Vertical Transmission of Hepatitis B and C—Then and Now—A Comprehensive Literature Systematic Review
Abstract
1. Introduction
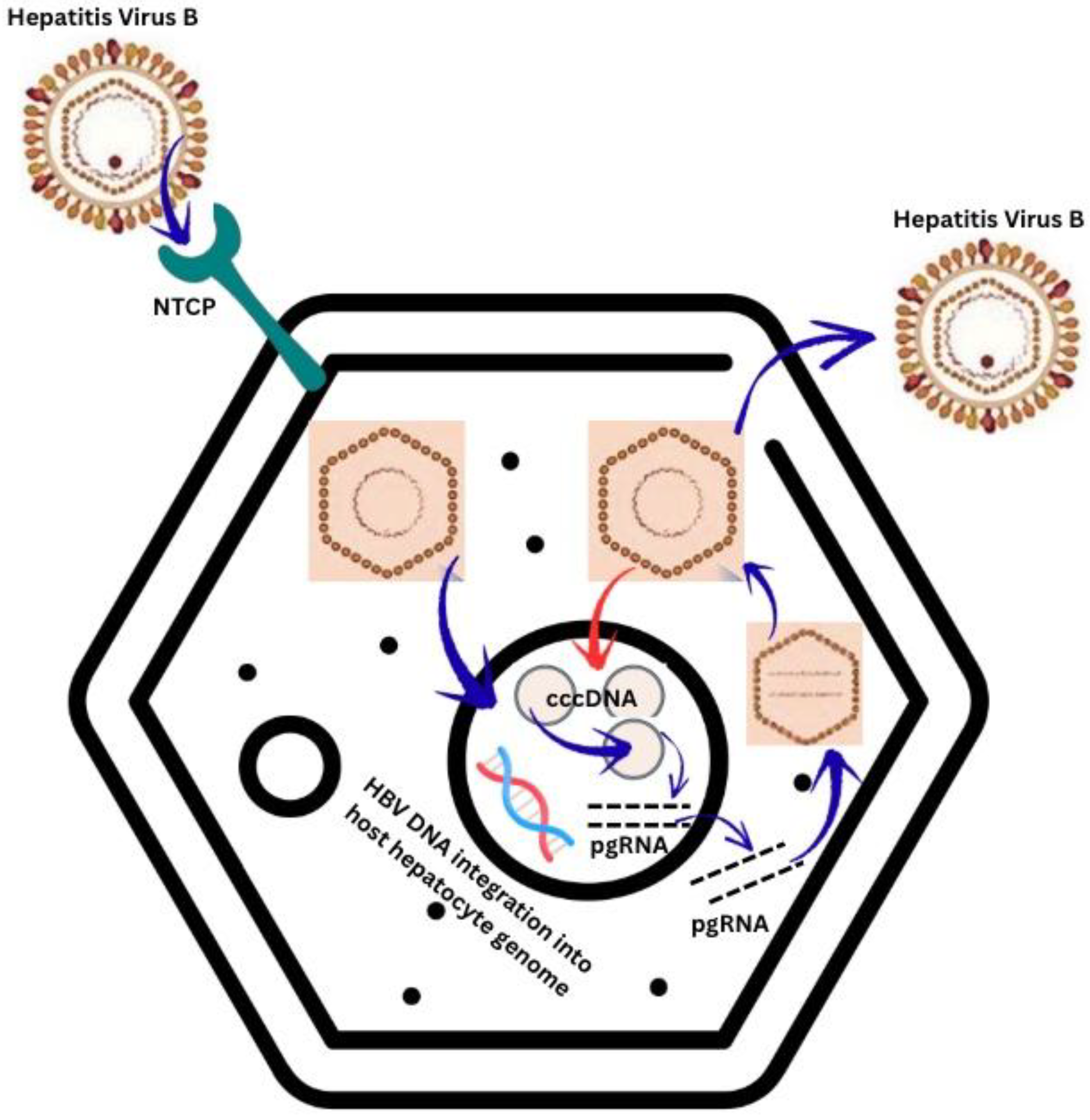
2. Materials and Methods
3. Screening Techniques
3.1. Screening of Pregnant Women with HBV Infection
3.2. Screening of Pregnant Women with HCV Infection
4. Mother-to-Child Transmission of Viral Infection
4.1. Vertical Transmission of HBV Infection
4.1.1. Transplacental Transmission of HBV Infection
4.1.2. Perinatal Transmission
4.1.3. Postnatal Transmission
4.2. Vertical Transmission of HCV Infection
5. Risk Factors for Mother-to-Child Viral Transmission
5.1. Risk Factors for HBV Vertical Transmission
5.1.1. Positive Status of HBeAg
5.1.2. HBV-DNA Viral Load in Maternal Blood
5.1.3. HBV Mutant Variants
5.1.4. Delivery Type
5.1.5. HIV Co-Infection
5.1.6. HBV Genotype
5.2. Risk Factors for HCV Vertical Transmission
5.2.1. High Maternal Serum Viral Load
5.2.2. Serum Levels of ALT
5.2.3. Prolonged Membrane Rupture, Prolonged Delivery and Obstetrical Procedures
5.2.4. Gender of Neonate
5.2.5. HIV Co-Infection
5.2.6. Twin Pregnancies
6. Prevention of Vertical Transmission
6.1. Management Strategies for Prevention of HBV VT During Pregnancy
6.1.1. Maternal Screening
6.1.2. Hepatitis B Vaccine During Pregnancy
6.1.3. Hepatitis B Immunoglobulin (HBIG) During Pregnancy
6.1.4. Antiviral Therapy During Pregnancy
6.2. Management Strategies for Prevention of HBV VT at Birth
6.3. Management Strategies for Prevention of HCV VT
7. Assessment of Infants Born to Infected Mothers
7.1. Assessment of Newborns from HBV-Positive Mothers
7.2. Assessment of Newborns from HCV-Positive Mothers
8. Future Directions
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AASLD | the American Association for the Study of Liver Diseases |
| ALT | alanine aminotransferase |
| Anti-HBc antibodies | antibodies against hepatitis B core antigen |
| Anti-HBe antibodies | antibodies against hepatitis B e antigen |
| Anti-HBs antibodies | antibodies against hepatitis B surface antigen |
| Anti-HCV antibodies | antibodies against hepatitis C virus |
| Anti-HIV antibodies | antibodies against human immunodeficiency virus |
| APSAL | the Asian Pacific Association for the Study of the Liver |
| BD | birth dose HBV vaccination |
| cccDNA | covalently closed circular DNA |
| CMA | the Chinese Medical Association |
| DNA | deoxyribonucleic acid |
| EASL | the European Association for the Study of the Liver |
| GI | gastrointestinal tract |
| HBeAg | hepatitis B e antigen |
| HBIG | hepatitis B immunoglobulin |
| HBsAg | hepatitis B surface antigen |
| HBV | hepatitis B virus |
| HBV-DNA | deoxyribonucleic acid of hepatitis B virus |
| HCV | hepatitis C virus |
| HIV | human immunodeficiency virus |
| HLA | human leukocyte antigen |
| IUT | intrauterine transmission |
| IV | intravenous |
| MTCT | mother-to-child transmission |
| NIHCE | the National Institute for Health and Care Excellence |
| PAP | prenatal antiviral prophylaxis |
| PVST | postvaccination serologic testing |
| HCV-RNA | ribonucleic acid of hepatitis C virus |
| STD | sexually transmitted disease |
| SVC | spontaneous viral clearance |
| TAF | tenofovir alafenamide fumarate |
| TDF | tenofovir disoproxil fumarate |
| VT | vertical transmission |
| WHO | World Health Organization |
References
- Jourdain, G.; Ngo-Giang-Huong, N.; Harrison, L.; Decker, L.; Khamduang, W.; Tierney, C.; Salvadori, N.; Cressey, T.R.; Sirirungsi, W.; Achalapong, J.; et al. Tenofovir versus Placebo to Prevent Perinatal Transmission of Hepatitis B. N. Engl. J. Med. 2018, 378, 911–923. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unal, E.R.; Lazenby, G.B.; Lintzenich, A.E.; Simpson, K.N.; Newman, R.; Goetzl, L. Cost-Effectiveness of Maternal Treatment to Prevent Perinatal Hepatitis B Virus Transmission. Obstet. Gynecol. 2011, 118, 655–662. [Google Scholar] [CrossRef]
- Chilaka, V.N.; Konje, J.C. Viral Hepatitis in pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Extremera, Á.; Díaz-Alcázar, M.D.M.; Muñoz-Gámez, J.A.; Cabrera-Lafuente, M.; Martín, E.; Arias-Llorente, R.P.; Carretero, P.; Gallo-Vallejo, J.L.; Romero-Narbona, F.; Salmerón-Ruiz, M.A.; et al. Seroprevalence and epidemiology of hepatitis B and C viruses in pregnant women in Spain. Risk factors for vertical transmission. PLoS ONE 2020, 15, e0233528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ndzie Ondigui, J.L.; Mafopa Goumkwa, N.; Lobe, C.; Wandji, B.; Awoumou, P.; Voussou Djivida, P.; Peyonga, P.; Manju Atah, S.; Verbe, V.; Kamgaing Simo, R.; et al. Prevalence and risk factors of transmission of hepatitis delta virus in pregnant women in the Center Region of Cameroon. PLoS ONE 2024, 19, e0287491. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Bendary, M.; Neamatallah, M.; Elalfy, H.; Besheer, T.; Kamel, E.; Mousa, H.; Eladl, A.H.; El-Setouhy, M.; El-Gilany, A.H.; El-Waseef, A.; et al. HLA Class II-DRB1 Alleles with Hepatitis C Virus Infection Outcome in Egypt: A Multicentre Family-based Study. Ann. Hepatol. 2019, 18, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Wasuwanich, P.; So, J.M.; Presnell, B.; Karnsakul, W.; Egerman, R.S.; Wen, T.S. A Composite Score for Predicting Vertical Transmission of Hepatitis C: A Multicenter Study. Pathogens 2024, 13, 45. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Global Hepatitis Report 2024: Action for Access in Low- and Middle-Income Countries; World Health Organization: Geneva, Switzerland, 2024; Available online: https://www.who.int/publications/i/item/9789240091672 (accessed on 2 April 2025).
- Belopolskaya, M.; Avrutin, V.; Kalinina, O.; Dmitriev, A.; Gusev, D. Chronic hepatitis B in pregnant women: Current trends and approaches. World J. Gastroenterol. 2021, 27, 3279–3289. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunkelberg, J.C.; Berkley, E.M.; Thiel, K.W.; Leslie, K.K. Hepatitis B and C in pregnancy: A review and recommendations for care. J. Perinatol. 2014, 34, 882–891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Belete, D.; Fekadie, E.; Kassaw, M.; Fenta, M.; Jegnie, A.; Mulu, T.; Adane, G.; Abebe, W.; Amare, A. Seroprevalence of hepatitis B virus and hepatitis C virus infection among pregnant women attending antenatal care at Guhala Primary Hospital, Northwestern Ethiopia. BMC Pregnancy Childbirth 2024, 24, 512. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, H.; Cao, W.; Zhang, L.; Yang, L.; Bi, X.; Lin, Y.; Deng, W.; Jiang, T.; Sun, F.; Zeng, Z.; et al. Effects of hepatitis B virus infection and strategies for preventing mother-to-child transmission on maternal and fetal T-cell immunity. Front. Immunol. 2023, 14, 1122048. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.N.; Kuo, C.F.; Ou, J.J. Mechanisms of Hepatitis B Virus Persistence. Trends Microbiol. 2018, 26, 33–42. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Păcurar, D.; Dinulescu, A.; Jugulete, G.; Păsărică, A.-S.; Dijmărescu, I. Hepatitis B in Pediatric Population: Observational Retrospective Study in Romania. Life 2024, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Schillie, S.; Vellozzi, C.; Reingold, A.; Harris, A.; Haber, P.; Ward, J.W.; Nelson, N.P. Prevention of Hepatitis B Virus Infection in the United States: Recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2018, 67, 1–31. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Niţă, A.F.; Păcurar, D. Adequacy of scoring systems in diagnosing paediatric autoimmune hepatitis: Retrospective study using a control group children with Hepatitis B infection. Acta Paediatr. 2019, 108, 1717–1724. [Google Scholar] [CrossRef]
- Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241565455 (accessed on 5 April 2025).
- Wu, J.; Wang, H.; Xiang, Z.; Jiang, C.; Xu, Y.; Zhai, G.; Ling, Z.; Chinese Consortium for the Study of Hepatitis E (CCSHE). Role of viral hepatitis in pregnancy its triggering mechanism. J. Transl. Int. Med. 2024, 12, 344–354. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fujiko, M.; Chalid, M.T.; Turyadi Ie, S.I.; Maghfira Syafri Wahyuni, R.; Roni, M.; Patellongi, I.; Massi, M.N.; Muljono, D.H. Chronic hepatitis B in pregnant women: Is hepatitis B surface antigen quantification useful for viral load prediction? Int. J. Infect. Dis. 2015, 41, 83–89. [Google Scholar] [CrossRef] [PubMed]
- di Filippo Villa, D.; Navas, M.C. Vertical Transmission of Hepatitis B Virus-An Update. Microorganisms 2023, 11, 1140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sintusek, P.; Wanlapakorn, N.; Poovorawan, Y. Strategies to Prevent Mother-to-child Transmission of Hepatitis B Virus. J. Clin. Transl. Hepatol. 2023, 11, 967–974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jaramillo, C.M.; De La Hoz, F.; Porras, A.; Di Filippo, D.; Choconta-Piraquive, L.A.; Payares, E.; Montes, N.; Navas, M.C. Characterization of Hepatitis B Virus in Amerindian Children and Mothers from Amazonas State, Colombia. PLoS ONE 2017, 12, e0181643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Chen, T.Y.; Zhao, Y.R. Vertical transmission of hepatitis B virus: Propositions and future directions. Chin. Med. J. 2021, 134, 2825–2831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Veronese, P.; Dodi, I.; Esposito, S.; Indolfi, G. Prevention of vertical transmission of hepa titis B virus infection. World J. Gastroenterol. 2021, 27, 4182–4193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tovo, P.A.; Calitri, C.; Scolfaro, C.; Gabiano, C.; Garazzino, S. Vertically acquired hepatitis C virus infection: Correlates of transmission and disease progression. World J. Gastroenterol. 2016, 22, 1382–1392. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Shabrawi, M.H.F.; Kamal, N.M.; Mogahed, E.A.; Elhusseini, M.A.; Aljabri, M.F. Perinatal transmission of hepatitis C virus: An update. Arch. Med. Sci. 2019, 16, 1360–1369. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Le Campion, A.; Larouche, A.; Fauteux-Daniel, S.; Soudeyns, H. Pathogenesis of hepatitis C during pregnancy and childhood. Viruses 2012, 4, 3531–3550. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dustin, L.B.; Bartolini, B.; Capobianchi, M.R.; Pistello, M. Hepatitis C virus: Life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin. Microbiol. Infect. 2016, 22, 826–832. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Z.; Cui, F.; Ding, Y.; Gao, Y.; Han, G.; Jia, J.; Li, J.; Li, Z.; Liu, Y.; et al. Management Algorithm for Prevention of Mother-to-child Transmission of Hepatitis B Virus (2022). J. Clin. Transl. Hepatol. 2022, 10, 1004–1010. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jhaveri, R.; Hashem, M.; El-Kamary, S.S.; Saleh, D.A.; Sharaf, S.A.; El-Mougy, F.; Abdelsalam, L.; Ehab, M.; El-Ghazaly, H. Hepatitis C Virus (HCV) Vertical Transmission in 12-Month-Old Infants Born to HCV-Infected Women and Assessment of Maternal Risk Factors. Open Forum Infect. Dis. 2015, 2, ofv089. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, L.Y.; Lee, G.H.; Mattar, C.; Saw, S.; Aw, M. Maternal HBeAg positivity and viremia associated with umbilical cord blood hepatitis B viremia. Pediatr. Neonatol. 2019, 60, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Mavilia, M.G.; Wu, G.Y. Mechanisms and Prevention of Vertical Transmission in Chronic Viral Hepatitis. J. Clin. Transl. Hepatol. 2017, 5, 119–129. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, H.; Sushmitha, S.; Kumar, R. Management and Outcomes of Chronic Hepatitis B in Pregnancy: A Retrospective Study from a Tertiary Center in Singapore. Cureus 2025, 17, e88384. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walker, T.Y.; Smith, E.A.; Fenlon, N.; Lazaroff, J.E.; Dusek, C.; Fineis, P.; Crowley, S.A.; Benson, R.; Veselsky, S.L.; Murphy, T.V. Characteristics of Pregnant Women with Hepatitis B Virus Infection in 5 US Public Health Jurisdictions, 2008–2012. Public Health Rep. 2016, 131, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Matthews, P.C.; Ocama, P.; Wang, S.; El-Sayed, M.; Turkova, A.; Ford, D.; Torimiro, J.; Garcia Ferreira, A.C.; Espinosa Miranda, A.; De La Hoz Restrepo, F.P.; et al. Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus. JHEP Rep. 2023, 5, 100777. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, Y.; Yu, H. Prevention strategies of mother-to-child transmission of hepatitis B virus (HBV) infection. Pediatr. Investig. 2020, 4, 133–137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yi, P.; Chen, R.; Huang, Y.; Zhou, R.R.; Fan, X.G. Management of mother-to-child transmission of hepatitis B virus: Propositions and challenges. J. Clin. Virol. 2016, 77, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Sayre, W.; Thompson, P. Prevention of Vertical Transmission of Hepatitis B Within a North Carolina Hospital System. Clin. Ther. 2021, 43, 1786–1791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aslam, A.; Campoverde Reyes, K.J.; Malladi, V.R.; Ishtiaq, R.; Lau, D.T.Y. Management of chronic hepatitis B during pregnancy. Gastroenterol. Rep. 2018, 6, 257–262. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maraolo, A.E.; Gentile, I.; Buonomo, A.R.; Pinchera, B.; Borgia, G. Current evidence on the management of hepatitis B in pregnancy. World J. Hepatol. 2018, 10, 585–594. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pan, C.Q.; Zhu, B.S.; Xu, J.P.; Li, J.X.; Sun, L.J.; Tian, H.X.; Zhang, X.H.; Li, S.W.; Dai, E.H. Pregnancy and fetal outcomes of chronic hepatitis C mothers with viremia in China. World J. Gastroenterol. 2022, 28, 5023–5035. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Post, J.J. Update on hepatitis C and implications for pregnancy. Obstet. Med. 2017, 10, 157–160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dieye, N.L.; Varol, M.; Zorich, S.C.; Millen, A.E.; Yu, K.O.A.; Gómez-Duarte, O.G. Retrospective analysis of vertical Hepatitis C exposure and infection in children in Western New York. BMC Gastroenterol. 2023, 23, 242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Epstein, R.L.; Sabharwal, V.; Wachman, E.M.; Saia, K.A.; Vellozzi, C.; Hariri, S.; Linas, B.P. Perinatal Transmission of Hepatitis C Virus: Defining the Cascade of Care. J. Pediatr. 2018, 203, 34.e1–40.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aebi-Popp, K.; Duppenthaler, A.; Rauch, A.; De Gottardi, A.; Kahlert, C. Vertical transmission of hepatitis C: Towards universal antenatal screening in the era of new direct acting antivirals (DAAs)? Short review and analysis of the situation in Switzerland. J. Virus Erad. 2016, 2, 52–54. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ades, A.E.; Gordon, F.; Scott, K.; Collins, I.J.; Claire, T.; Pembrey, L.; Chappell, E.; Mariné-Barjoan, E.; Butler, K.; Indolfi, G.; et al. Overall Vertical Transmission of Hepatitis C Virus, Transmission Net of Clearance, and Timing of Transmission. Clin. Infect. Dis. 2023, 76, 905–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tziomalos, K.; Neokosmidis, G.; Mavromatidis, G.; Dinas, K. Novel insights in the prevention of perinatal transmission of hepatitis B. World J. Hepatol. 2018, 10, 795–798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- McCluskey, J.M.; Sato, A.I. Vertical Transplacental Infections. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Vyas, A.K.; Negi, P.; Patra, S.; Maras, J.S.; Ramakrishna, G.; Sarin, S.K.; Trehanpati, N. Maternal Immunity Influences Vertical Transmission of Hepatitis B to Newborns. Hepatol. Commun. 2019, 3, 795–811. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, C.P.; Zeng, Y.L.; Zhou, M.; Chen, L.L.; Hu, R.; Wang, L.; Tang, H. Factors associated with mother-to-child transmission of hepatitis B virus despite immunoprophylaxis. Intern. Med. 2015, 54, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Liu, H.H.; Zhong, Y.W.; Liu, C.; Wang, Y.; Jia, L.L.; Qiao, F.; Li, X.X.; Zhang, C.F.; Li, S.L.; et al. Peripheral Blood Mononuclear Cell Traffic Plays a Crucial Role in Mother-to-Infant Transmission of Hepatitis B Virus. Int. J. Biol. Sci. 2015, 11, 266. [Google Scholar] [CrossRef]
- Eke, A.C.; Eleje, G.U.; Eke, U.A.; Xia, Y.; Liu, J. Hepatitis B immunoglobulin during pregnancy for prevention of mother-to-child transmission of hepatitis B virus. Cochrane Database Syst. Rev. 2017, 2, CD008545. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hu, X.; Yang, Y.; Feng, G.; Zhou, X.; Tang, M.; Yan, H.; Li, M.; Liu, A.; Zhu, Y. Hepatitis B virus in oocytes and embryos: Pregnancy outcomes and children’s health. F S Rep. 2024, 5, 272–278. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, L.; Nie, R.; Li, Y.; Xiao, N.; Zhu, L.; Zhu, G. Hepatitis B surface antigen in oocytes and embryos may not result in vertical transmission to offspring of hepatitis B virus carriers. Fertil. Steril. 2016, 105, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Benova, L.; Awad, S.F.; Miller, F.D.; Abu-Raddad, L.J. Estimation of hepatitis C virus infections resulting from vertical transmission in Egypt. Hepatology 2015, 61, 834–842. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheng, Q.; Ding, Y.; Li, B.; Han, C.; Li, Y.; Zhang, C.; Bai, H.; Wang, J.; Zhao, L.; Xia, T.; et al. Efficacy and safety of nucleos(t)ide analogues to prevent hepatitis B virus mother-to-child transmission in pregnant women with high viremia: Real life practice from China. Int. J. Med. Sci. 2018, 15, 796–801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, X.; Chen, J.; Wen, J.; Xu, C.; Zhang, S.; Zhou, Y.H.; Hu, Y. Breastfeeding is not a risk factor for mother-to-child transmission of hepatitis B virus. PLoS ONE 2013, 8, e55303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Funk, A.L.; Lu, Y.; Yoshida, K.; Zhao, T.; Boucheron, P.; van Holten, J.; Chou, R.; Bulterys, M.; Shimakawa, Y. Efficacy and safety of antiviral prophylaxis during pregnancy to prevent mother-to-child transmission of hepatitis B virus: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Prevention of Mother-to-Child Transmission of Hepatitis B Virus: Guidelines on Antiviral Prophylaxis in Pregnancy; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/978-92-4-000270-8 (accessed on 5 April 2025).
- Giugliano, S.; Petroff, M.G.; Warren, B.D.; Jasti, S.; Linscheid, C.; Ward, A.; Kramer, A.; Dobrinskikh, E.; Sheiko, M.A.; Gale, M., Jr.; et al. Hepatitis C Virus Sensing by Human Trophoblasts Induces Innate Immune Responses and Recruitment of Maternal NK Cells: Potential Implications for Limiting Vertical Transmission. J. Immunol. 2015, 195, 3737–3747, Erratum in J. Immunol. 2017, 198, 972. https://doi.org/10.4049/jimmunol.1601926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tsai, K.N.; Ou, J.J. Hepatitis B virus e antigen and viral persistence. Curr. Opin. Virol. 2021, 51, 158–163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ansari, A.; Vincent, J.P.; Moorhouse, L.; Shimakawa, Y.; Nayagam, S. Risk of early horizontal transmission of hepatitis B virus in children of uninfected mothers in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e715–e728. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Cui, F.; Ding, Y.; Dou, X.; Duan, Z.; Han, G.; Jia, J.; Mao, Q.; Li, J.; Li, Z.; et al. Management Algorithm for Interrupting Mother-to-Child Transmission of Hepatitis B Virus. Clin. Gastroenterol. Hepatol. 2019, 17, 1929.e1–1936.e1. [Google Scholar] [CrossRef] [PubMed]
- Machaira, M.; Papaevangelou, V.; Vouloumanou, E.K.; Tansarli, G.S.; Falagas, M.E. Hepatitis B Vaccine Alone or with Hepatitis B Immunoglobulin in Neonates of HBsAg+/HBeAg− Mothers: A Systematic Review and Meta-Analysis. J. Antimicrob. Chemother. 2015, 70, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Stevens, C.E.; Toy, P.; Kamili, S.; Taylor, P.E.; Tong, M.J.; Xia, G.L.; Vyas, G.N. Eradicating hepatitis B virus: The critical role of preventing perinatal transmission. Biologicals 2017, 50, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.S.; Gao, S.; Castillo, E.; Coffin, C.S. Presence of Precore (C)/C Promoter Mutants in Peripheral Blood Mononuclear Cells of Chronic Hepatitis B (CHB) Carriers During Pregnancy Does Not Correlate with Increased Risk of Liver Disease in 4 Years of Follow-Up. Dig. Dis. Sci. 2020, 65, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Zhang, P.; Tan, Z.; Zhou, J.; Wu, L.; Hou, H. The Association of Pre-S/S Gene Mutations and Hepatitis B Virus Vertical Transmission. Hepat. Mon. 2016, 16, 32160. [Google Scholar] [CrossRef]
- He, R.; Wen, P.; Xiong, M.; Fan, Z.; Li, F.; Luo, D.; Xie, X. Cesarean section in reducing mother-to-child HBV transmission: A meta-analysis. J. Matern. Fetal Neonatal Med. 2022, 35, 3424–3432. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Cai, J.Y.; Song, Y.P.; Zha, M.L.; Qin, G. Vaginal delivery and HBV mother to child transmission risk after immunoprophylaxis: A systematic review and a meta-analysis. Midwifery 2019, 74, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Qin, Q.; Fang, Q.; Jiang, L.; Nie, S. Cesarean section to prevent mother-to-child transmission of hepatitis B virus in China: A meta-analysis. BMC Pregnancy Childbirth 2017, 17, 303. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chasela, C.S.; Kourtis, A.P.; Wall, P.; Drobeniuc, J.; King, C.C.; Thai, H.; Teshale, E.H.; Hosseinipour, M.; Ellington, S.; Codd, M.B.; et al. Hepatitis B virus infection among HIV-infected pregnant women in Malawi and transmission to infants. J. Hepatol. 2014, 60, 508–514. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sookoian, S. Liver disease during pregnancy: Acute viral hepatitis. Ann. Hepatol. 2006, 5, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Stasi, C.; Milli, C.; Voller, F.; Silvestri, C. The Epidemiology of Chronic Hepatitis C: Where We Are Now. Livers 2024, 4, 172–181. [Google Scholar] [CrossRef]
- Inui, A.; Fujisawa, T.; Sogo, T.; Komatsu, H.; Isozaki, A.; Sekine, I. Different outcomes of vertical transmission of hepatitis C virus in a twin pregnancy. J. Gastroenterol. Hepatol. 2002, 17, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.; Morgan, C.E.; Ngimbi, P.; Mwandagalirwa, K.; Ravelomanana, N.L.R.; Tabala, M.; Fathy, M.; Kawende, B.; Muwonga, J.; Misingi, P.; et al. Arresting vertical transmission of hepatitis B virus (AVERT-HBV) in pregnant women and their neonates in the Democratic Republic of the Congo: A feasibility study. Lancet Glob. Health. 2021, 9, e1600–e1609, Erratum in Lancet Glob. Health 2021, 9, e1507. https://doi.org/10.1016/S2214-109X(21)00468-X. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- World Health Organization (WHO). Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 2 December 2022).
- Guidelines on Hepatitis B and C Testing; World Health Organization: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/10665/254621/1/9789241549981-eng.pdf?ua=1 (accessed on 2 April 2020).
- Perinatal Hepatitis B Prevention Program Manual. Available online: https://doh.wa.gov/sites/default/files/legacy/Documents/Pubs//348-165_PerinatalHepatitisBPreventionProgramGuidelines.pdf#:~:text=Post%2DVaccination%20Serology%20Testing%20PVST%20helps%20identify%20infants,vaccine%20series%20if%20the%20series%20is%20delayed) (accessed on 11 April 2025).
- Management of Infants Born to Women with Hepatitis B Virus Infection for Pediatricians. Available online: https://www.cdc.gov/vaccines/programs/perinatal-hepb/downloads/HepB-Provider-tipsheet-508.pdf (accessed on 2 September 2025).
- Tamandjou Tchuem, C.R.; Andersson, M.I.; Wiysonge, C.S.; Mufenda, J.; Preiser, W.; Cleary, S. Prevention of hepatitis B mother-to-child transmission in Namibia: A cost- effectiveness analysis. Vaccine 2021, 39, 3141–3151. [Google Scholar] [CrossRef] [PubMed]
- Dionne-Odom, J.; Njei, B.; Tita, A.T.N. Elimination of Vertical Transmission of Hepatitis B in Africa: A Review of Available Tools and New Opportunities. Clin. Ther. 2018, 40, 1255–1267. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Breakwell, L.; Tevi-Benissan, C.; Childs, L.; Mihigo, R.; Tohme, R. The Status of Hepatitis B Control in the African Region. Pan Afr. Med. J. 2017, 27, 17. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, S.; de Villiers, M.J.; Shimakawa, Y.; Lemoine, M.; Thursz, M.R.; Walsh, N.; Hallett, T.B. Impact and cost-effectiveness of hepatitis B virus prophylaxis in pregnancy: A dynamic simulation modelling study. Lancet Gastroenterol. Hepatol. 2023, 8, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Enebe, J.T.; Enebe, N.O.; Onwujekwe, O.E. Willingness to pay for hepatitis B immunoglobulin among pregnant women in Enugu metropolis, South-East, Nigeria: A cross-sectional study. BMC Pregnancy Childbirth 2025, 25, 266. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dionne-Odom, J.; Cozzi, G.D.; Franco, R.A.; Njei, B.; Tita, A.T.N. Treatment and prevention of viral hepatitis in pregnancy. Am. J. Obstet. Gynecol. 2022, 226, 335–346. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bierhoff, M.; Nelson, K.E.; Guo, N.; Jia, Y.; Angkurawaranon, C.; Jittamala, P.; Carrara, V.; Watthanaworawit, W.; Ling, C.; Tongprasert, F.; et al. Prevention of mother-to-child transmission of hepatitis B virus: Protocol for a one-arm, open-label intervention study to estimate the optimal timing of tenofovir in pregnancy. BMJ Open 2020, 10, e038123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, H.T.; Thavorncharoensap, M.; Phung, T.L.; Anothaisintawee, T.; Chaikledkaew, U.; Sobhonslidsuk, A.; Talungchit, P.; Chaiyakunapruk, N.; Attia, J.; McKay, G.J.; et al. Comparative efficacy and safety of pharmacologic interventions to prevent mother-to-child transmission of hepatitis B virus: A systematic review and network meta-analysis. Am. J. Obstet. Gynecol. 2022, 227, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, L.; Hu, J.; Zhai, P.; Ou, X.; He, F.; Pan, C.Q. Tenofovir Alafenamide Therapy Throughout Pregnancy in Mothers with Hepatitis B. Aliment. Pharmacol. Ther. 2025, 62, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.Q. The role of tenofovir disoproxil fumarate for preventing vertical transmission of hepatitis B. Antivir. Ther. 2022, 27, 13596535221076640. [Google Scholar] [CrossRef] [PubMed]
- Delamare, H.; Ishii-Rousseau, J.E.; Rao, A.; Cresta, M.; Vincent, J.P.; Ségéral, O.; Nayagam, S.; Shimakawa, Y. Proportion of pregnant women with HBV infection eligible for antiviral prophylaxis to prevent vertical transmission: A systematic review and meta-analysis. JHEP Rep. 2024, 6, 101064. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garcia, D.; Porras, A.; Rico Mendoza, A.; Alvis, N.; Navas, M.C.; De La Hoz, F.; De Neira, M.; Osorio, E.; Valderrama, J.F. Hepatitis B Infection Control in Colombian Amazon after 15 Years of Hepatitis B Vaccination. Effectiveness of Birth Dose and Current Prevalence. Vaccine 2018, 36, 2721–2726. [Google Scholar] [CrossRef]
- Guidance on the Hepatitis B Antenatal Screening and Selective Neonatal Immunization Pathway. Updated 13 June 2025. Available online: https://www.gov.uk/government/publications/hepatitis-b-antenatal-screening-and-selective-neonatal-immunisation-pathway/guidance-on-the-hepatitis-b-antenatal-screening-and-selective-neonatal-immunisation-pathway--2#:~:text=The%20DBS%20sample%20will%20be%20tested%20for,test%20for%20antibody%20response%20to%20vaccination%20(anti%2DHBs) (accessed on 2 September 2025).
- Meng, X.; Zhang, W.; Yan, B.; Feng, Y.; Lu, J.; Zhang, L. Progress toward elimination mother-to-child transmission of HBV in Shandong Province, China: Coverage trend in hepatitis B vaccination and HBIG administration, 2017–2024. Hum. Vaccines Immunother. 2025, 21, 2554029. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ECDC Surveillance Report Hepatitis B Annual Epidemiological Report for 2022. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/AER%20HEPB%202022_0.pdf (accessed on 2 September 2025).
- Panagiotakopoulos, L.; Sandul, A.L.; Conners, E.E.; Foster, M.A.; Nelson, N.P.; Wester, C. CDC Recommendations for Hepatitis C Testing Among Perinatally Exposed Infants and Children—United States, 2023. Recomm. Rep. 2023, 72, 1–19. [Google Scholar] [CrossRef]
- Thilakanathan, C.; Wark, G.; Maley, M.; Davison, S.; Lawler, J.; Lee, A.; Shackel, N.; Nguyen, V.; Jackson, K.; Glass, A.; et al. Mother to Child Transmission of Hepatitis B: Examining Viral Cut Offs, Maternal HBsAg Serology and Infant Testing. Liver Int. 2018, 38, 1212–1219. [Google Scholar] [CrossRef]
- Biondi, M.J.; Flemming, J.; van Gennip, J.; Barnett, T.; Masterman, C.; Mendlowitz, A.; Mooney, M.; Fontaine, G.; Feld, J.J. Hepatitis C virus testing in infants, a move to early screening by HCV RNA at 2 months of age. Paediatr. Child. Health 2025, 30, 373–378. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hachicha-Maalej, N.; Lepers, C.; Collins, I.J.; Mostafa, A.; Ades, A.E.; Judd, A.; Scott, K.; Gibb, D.M.; Pett, S.; Indolfi, G.; et al. Modelling the potential clinical and economic impact of universal antenatal hepatitis C (HCV) screening and providing treatment for pregnant women with HCV and their infants in Egypt: A cost-effectiveness study. BMJ Public Health 2024, 2, e000517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Z.; Li, M.; Hutton, D.W.; Wagner, A.L.; Yao, Y.; Zhu, W.; Cao, L.; Tang, S.; Pan, J.; Wang, Y.; et al. Impact of the national hepatitis B immunization program in China: A modeling study. Infect. Dis. Poverty 2022, 11, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Liang, W.; Jing, W.; Liu, M. Countdown to 2030: Eliminating hepatitis B disease, China. Bull. World Health Organ. 2019, 97, 230–238. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yan, B.; Zhang, X.; Lv, J.; Feng, Y.; Meng, X.; Lin, X.; Zhang, Y.; Wang, S.; Ji, F.; Chen, M.; et al. Seroprevalence of hepatitis B among the general population in Shandong Province, Eastern China, an update 30 years after the implementation of the neonatal vaccination program. BMC Infect. Dis. 2024, 24, 1433. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ni, Y.H.; Chang, M.H.; Jan, C.F.; Hsu, H.Y.; Chen, H.L.; Wu, J.F.; Chen, D.S. Continuing Decrease in Hepatitis B Virus Infection 30 Years After Initiation of Infant Vaccination Program in Taiwan. Clin. Gastroenterol. Hepatol. 2016, 14, 1324–1330. [Google Scholar] [CrossRef] [PubMed]





| Serologic and Molecular Markers | Significance | Vertical Transmission Risk |
|---|---|---|
| HBsAg+ | First infection marker Positive after 6 months means chronic infection | Very high |
| Anti-HBs antibodies | After vaccination or clinical resolution of acute infection | Low |
| HBeAg+ | Elevated viral replication Related to immune tolerance phase | Very high |
| Anti-HBe antibodies | Indicates resolution of infection if it is associated with anti-HBs+ | Low |
| IgM Anti-HBc antibodies | Indicates acute or recent infection Can reappear during chronic infection reactivation (pregnancy) | Very high |
| HBV-DNA | Indicates viral replication | Depends on the viral load |
| Infection Markers | Management Approach |
|---|---|
| HBV-DNA+ ALT ≥ 5 × ULN | Requires further examination (abdominal ultrasound, fibrosis score) and antiviral therapy as primary recommendation |
| HBV-DNA+ ALT ≥ 1 and ≤ 5 × ULN and Total bilirubin ≤ 2 × ULN | “Wait and watch” approach until 24 weeks of gestation If levels remain the same, antiviral therapy should be administered |
| HBV-DNA+ ALT ≥ 5 × ULN or Total bilirubin ≥ 2 × ULN | Requires further examination (abdominal ultrasound, fibrosis score) and antiviral therapy as primary recommendation |
| HBV-DNA+ Normal levels for ALT No manifestations of liver cirrhosis | “Wait and watch” approach until 24 weeks of gestation If ALT ≥ 1× ULN at follow-up, further testing required and, according to ALT value, consider antiviral therapy |
| Undetectable levels | Test again for HBV-DNA levels at 24 weeks of gestation |
| HBV-DNA Level | TDF Therapy | Alternative Drugs |
|---|---|---|
| >2 × 105 UI/mL | Start at 28 weeks of gestation | TAF or telbivudine if mother has osteoporosis, kidney damage, severe GI symptoms |
| <2 × 105 UI/mL | NOT recommended (perform standard active and passive immunization of newborn) | NOT recommended |
| ≥2 × 105 UI/mL First follow-up after 28 weeks of gestation | Immediate initiation of TDF | TAF or telbivudine if mother has osteoporosis, kidney damage, severe GI symptoms |
| Societies | Time for Treatment Cessation |
|---|---|
| AASLD | At birth to 3 months |
| EASL | Up to 3 months after delivery |
| APASL | At delivery |
| NIHCE | 1 to 3 months after delivery |
| CMA | At delivery |
| Strategy Type | Intervention |
|---|---|
Strategy 1
|
|
Strategy 2
|
|
Strategy 3
|
|
Strategy 4
|
|
| Newborn’s General Status and Mother’s HBsAg Status | General Recommendations |
|---|---|
| Routine vaccination |
|
| Normal newborn HBsAg-positive mother |
|
| Normal newborn Mother’s HBsAg status unknown |
|
| Normal newborn HBsAg-negative mother |
|
| Low-birth-weight newborn (under 2000 g) Preterm newborn (under 37 weeks of gestation) HBsAg-positive mother |
|
| Low-birth-weight newborn (under 2000 g) Preterm newborn (under 37 weeks of gestation) Mother’s HBsAg status unknown |
|
| Low-birth-weight newborn (under 2000 g) Preterm newborn (under 37 weeks of gestation) HBsAg-negative mother |
|
| Very low birth weight (under 1500 g) Severe birth defects Neonatal hypoxia Respiratory distress syndrome HBsAg-positive mother |
|
| Very low birth weight (under 1500 g) Severe birth defects Neonatal hypoxia Respiratory distress syndrome Mother’s HBsAg status unknown |
|
| Very low birth weight (under 1500 g) Severe birth defects Neonatal hypoxia Respiratory distress syndrome HBsAg-negative mother |
|
| Delayed vaccination |
|
| HVC Infection Markers | HVC Infection Status |
|---|---|
| Two positive HCV-RNA samples at least 1 month apart | HVC infection |
| Anti-HVC antibodies at/beyond 18 months of age | HVC infection |
| Two negative HCV-RNA samples at least 1 month apart | Non-infected child |
| Negative anti-HVC antibodies at any age | Non-infected child |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dobritoiu, R.; Pacurar, D.; Vlad, R.M.; Plesca, D.A. Vertical Transmission of Hepatitis B and C—Then and Now—A Comprehensive Literature Systematic Review. Viruses 2025, 17, 1395. https://doi.org/10.3390/v17101395
Dobritoiu R, Pacurar D, Vlad RM, Plesca DA. Vertical Transmission of Hepatitis B and C—Then and Now—A Comprehensive Literature Systematic Review. Viruses. 2025; 17(10):1395. https://doi.org/10.3390/v17101395
Chicago/Turabian StyleDobritoiu, Ruxandra, Daniela Pacurar, Raluca Maria Vlad, and Doina Anca Plesca. 2025. "Vertical Transmission of Hepatitis B and C—Then and Now—A Comprehensive Literature Systematic Review" Viruses 17, no. 10: 1395. https://doi.org/10.3390/v17101395
APA StyleDobritoiu, R., Pacurar, D., Vlad, R. M., & Plesca, D. A. (2025). Vertical Transmission of Hepatitis B and C—Then and Now—A Comprehensive Literature Systematic Review. Viruses, 17(10), 1395. https://doi.org/10.3390/v17101395












