F.; Taber, R.L.; Zeigel, R.F. Characterization of virus-like particles released from the hamster cell line CHO-K1 after treatment with 5-bromodeoxyuridine. J. Gen. Virol. 1978, 39, 505–517. [Google Scholar] [CrossRef]
- Anderson, K.P.; Low, M.A.; Lie, Y.S.; Keller, G.A.; Dinowitz, M. Endogenous origin of defective retroviruslike particles from a recombinant Chinese hamster ovary cell line. Virology 1991, 181, 305–311. [Google Scholar] [CrossRef]
- Lie, Y.S.; Penuel, E.M.; Low, M.A.; Nguyen, T.P.; Mangahas, J.O.; Anderson, K.P.; Petropoulos, C.J. Chinese hamster ovary cells contain transcriptionally active full-length type C proviruses. J. Virol. 1994, 68, 7840–7849. [Google Scholar] [CrossRef] [PubMed]
- Duroy, P.O.; Bosshard, S.; Schmid-Siegert, E.; Neuenschwander, S.; Arib, G.; Lemercier, P.; Masternak, J.; Roesch, L.; Buron, F.; Girod, P.A.; et al. Characterization and mutagenesis of Chinese hamster ovary cells endogenous retroviruses to inactivate viral particle release. Biotechnol. Bioeng. 2020, 117, 466–485. [Google Scholar] [CrossRef] [PubMed]
- Dinowitz, M.; Lie, Y.S.; Low, M.A.; Lazar, R.; Fautz, C.; Potts, B.; Sernatinger, J.; Anderson, K. Recent studies on retrovirus-like particles in Chinese hamster ovary cells. Dev. Biol. Stand. 1991, 76, 201–207. [Google Scholar]
- Emanoil-Ravier, R.; Hojman, F.; Servenay, M.; Lesser, J.; Bernardi, A.; Peries, J. Biological and molecular studies of endogenous retrovirus-like genes in Chinese hamster cell lines. Dev. Biol. Stand. 1991, 75, 113–122. [Google Scholar]
- Todaro, G.J. Type C virogenes: Modes of transmission and evolutionary aspects. Hamatol. Bluttransfus. 1976, 19, 357–374. [Google Scholar]
- de Harven, E. Remarks on the ultrastructure of type A, B, and C virus particles. Adv. Virus Res. 1974, 19, 221–264. [Google Scholar]
- Lesser, J.; Bittoun, P.; Emanoil-Ravier, R.; Peries, J. Biological and molecular studies on Syrian hamster intracisternal R-type particles. Arch. Virol. 1995, 140, 95–109. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Toh, H.; Miyata, T.; Awaya, T. Nucleotide sequence of the Syrian hamster intracisternal A-particle gene: Close evolutionary relationship of type A particle gene to types B and D oncovirus genes. J. Virol. 1985, 55, 387–394. [Google Scholar] [CrossRef]
- Suzuki, A.; Kitasato, H.; Kawakami, M.; Ono, M. Molecular cloning of retrovirus-like genes present in multiple copies in the Syrian hamster genome. Nucleic Acids Res. 1982, 10, 5733–5746. [Google Scholar] [CrossRef][Green Version]
- Lueders, K.K.; Kuff, E.L. Comparison of the sequence organization of related retrovirus-like multigene families in three evolutionarily distant rodent genomes. Nucleic Acids Res. 1983, 11, 4391–4408. [Google Scholar] [CrossRef]
- Hawley, R.G.; Shulman, M.J.; Murialdo, H.; Gibson, D.M.; Hozumi, N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc. Natl. Acad. Sci. USA 1982, 79, 7425–7429. [Google Scholar] [CrossRef] [PubMed]
- Canaani, E.; Dreazen, O.; Klar, A.; Rechavi, G.; Ram, D.; Cohen, J.B.; Givol, D. Activation of the c-mos oncogene in a mouse plasmacytoma by insertion of an endogenous intracisternal A-particle genome. Proc. Natl. Acad. Sci. USA 1983, 80, 7118–7122. [Google Scholar] [CrossRef]
- Kuff, E.L.; Feenstra, A.; Lueders, K.; Smith, L.; Hawley, R.; Hozumi, N.; Shulman, M. Intracisternal A-particle genes as movable elements in the mouse genome. Proc. Natl. Acad. Sci. USA 1983, 80, 1992–1996. [Google Scholar] [CrossRef]
- Burt, D.W.; Reith, A.D.; Brammar, W.J. A retroviral provirus closely associated with the Ren-2 gene of DBA/2 mice. Nucleic Acids Res. 1984, 12, 8579–8593. [Google Scholar] [CrossRef]
- Hawley, R.G.; Trimble, W.S.; Shulman, M.J.; Hozumi, N. Transposition of intracisternal A-particle genes in mouse hybridomas. J. Cell Physiol. Suppl. 1984, 3, 29–38. [Google Scholar] [CrossRef]
- Greenberg, R.; Hawley, R.; Marcu, K.B. Acquisition of an intracisternal A-particle element by a translocated c-myc gene in a murine plasma cell tumor. Mol. Cell Biol. 1985, 5, 3625–3628. [Google Scholar]
- Li, X.; Gao, Y.; Ye, Z. A Single Amino Acid Substitution at Residue 218 of Hemagglutinin Improves the Growth of Influenza A(H7N9) Candidate Vaccine Viruses. J. Virol. 2019, 93, e00570-19. [Google Scholar] [CrossRef]
- Gardner, M.B.; Henderson, B.E.; Estes, J.D.; Menck, H.; Parker, J.C.; Huebner, R.J. Unusually high incidence of spontaneous lymphomas in wild house mice. J. Natl. Cancer Inst. 1973, 50, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Gardner, M.B.; Henderson, B.E.; Officer, J.E.; Rongey, R.W.; Parker, J.C.; Oliver, C.; Estes, J.D.; Huebner, R.J. A spontaneous lower motor neuron disease apparently caused by indigenous type-C RNA virus in wild mice. J. Natl. Cancer Inst. 1973, 51, 1243–1254. [Google Scholar] [CrossRef]
- Officer, J.E.; Tecson, N.; Estes, J.D.; Fontanilla, E.; Rongey, R.W.; Gardner, M.B. Isolation of a neurotropic type C virus. Science 1973, 181, 945–947. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Gardner, M.B.; Chan, E. Amphotropic host range of naturally occuring wild mouse leukemia viruses. J. Virol. 1976, 19, 13–18. [Google Scholar] [CrossRef]
- Risser, R.; Horowitz, J.M.; McCubrey, J. Endogenous mouse leukemia viruses. Annu. Rev. Genet. 1983, 17, 85–121. [Google Scholar] [CrossRef]
- Hartley, J.W.; Rowe, W.P. Naturally occurring murine leukemia viruses in wild mice: Characterization of a new “amphotropic” class. J. Virol. 1976, 19, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.K.; Khan, A.S. Evaluation of different RT enzyme standards for quantitation of retroviruses using the single-tube fluorescent product-enhanced reverse transcriptase assay. J. Virol. Methods 2009, 157, 133–140. [Google Scholar] [CrossRef]
- Paul, J. Cell and Tissue Culture, 5th ed.; Churchill Livingstone: Edinburgh, UK, 1975. [Google Scholar]
- Ma, H.; Bae, E.H.; Chin, P.J.; Khan, A.S. Characterization of Endogenous Retroviral-like Particles Expressed from the Spodoptera frugiperda Sf9 Cell Line. Viruses 2025, 17, 136. [Google Scholar] [CrossRef]
- Khan, A.S.; Sears, J.F. Pert analysis of endogenous retroviruses induced from K-BALB mouse cells treated with 5-iododeoxyuridine: A potential strategy for detection of inducible retroviruses from vaccine cell substrates. Dev. Biol. 2001, 106, 387–392; discussion 392–393. [Google Scholar]
- Edmondson, M.; Jana, S.; Meng, F.; Strader, M.B.; Baek, J.H.; Gao, Y.; Buehler, P.W.; Alayash, A.I. Redox states of hemoglobin determine left ventricle pressure recovery and activity of mitochondrial complex IV in hypoxic rat hearts. Free Radic. Biol. Med. 2019, 141, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Ma, H.; Ma, Y.; Ma, W.; Williams, D.K.; Galvin, T.A.; Khan, A.S. Chemical induction of endogenous retrovirus particles from the vero cell line of African green monkeys. J. Virol. 2011, 85, 6579–6588. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Ma, W.; Ma, Y.; Kumar, A.; Williams, D.K.; Muller, J.; Ma, H.; Galvin, T.A. Proposed algorithm to investigate latent and occult viruses in vaccine cell substrates by chemical induction. Biologicals 2009, 37, 196–201. [Google Scholar] [CrossRef]
- Thion, C.; Green, M. Cyclic AMP-amplified replication of RNA tumour virus-like particles in Chinese hamster ovary cells. Nat. New Biol. 1973, 244, 227–231. [Google Scholar] [CrossRef]
- Lieber, M.M.; Livingston, D.M.; Todaro, G.J. Superinduction of endogenous type C virus by 5-bromodeoxyuridine from transformed mouse clones. Science 1973, 181, 443–444. [Google Scholar] [CrossRef]
- Lieber, M.M.; Todaro, G.J. Spontaneous and induced production of endogenous type-C RNA virus from a clonal line of spontaneously transformed BALB-3T3. Int. J. Cancer 1973, 11, 616–627. [Google Scholar] [CrossRef]
- Kuff, E.L.; Lueders, K.K.; Orenstein, J.M.; Wilson, S.H. Differential response of type C and intracisternal type A particle markers in cells treated with iododeoxyuridine and dexamethasone. J. Virol. 1976, 19, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Hojman, F.; Emanoil-Ravier, R.; Lesser, J.; Peries, J. Biological and molecular characterization of an endogenous retrovirus present in CHO/HBs-A Chinese hamster cell line. Dev. Biol. Stand. 1989, 70, 195–202. [Google Scholar]
- Vogt, V.M. Retroviral Virions and Genomes. In Retroviruses; John, M., Coffin, S.H.H., Harold, E., Varmus, H.E., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997; pp. 27–69. [Google Scholar]
- Khan, A.S.; Sears, J.F.; Muller, J.; Galvin, T.A.; Shahabuddin, M. Sensitive assays for isolation and detection of simian foamy retroviruses. J. Clin. Microbiol. 1999, 37, 2678–2686. [Google Scholar] [CrossRef]
- Fuentes, S.M.; Bae, E.H.; Nandakumar, S.; Williams, D.K.; Khan, A.S. Genome Analysis and Replication Studies of the African Green Monkey Simian Foamy Virus Serotype 3 Strain FV2014. Viruses 2020, 12, 403. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry. Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications. 2010. Available online: http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/vaccines/ucm202439.pdf (accessed on 2 September 2025).
- Berting, A.; Farcet, M.R.; Kreil, T.R. Virus susceptibility of Chinese hamster ovary (CHO) cells and detection of viral contaminations by adventitious agent testing. Biotechnol. Bioeng. 2010, 106, 598–607. [Google Scholar] [CrossRef] [PubMed]
- International Council for Hamonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Q5A(R2): Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin. 2023. Available online: https://database.ich.org/sites/default/files/ICH_Q5A(R2)_Guideline_2023_1101.pdf (accessed on 2 September 2025).
- Barone, P.W.; Wiebe, M.E.; Leung, J.C.; Hussein, I.T.M.; Keumurian, F.J.; Bouressa, J.; Brussel, A.; Chen, D.; Chong, M.; Dehghani, H.; et al. Viral contamination in biologic manufacture and implications for emerging therapies. Nat. Biotechnol. 2020, 38, 563–572. [Google Scholar] [CrossRef]
- Anderson, K.P.; Lie, Y.S.; Low, M.A.; Williams, S.R.; Wurm, F.M.; Dinowitz, M. Defective endogenous retrovirus-like sequences and particles of Chinese hamster ovary cells. Dev. Biol. Stand. 1991, 75, 123–132. [Google Scholar]
- Stoye, J.P.; Coffin, J.M. The four classes of endogenous murine leukemia virus: Structural relationships and potential for recombination. J. Virol. 1987, 61, 2659–2669. [Google Scholar] [CrossRef]
- Kawana, A.; Iwamoto, A.; Odawara, T.; Yoshikura, H. Host range conversion of murine leukemia virus resulting from recombination with endogenous virus. Arch. Virol. 1997, 142, 139–149. [Google Scholar] [CrossRef]
- Zhuang, J.; Mukherjee, S.; Ron, Y.; Dougherty, J.P. High rate of genetic recombination in murine leukemia virus: Implications for influencing proviral ploidy. J. Virol. 2006, 80, 6706–6711. [Google Scholar] [CrossRef]
- Hawley, R.G.; Shulman, M.J.; Hozumi, N. Transposition of two different intracisternal A particle elements into an immunoglobulin kappa-chain gene. Mol. Cell Biol. 1984, 4, 2565–2572. [Google Scholar]
- Aaronson, S.A.; Dunn, C.Y. Endogenous C-type viruses of BALB-c cells: Frequencies of spontaneous and chemical induction. J. Virol. 1974, 13, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.A.; Stephenson, J.R. Independent segregation of loci for activation of biologically distinguishable RNA C-type viruses in mouse cells. Proc. Natl. Acad. Sci. USA 1973, 70, 2055–2058. [Google Scholar] [CrossRef] [PubMed]
- Aaronson, S.A.; Todaro, G.J.; Scolnick, E.M. Induction of murine C-type viruses from clonal lines of virus-free BALB-3T3 cells. Science 1971, 174, 157–159. [Google Scholar] [CrossRef]
- Khan, A.S.; Muller, J.; Sears, J.F. Early detection of endogenous retroviruses in chemically induced mouse cells. Virus Res. 2001, 79, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Khan, A.S. Detection of Latent Retroviruses in Vaccine-related Cell Substrates: Investigation of RT Activity Produced by Chemical Induction of Vero Cells. PDA J. Pharm. Sci. Technol. 2011, 65, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Barone, P.W.; Avgerinos, S.; Ballard, R.; Brussel, A.; Clark, P.; Dowd, C.; Gerentes, L.; Hart, I.; Keumurian, F.J.; Kindermann, J.; et al. Biopharmaceutical Industry Approaches to Facility Segregation for Viral Safety: An Effort from the Consortium on Adventitious Agent Contamination in Biomanufacturing. PDA J. Pharm. Sci. Technol. 2019, 73, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.K.; Hartley, J.W.; Lander, M.R.; Kramer, B.S.; Rowe, W.P. Biochemical characterization of the amphotropic group of murine leukemia viruses. J. Virol. 1978, 26, 29–39. [Google Scholar] [CrossRef]
| GenBank Accession No. | Description |
|---|---|
| none | DNA_CS, control lambda DNA (Nanopore) |
| DQ222453.1 | Bos taurus 18S ribosomal RNA gene, internal transcribed spacer 1, 5.8S ribosomal RNA gene, internal transcribed spacer 2, and 28S ribosomal RNA gene, complete sequence |
| J00053.1 | Chinese hamster Alu-equivalent repeat (clone 34) DNA |
| J00054.1 | Chinese hamster Alu-equivalent repeat (clone 49a) DNA |
| J00055.1 | Chinese hamster Alu-equivalent repeat (clone 49b) DNA |
| J00056.1 | Chinese hamster Alu-equivalent type 2 repeat (clone 49c) DNA |
| J00057.1 | Chinese hamster Alu-equivalent repeat (clone 49d) DNA |
| J00058.1 | Chinese hamster Alu-equivalent repeat (clone 63) DNA |
| J00628.1 | mouse b1 ubiquitous repeat (copy a) mRNA and flanks |
| J00631.1 | mouse b1 ubiquitous repeat (copy b) mRNA and flanks |
| J00632.1 | mouse b1 ubiquitous repeat (copy c) mRNA and flanks |
| KY962518.1 | Homo sapiens external transcribed spacer 18S ribosomal RNA gene, internal transcribed spacer 1, 5.8S ribosomal RNA gene, internal transcribed spacer 2, 28S ribosomal RNA gene, external transcribed spacer, and CDC27-like protein pseudogene, complete sequence |
| NC_007936.1 | Cricetulus griseus mitochondrion, complete genome |
| NM_001244575.1 | Cricetulus griseus actin beta (Actb), mRNA |
| NR_046239.1 | Rattus norvegicus 45S pre-ribosomal RNA (Rn45s), ribosomal RNA |
| NR_046263.1 | Cricetulus griseus 45S ribosomal RNA (Rn45s), ribosomal RNA |
| X52123.1 | Cricetus cricetus mRNA for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (EC 1.2.1.12) |
| XM_027388602.2 | PREDICTED: Cricetulus griseus alpha tubulin acetyl-transferase 1 (Atat1), transcript variant X1, mRNA |
| XM_027427709.2 | PREDICTED: Cricetulus griseus keratin 16 (Krt16), mRNA |
| XM_027427711.2 | PREDICTED: Cricetulus griseus keratin 14 (Krt14), transcript variant X1, mRNA |
| XR_005974209.1 | PREDICTED: Microtus oregoni 5S ribosomal RNA (LOC121447662), rRNA |
| U78038.1 (nt 1-1164 and nt 1222-2068) | Mus musculus beige gene, LINE 1 repetitive element |
| U09104.1 (nt 83-344) and nt 8768-8892) | CHO SINE element sequences |
| In-house | CHO_related 7SL sequence |
| Sample | Dilution Factor | Cq b Average | RT (pU/µL) Average |
|---|---|---|---|
| AzaC + IdU (2.5 + 30 µg/mL) treated cells: d8 resuspended pellet | 10× | 27.48 | 339,767.33 |
| 100× | 22.58 | 5,755,904.90 | |
| 1000× | 26.95 | 325,327.98 | |
| Untreated cells: d7 resuspended pellet | 10× | 33.18 | 5735.42 |
| 100× | 31.22 | 19,729.09 | |
| 1000× | 35.25 | 1482.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattson, N.B.; Bosma, T.J.; Gao, Y.; Fuentes, S.M.; Chin, P.-J.; Khan, A.S. Characterization of Chemically-Induced Endogenous Retroviral Particles in the CHO-K1 Cell Line. Viruses 2025, 17, 1408. https://doi.org/10.3390/v17111408
Mattson NB, Bosma TJ, Gao Y, Fuentes SM, Chin P-J, Khan AS. Characterization of Chemically-Induced Endogenous Retroviral Particles in the CHO-K1 Cell Line. Viruses. 2025; 17(11):1408. https://doi.org/10.3390/v17111408
Chicago/Turabian StyleMattson, Nicholas B., Trent J. Bosma, Yamei Gao, Sandra M. Fuentes, Pei-Ju Chin, and Arifa S. Khan. 2025. "Characterization of Chemically-Induced Endogenous Retroviral Particles in the CHO-K1 Cell Line" Viruses 17, no. 11: 1408. https://doi.org/10.3390/v17111408
APA StyleMattson, N. B., Bosma, T. J., Gao, Y., Fuentes, S. M., Chin, P.-J., & Khan, A. S. (2025). Characterization of Chemically-Induced Endogenous Retroviral Particles in the CHO-K1 Cell Line. Viruses, 17(11), 1408. https://doi.org/10.3390/v17111408






