Reinfection Dynamics of Disease-Free Cassava Plants in Three Agroecological Regions of Côte d’Ivoire
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
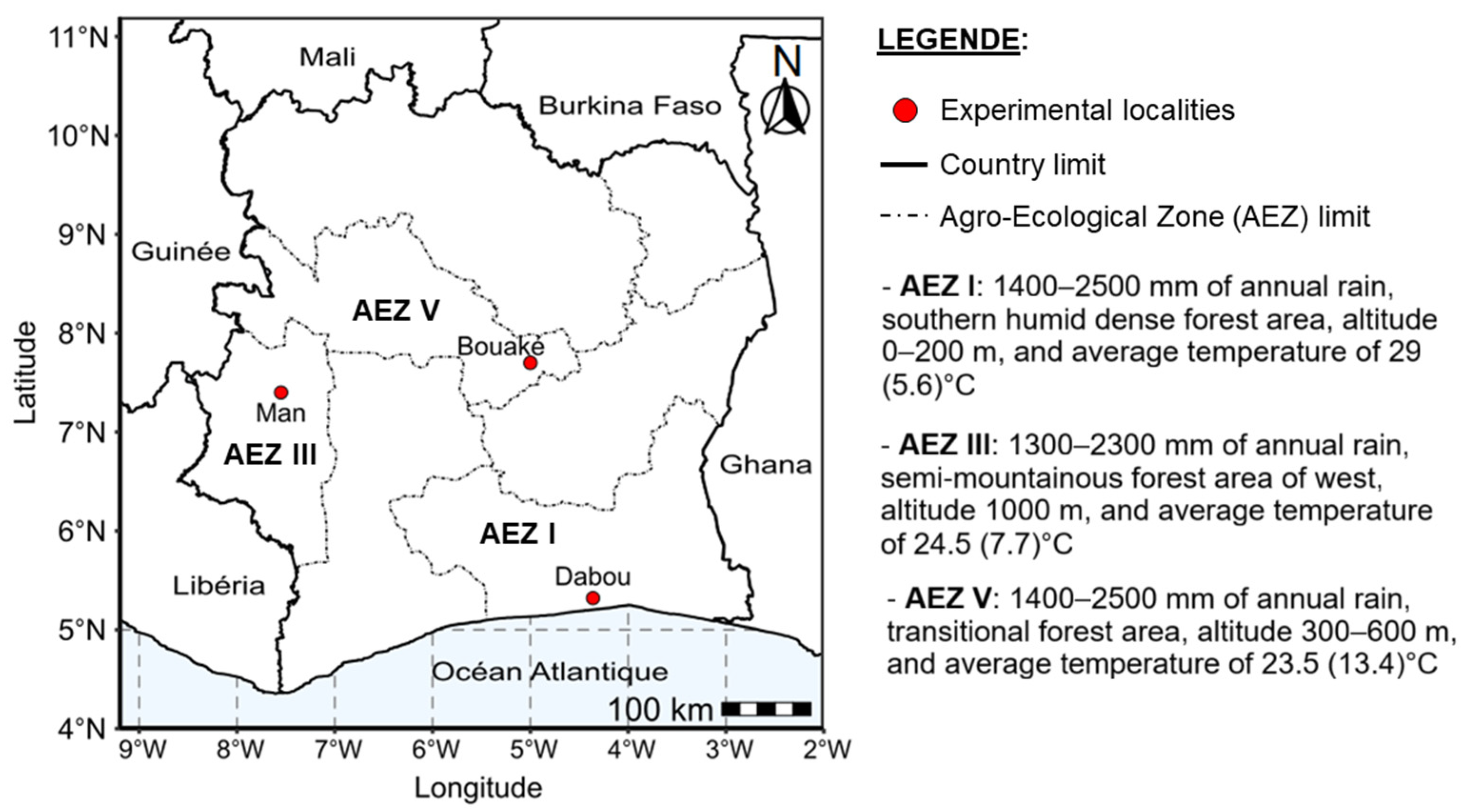
2.2. Characteristics of the Study Sites and Experimental Design
2.3. Assessment of CMD Epidemiological Parameters
2.4. Sample Collection
2.5. Molecular Analysis
2.5.1. Total DNA Extraction and CMB Detection by Polymerase Chain Reaction (PCR)
2.5.2. DNA Extraction and PCR Amplification of the Whiteflies’ Mitochondrial Cytochrome Oxidase I (mtCOI) Gene and CMBs Present in Field-Collected Whiteflies
2.6. Sequencing and Phylogenetic Analysis
2.7. Statistical Analysis
3. Results
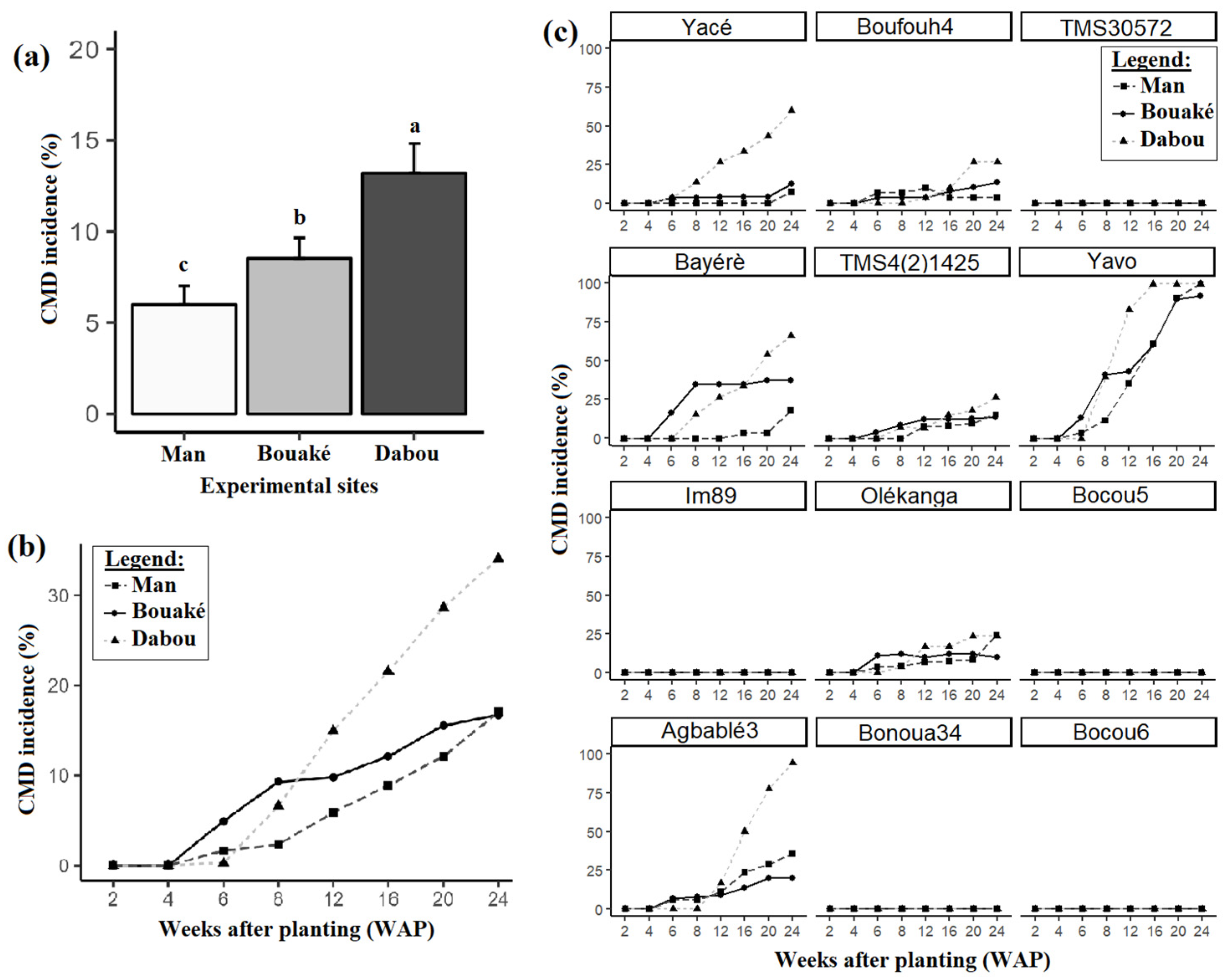
3.1. CMD Incidence
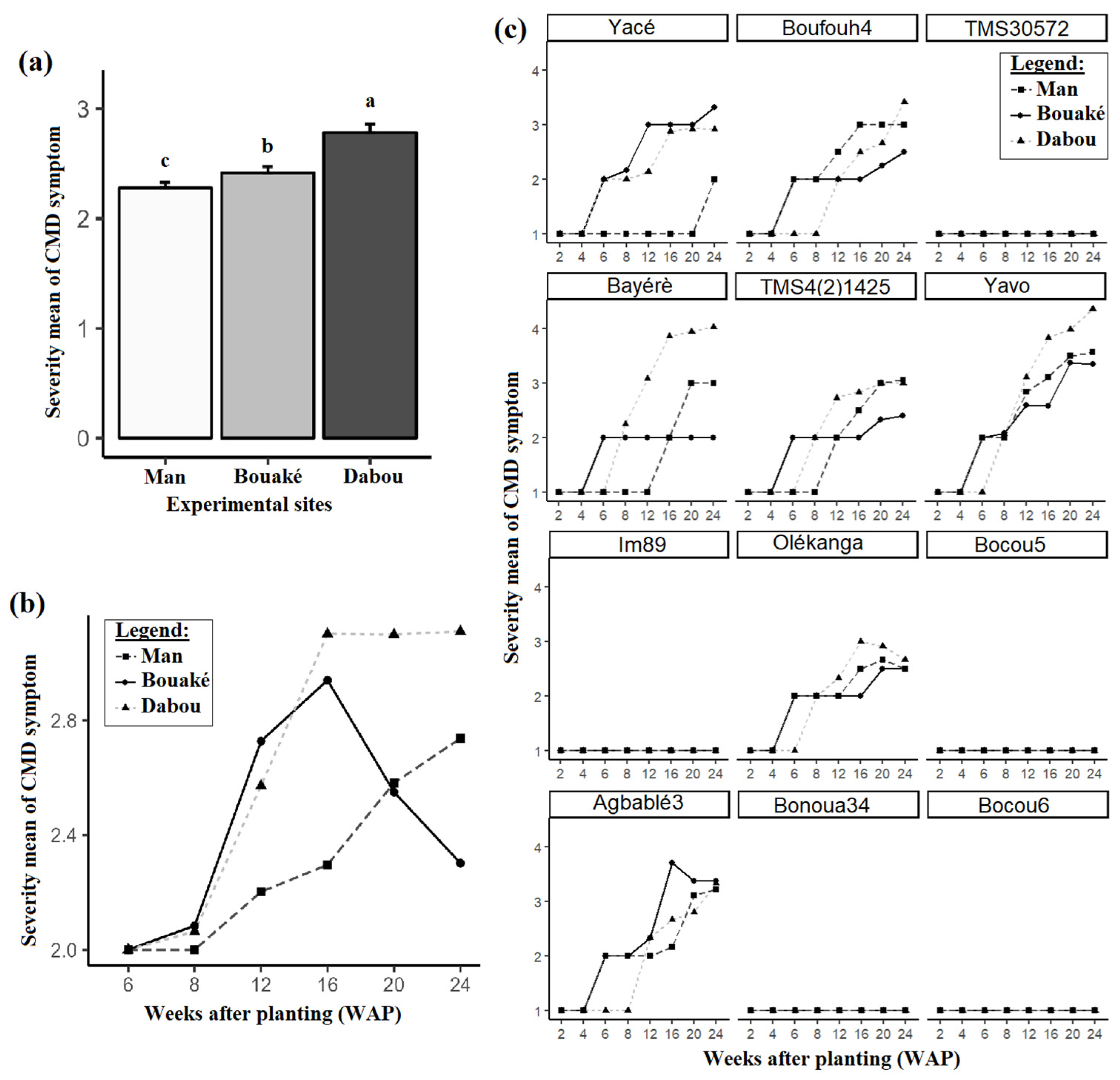
3.2. CMD Symptom Severity
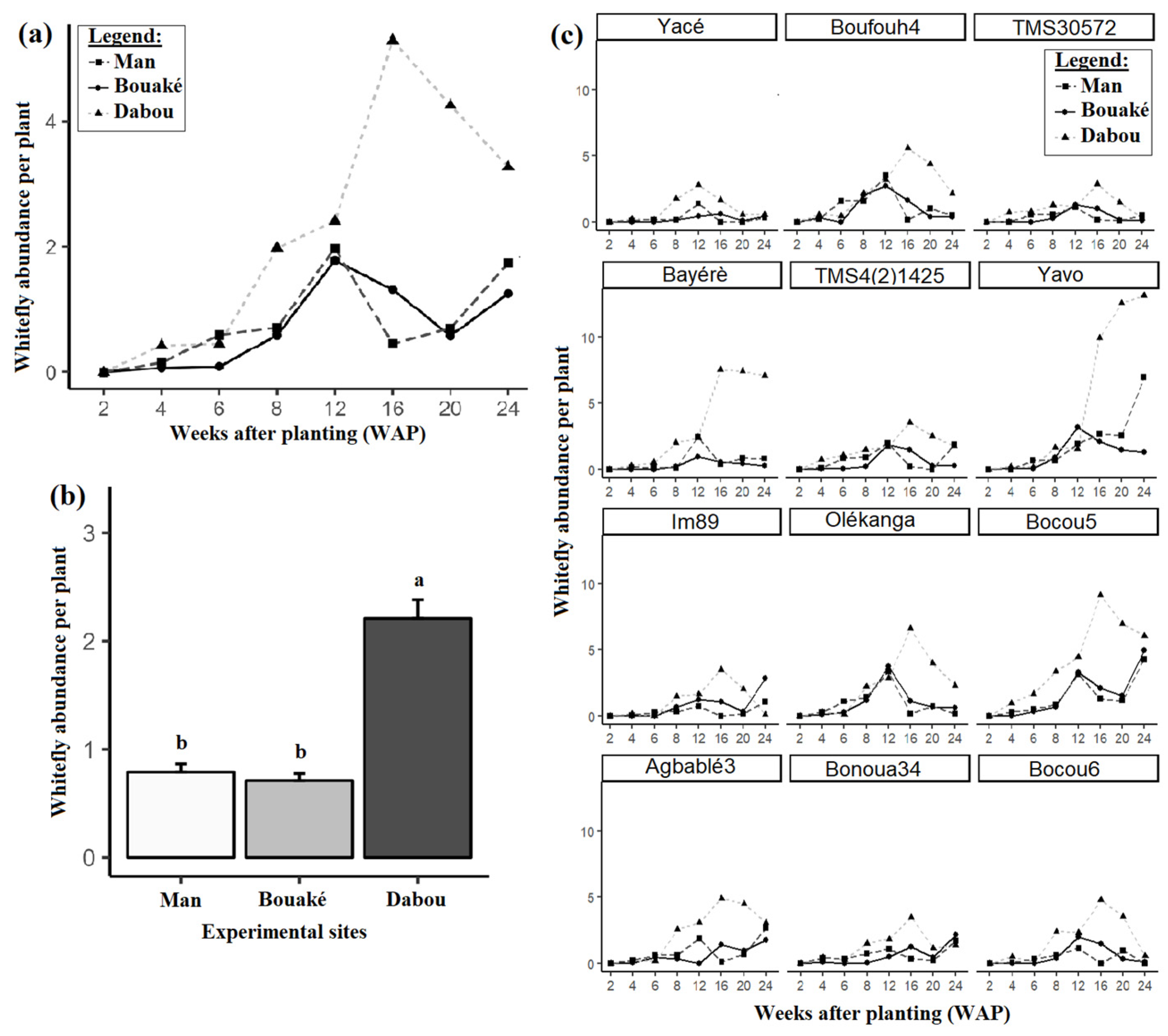
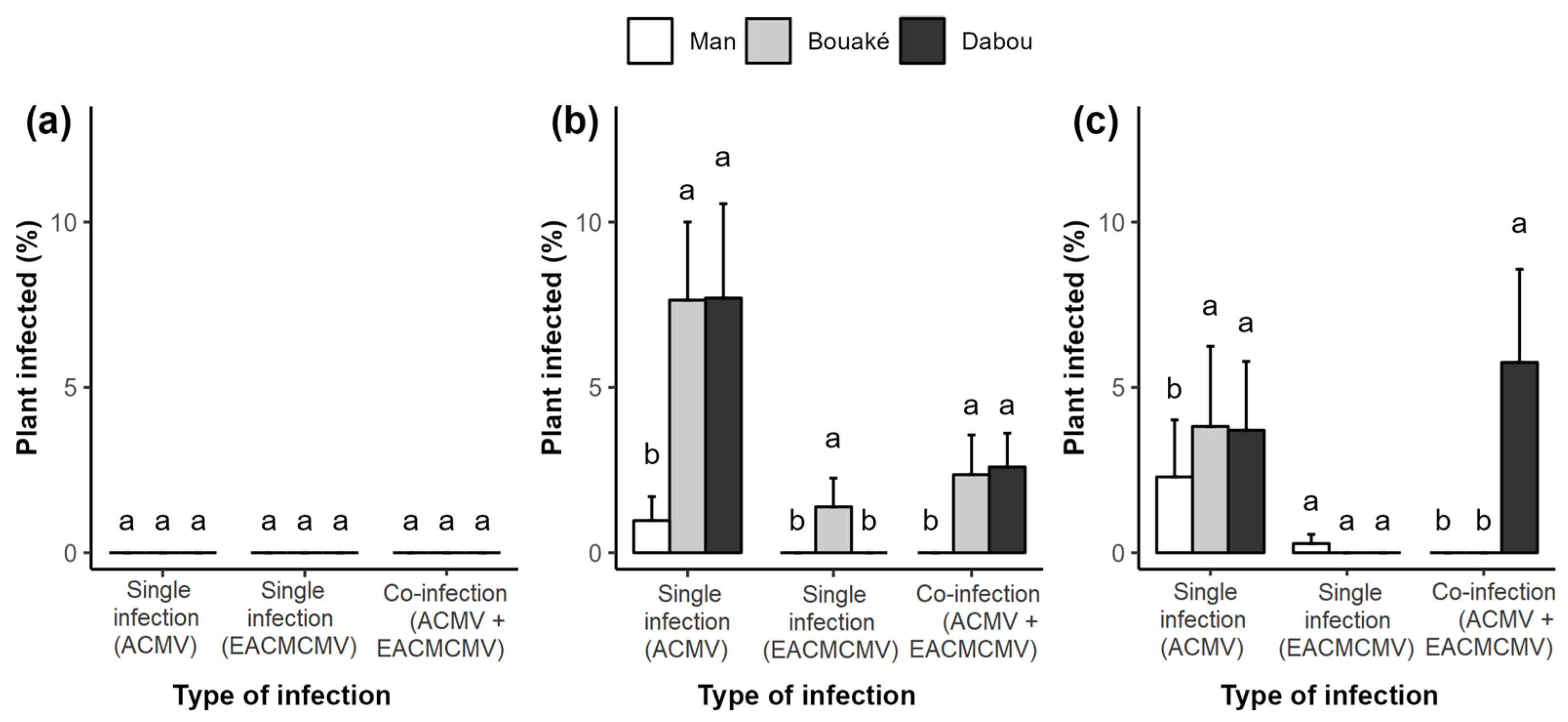
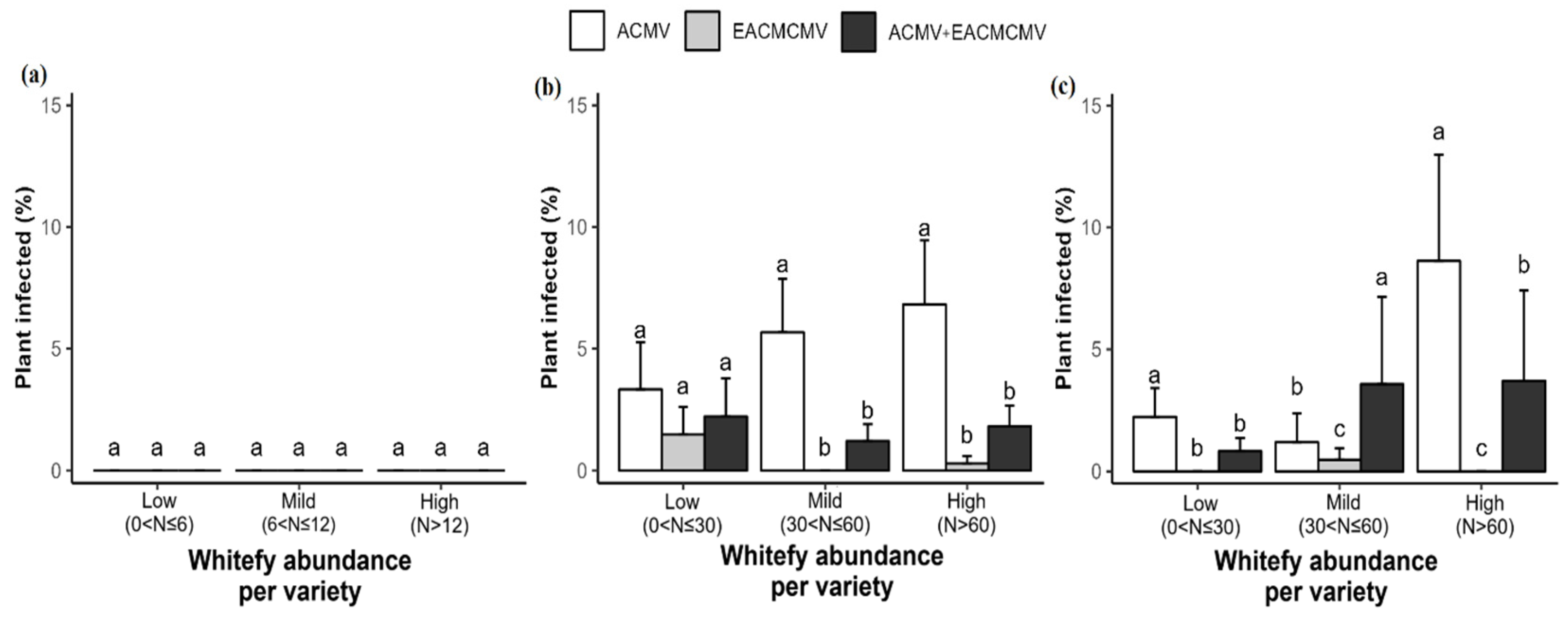
3.3. Mode of Infection, Whitefly Abundance per Plant, and Their Relationship with CMD Incidence and Severity of CMD Symptoms
3.4. Detection of ACMV and EACMCMV from Cassava Leaf Samples
3.5. Presence of ACMV and/or EACMCMV, CMD Symptom Severity, and Plant Phytosanitary Status
3.6. Correlation Between CMB Infection, Study Sites, and Whitefly Abundance
3.7. Type of Infection and Level of CMD Resistance of Different Varieties
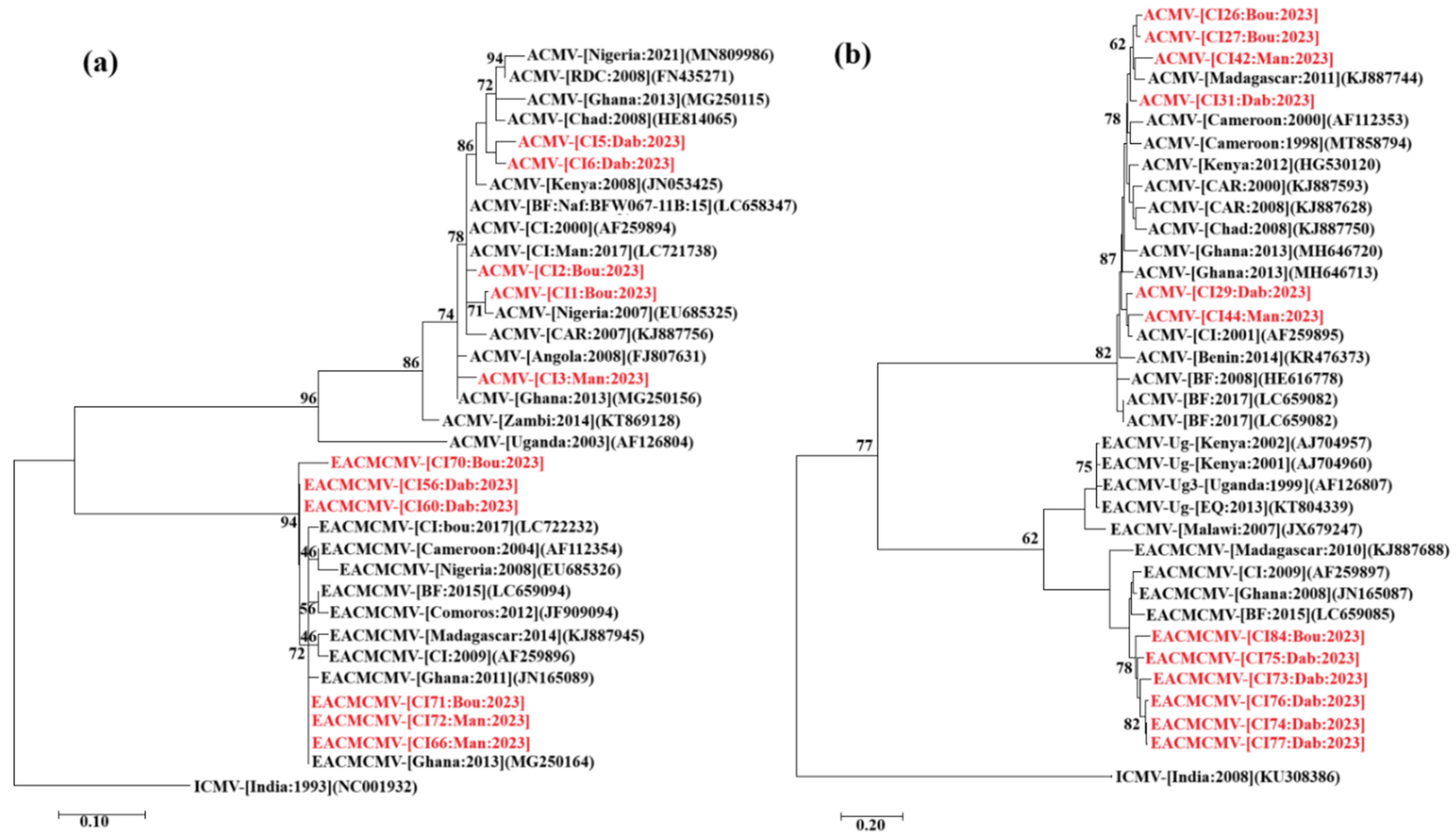
3.8. Phylogeny of the CMBs Detected in the Tested Leaf Samples
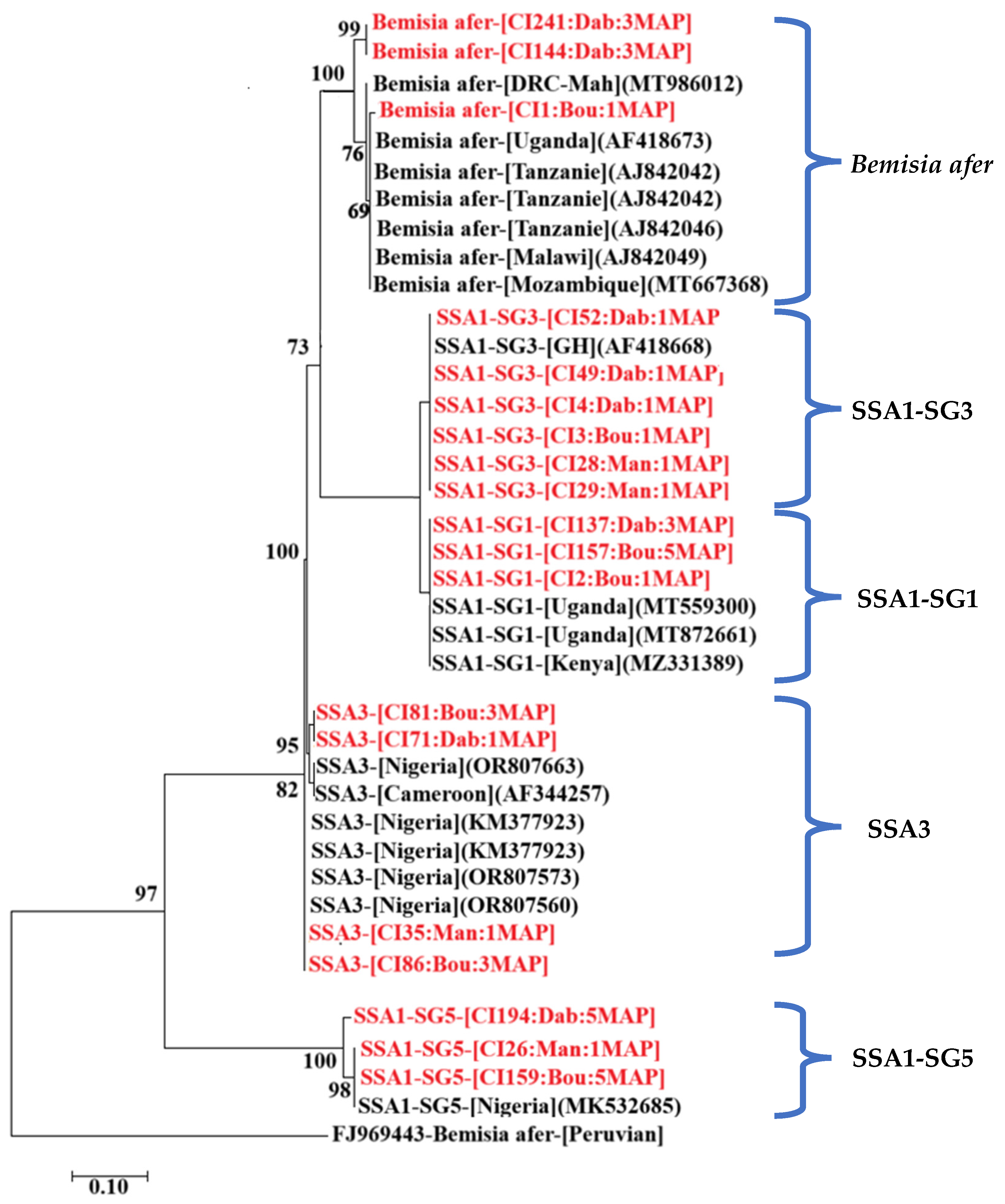
3.9. Identification of Whitefly Species and Characterisation of the CMBs They Carry
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosenthal, D.M.; Ort, D.R. Examining Cassava’s Potential to Enhance Food Security Under Climate Change. Trop. Plant Biol. 2012, 5, 30–38. [Google Scholar] [CrossRef]
- FAOSTAT Food and Agriculture Organization of the United Nations Statistics. Available online: https://www.fao.org/faostat/fr/#data/QCL/visualize (accessed on 15 May 2025).
- Coulibaly, O.; Arinloye, A.D.; Faye, M.; Abdoulaye, T.; Calle-Goulivas, A.; Ahoyo, R. Analyse des Chaines de Valeur Regionales du Manioc en Afrique de l’Ouest; CORAF/WECARD: Dakar, Senegal, 2014. [Google Scholar]
- Kouassi, K.M.; Mahyao, A.; N’zue, B.; Koffi, E.; Koffi, C. Status of Cassava (Manihot esculenta Crantz) in Côte d’Ivoire: From Production to Consumption and Evaluation of Technology Adoption. Eur. Sci. J. ESJ 2018, 14, 285–299. [Google Scholar]
- Vernier, P.; N’Zué, B.; Zakhia-Rozis, N. Le Manioc, Entre Culture Alimentaire et Filière Agro-Industrielle; Editions Quae: Versailles, France, 2018. [Google Scholar]
- Zinga, I.; Chiroleu, F.; Valam Zango, A.; Ballot, C.S.A.; Harimalala, M.; Kosh Komba, E.; Yandia, P.S.; Semballa, S.; Reynaud, B.; Lefeuvre, P.; et al. Evaluation of Cassava Cultivars for Resistance to Cassava Mosaic Disease and Yield Potential in Central African Republic. J. Phytopathol. 2016, 164, 913–923. [Google Scholar] [CrossRef]
- ICTV. Virus Taxonomy: 2024 Release; ICTV: Bari, Italy, 2024; Available online: https://ictv.global/taxonomy/visual-browser (accessed on 19 July 2025).
- Amoakon, W.J.-L.; Yoboué, A.A.N.; Pita, J.S.; Mutuku, J.M.; N’Zué, B.; Combala, M.; Otron, D.H.; Koné, M.; Kouassi, N.K.; Sié, R. Occurrence of Cassava Mosaic Begomoviruses in National Cassava Germplasm Preserved in Two Agro-ecological Zones of Ivory Coast. Plant Pathol. 2023, 72, 1011–1021. [Google Scholar] [CrossRef]
- Kouakou, B.S.M.; Yoboué, A.A.N.; Pita, J.S.; Mutuku, J.M.; Otron, D.H.; Kouassi, N.K.; Kouassi, K.M.; Vanié-Léabo, L.P.L.; Ndougonna, C.; Zouzou, M.; et al. Gradual Emergence of East African Cassava Mosaic Cameroon Virus in Cassava Farms in Côte d’Ivoire. Agronomy 2024, 14, 418. [Google Scholar] [CrossRef]
- Pita, J.S.; Fondong, V.N.; Sangaré, A.; Kokora, R.N.N.; Fauquet, C.M. Genomic and Biological Diversity of the African Cassava Geminiviruses. Euphytica 2001, 120, 115–125. [Google Scholar] [CrossRef]
- Toualy, M.N.; Akinbade, S.; Koutoua, S.; Diallo, H.; Kumar, P. Incidence and Distribution of Cassava Mosaic Begomoviruses in Côte d’Ivoire. Int. J. Agron. Agric. Res. 2014, 4, 131–139. [Google Scholar]
- Dubern, J. Transmission of African Cassava Mosaic Geminivirus by the Whitefly (Bemisia tabaci). Trop. Sci. 1994, 34, 82–91. [Google Scholar]
- Romba, R.; Gnankine, O.; Drabo, S.F.; Tiendrebeogo, F.; Henri, H.; Mouton, L.; Vavre, F. Abundance of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) and Its Parasitoids on Vegetables and Cassava Plants in Burkina Faso (West Africa). Ecol. Evol. 2018, 8, 6091–6103. [Google Scholar] [CrossRef]
- Amoakon, W.J.-L.; Combala, M.; Pita, J.S.; Mutuku, J.M.; N’Zué, B.; Otron, D.H.; Yéo, E.F.; Kouassi, N.K.; Sié, R. Phenotypic Screening and Molecular Characterization of Cassava Mosaic Disease Resistance in Côte d’Ivoire Cassava Germplasm. Front. Sustain. Food Syst. 2023, 6, 1052437. [Google Scholar] [CrossRef]
- Combala, M.; Pita, J.S.; Gbonamou, M.; Samura, A.E.; Amoakon, W.J.-L.; Kouakou, B.S.M.; Onile-ere, O.; Sawadogo, S.; Eboulem, G.R.; Otron, D.H.; et al. An Alarming Eastward Front of Cassava Mosaic Disease in Development in West Africa. Viruses 2024, 16, 1691. [Google Scholar] [CrossRef]
- Djaha, K.E. Caractérisation Morphologique et Micropropagation des Cultivars de Manioc (Manihot esculenta Crantz) Assainis en Côte d’Ivoire. Ph.D. Thesis, Université Nangui Abrogoua, Abidjan, Côte d’Ivoire, 2019. [Google Scholar]
- Silué, O.; Kouadjo, C.G.Z.; Konan, K.J.L.; Konan, A.; Koffi, K.E. Bien Produire In Vitro des Plants de Manioc Indemnes du Virus de la Mosaïque; CNRA: Sacramento, CA, USA, 2022. [Google Scholar]
- Yéo, E.F.; Kouassi, M.K.; Pita, J.S.; Kouassi, N.K.; Koné, D.; N’guetta, S.P.A. Using Thermotherapy And Meristem Tip Culture For Producing Virus-Free Cassava Planting Material From Six Varieties Cultivated In Côte d’Ivoire. Int. J. Sci. Technol. 2020, 9, 1607–1612. [Google Scholar]
- Benke, A.P.; Krishna, R.; Khandagale, K.; Gawande, S.; Shelke, P.; Dukare, S.; Dhumal, S.; Singh, M.; Mahajan, V. Efficient Elimination of Viruses from Garlic Using a Combination of Shoot Meristem Culture, Thermotherapy, and Chemical Treatment. Pathogens 2023, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Akano, A.; Dixon, A.; Mba, C.; Barrera, E.; Fregene, M. Genetic Mapping of a Dominant Gene Conferring Resistance to Cassava Mosaic Disease. Theor. Appl. Genet. 2002, 105, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Okorogri, E.B.; Adetimirin, V.O.; Ssemakula, G.; Odu, B.; Dixon, A.G.O. Rate of Re-Infection of Tissue Culture-Derived Latin American and East and Southern African Cassava Genotypes by Mosaic Disease. Afr. J. Biotechnol. 2010, 9, 8748–8753. [Google Scholar]
- Bairu, M.W.; Amoo, S.O.; Van Staden, J. Comparative Phytochemical Analysis of Wild and in Vitro-Derived Greenhouse-Grown Tubers, in Vitro Shoots and Callus-like Basal Tissues of Harpagophytum procumbens. S. Afr. J. Bot. 2011, 77, 479–484. [Google Scholar] [CrossRef]
- Beyene, G.; Chauhan, R.D.; Wagaba, H.; Moll, T.; Alicai, T.; Miano, D.; Carrington, J.C.; Taylor, N.J. Loss of CMD2-Mediated Resistance to Cassava Mosaic Disease in Plants Regenerated through Somatic Embryogenesis. Mol. Plant Pathol. 2016, 17, 1095–1110. [Google Scholar] [CrossRef]
- Chauhan, R.D.; Beyene, G.; Taylor, N.J. Multiple Morphogenic Culture Systems Cause Loss of Resistance to Cassava Mosaic Disease. BMC Plant Biol. 2018, 18, 132. [Google Scholar] [CrossRef]
- Dibi, K.E.B.; Essis, B.S.; Kouamé, K.T.; Essy, A.R.-F.; Kouakou, A.M.; Dogbo, D.O.; N’Zue, B. Effet de la Durée de Conservation des Boutures sur le Rendement et la Qualité des Racines Tubéreuses du Manioc (Manihot esculenta Crantz) au Centre de la Côte d’Ivoire. Tropicultura 2024, 42, 2295–8010. [Google Scholar]
- Doubi, B.T.S.; Kouassi, K.I.; Kouakou, K.L.; Koffi, K.K.; Baudoin, J.-P.; Zoro, B.I.A. Existing Competitive Indices in the Intercropping System of Manihot esculenta Crantz and Lagenaria siceraria (Molina) Standley. J. Plant Interact. 2016, 11, 178–185. [Google Scholar] [CrossRef]
- N’zué, B.; Zouhouri, P.G.; Sangaré, A. Performances Agronomiques de Quelques Varietes de Manioc (Manihot esculenta Crantz) Dans Trois Zones Agroclimatiques de la Cote D’Ivoire. Agron. Afr. 2004, 16, 1–7. [Google Scholar]
- Boni, N.; Pamelas, O.M.; Michel, K.A.; Brice, D.K.E.; Pierre, Z.G.; Sidoine, E.B.; Alexandre, D.A. Morphological Characterization of Cassava (Manihot esculenta Crantz) Accessions Collected in the Centre-West, South-West and West of Côte d’Ivoire. Greener J. Agric. Sci. 2012, 4, 220–231. [Google Scholar] [CrossRef]
- Seka, J.S.S.; Kouassi, M.K.; Yéo, E.F.; Saki, F.M.; Otron, D.H.; Tiendrébéogo, F.; Eni, A.; Kouassi, N.K.; Pita, J.S. Removing Recalcitrance to the Micropropagation of Five Farmer-Preferred Cassava Varieties in Côte d’Ivoire by Supplementing Culture Medium with Kinetin or Thidiazuron. Front. Plant Sci. 2025, 16, 1538799. [Google Scholar] [CrossRef]
- Akano, A.O.; Atiri, G.I.; Ng, S.Y.C.; Asiedu, R. Effect of African Cassava Mosaic Disease on Growth and Yield Components of Virus-Tested Cassava Genotypes Derived from Meristem Culture in Early and Late Planting Periods in Three Agroecologies of Nigeria. Afr. J. Root Tuber. Crops 1997, 2, 44–48. [Google Scholar]
- Hahn, S.K.; Terry, E.R.; Leuschner, K. Breeding Cassava for Resistance to Cassava Mosaic Disease. Euphytica 1980, 29, 673–683. [Google Scholar] [CrossRef]
- Sseruwagi, P.; Sserubombwe, W.S.; Legg, J.P.; Ndunguru, J.; Thresh, J.M. Methods of Surveying the Incidence and Severity of Cassava Mosaic Disease and Whitefly Vector Populations on Cassava in Africa: A Review. Virus Res. 2004, 100, 129–142. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A Rapid DNA Isolation Procedure for Small Quantities of Fresh Leaf Tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Matic, S.; da Cunha, A.P.; Thompson, J.R.; Tepfer, M. An Analysis of Viruses Associated with Cassava Mosaic Disease in Three Angolan Provinces. J. Plant Pathol. 2012, 94, 443–450. [Google Scholar]
- Alabi, O.J.; Ogbe, F.O.; Bandyopadhyay, R.; Lava Kumar, P.; Dixon, A.G.; Hughes, J.A.; Naidu, R.A. Alternate Hosts of African Cassava Mosaic Virus and East African Cassava Mosaic Cameroon Virus in Nigeria. Arch. Virol. 2008, 153, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Fondong, V.N.; Pita, J.S.; Rey, M.E.C.; de Kochko, A.; Beachy, R.N.; Fauquet, C.M. Evidence of Synergism between African Cassava Mosaic Virus and a New Double-Recombinant Geminivirus Infecting Cassava in Cameroon. J. General. Virol. 2000, 81, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Mugerwa, H.; Seal, S.; Wang, H.-L.; Patel, M.V.; Kabaalu, R.; Omongo, C.A.; Alicai, T.; Tairo, F.; Ndunguru, J.; Sseruwagi, P. African Ancestry of New World, Bemisia tabaci-Whitefly Species. Sci. Rep. 2018, 8, 2734. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Wickham, H. Data Analysis. In ggplot2; Use R! Springer International Publishing: Cham, Switzerland, 2016; pp. 189–201. ISBN 978-3-319-24275-0. [Google Scholar]
- R Core Team; Venables, W.N.; Smith, D. An Introduction to R-Notes on R: A Programming Environment for Data Analysis and Graphics; Network Theory: Godalming, UK, 2024. [Google Scholar]
- Patil, B.L.; Fauquet, C.M. Cassava Mosaic Geminiviruses: Actual Knowledge and Perspectives. Mol. Plant Pathol. 2009, 10, 685–701. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Yearbook 2014 Africa Food and Agriculture. In Proceedings of the 2016 ASABE Annual International Meeting, Orlando, FL, USA, 17–20 July 2016; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2014. [Google Scholar]
- Otim-Nape, G.W.; Thresh, J.M. The Current Pandemic of Cassava Mosaic Virus Disease in Uganda. In The Epidemiology of Plant Diseases; Jones, D.G., Ed.; Springer: Dordrecht, The Netherlands, 1998; pp. 423–443. ISBN 978-94-017-3304-5. [Google Scholar]
- Harimalala, M.; Chiroleu, F.; Giraud-Carrier, C.; Hoareau, M.; Zinga, I.; Randriamampianina, J.A.; Velombola, S.; Ranomenjanahary, S.; Andrianjaka, A.; Reynaud, B.; et al. Molecular Epidemiology of Cassava Mosaic Disease in Madagascar. Plant Pathol. 2015, 64, 501–507. [Google Scholar] [CrossRef]
- Hasanuzzaman, A.T.M.; Islam, M.N.; Zhang, Y.; Zhang, C.-Y.; Liu, T.-X. Leaf Morphological Characters Can Be a Factor for Intra-Varietal Preference of Whitefly Bemisia tabaci (Hemiptera: Aleyrodidae) among Eggplant Varieties. PLoS ONE 2016, 11, e0153880. [Google Scholar] [CrossRef] [PubMed]
- Prado, J.C.; Peñaflor, M.F.G.V.; Cia, E.; Vieira, S.S.; Silva, K.I.; Carlini-Garcia, L.A.; Lourenção, A.L. Resistance of Cotton Genotypes with Different Leaf Colour and Trichome Density to Bemisia tabaci Biotype B. J. Appl. Entomol. 2016, 140, 405–413. [Google Scholar] [CrossRef]
- Silva, M.S.; Lourenção, A.L.; Souza-Dias, J.A.C.; Miranda Filho, H.S.; Ramos, V.J.; Schammass, E.A. Resistance of Potato Genotypes (Solanum spp.) to Bemisia tabaci Biotype B. Hortic. Bras. 2008, 26, 221–226. [Google Scholar] [CrossRef]
- Morales, F.J.; Jones, P.G. The Ecology and Epidemiology of Whitefly-Transmitted Viruses in Latin America. Virus Res. 2004, 100, 57–65. [Google Scholar] [CrossRef]
- Baulcombe, D.C. RNA as a Target and an Initiator of Post-Transcriptional Gene Silencing in Trangenic Plants. In Post-Transcriptional Control of Gene Expression in Plants; Springer: Dordrecht, The Netherlands, 1996; pp. 79–88. ISBN 978-94-009-0353-1. [Google Scholar]
- Napoli, C.; Lemieux, C.; Jorgensen, R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in Trans. Plant Cell 1990, 2, 279–289. [Google Scholar] [CrossRef]
- Waterhouse, P.M.; Wang, M.-B.; Lough, T. Gene Silencing as an Adaptive Defence against Viruses. Nature 2001, 411, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Vanitharani, R.; Chellappan, P.; Pita, J.S.; Fauquet, C.M. Differential Roles of AC2 and AC4 of Cassava Geminiviruses in Mediating Synergism and Suppression of Posttranscriptional Gene Silencing. J. Virol. 2004, 78, 9487–9498. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, P.; Vanitharani, R.; Fauquet, C.M. Short Interfering RNA Accumulation Correlates with Host Recovery in DNA Virus-Infected Hosts, and Gene Silencing Targets Specific Viral Sequences. J. Virol. 2004, 78, 7465–7477. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, Z.; Zhang, J.; Chen, C.; Liu, F. Application of PCR and PCR-Derived Technologies for the Detection of Pathogens Infecting Crops. Physiol. Mol. Plant Pathol. 2025, 136, 102589. [Google Scholar] [CrossRef]
- Allado, S.S.; Adjata, D.K.; Pita, J.S.; Mivedor, A.S.; Dansou-Kodjo, K.A.; Tozo, K. Multiplex PCR for Identification and Detection of Cassava Mosaic Begomoviruses in Togo. Adv. Microbiol. 2023, 13, 517–525. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in Virus Diagnostics: From ELISA to next Generation Sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef]
- Otron, D.H.; Filloux, D.; Brousse, A.; Hoareau, M.; Fenelon, B.; Hoareau, C.; Fernandez, E.; Tiendrébéogo, F.; Lett, J.-M.; Pita, J.S.; et al. Improvement of Nanopore Sequencing Provides Access to High Quality Genomic Data for Multi-Component CRESS-DNA Plant Viruses. Virol. J. 2025, 22, 78. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Fondong, V.N.; Rey, C.; Rogan, D.; Fauquet, C.M.; Brown, J.K. Molecular Evidence for Five Distinct Bemisia tabaci (Homoptera: Aleyrodidae) Geographic Haplotypes Associated with Cassava Plants in Sub-Saharan Africa. Ann. Entomol. Soc. Am. 2004, 97, 852–859. [Google Scholar] [CrossRef]
- Nwezeobi, J.; Onyegbule, O.; Nkere, C.; Onyeka, J.; Brunschot, S.; Seal, S.; Colvin, J. Cassava Whitefly Species in Eastern Nigeria and the Threat of Vector-Borne Pandemics from East and Central Africa. PLoS ONE 2020, 15, e0232616. [Google Scholar] [CrossRef]
- Efekemo, O.P.; Onile-ere, O.A.; Abegunde, I.O.; Otitolaye, F.T.; Pita, J.S.; Alicai, T.; Eni, A.O. Molecular Diversity and Distribution of Whiteflies (Bemisia tabaci) in Cassava Fields Across South West and North Central, Nigeria. Insects 2024, 15, 906. [Google Scholar] [CrossRef] [PubMed]

| Code | Variety Name | Type | Status of Resistance to Cassava Mosaic Disease |
|---|---|---|---|
| V1 | Yacé CNRA | Local | Susceptible |
| V2 | Boufouh4 | Local | Tolerant |
| V3 | TMS30572 | Improved | Resistant |
| V4 | Bayérè | Local | Susceptible |
| V5 | TMS4(2)1425 | Improved | Susceptible |
| V6 | Yavo (TME7) | Improved | Susceptible |
| V7 | Im89 | Improved | Resistant |
| V8 | Olékanga | Local | Tolerant |
| V9 | Bocou 5 (TMS 98/0581) | Improved | Resistant |
| V10 | Agbablé3 | Local | Susceptible |
| V11 | Bonoua 34 | Local | Resistant |
| V12 | Bocou 6 (M98/0068) | Improved | Resistant |
| Scale | CMD Symptoms |
|---|---|
| 1 | Plants without symptoms |
| 2 | Plants showing moderate chlorotic spots or some deformation at the base |
| 3 | Plants with spots all over the leaf surface and twisting of the leaves |
| 4 | Plants with a leaf blade (up to 2/3 of the leaf surface) with a deformed leaf |
| 5 | Plants showing numerous symptoms of CMD and/or deformation, total loss of 4/5 of the leaf surface, and stunting of the entire plant |
| Primers | Sequences (5′ to 3′) | Target Region | Size | Reference |
|---|---|---|---|---|
| WAVE-508F | AAGGCCCATGTAAGGTCCAG | ACMV DNA-A AV1/AC3 | 800 pb | [15] |
| WAVE-1307R | GAAGGAGCTGGGGATTCACA | |||
| ACMVB-F | TCGGGAGTGATACATGCGAAGGC | ACMV DNA-B (BV1/BC1) | 628 pb | [34] |
| ACMVB-R | GGCTACACCAGCTACCTGAAGCT | |||
| CMBRepF | CRT CAA TGA CGT TGT ACC A | EACMV DNA-A (AC1) | 650 pb | [35] |
| EACMVRepR | GGT TTG CAG AGA ACT ACA TC | |||
| WAVE-EB 1869F | TTCCAAGGGGAGGGTTCTGA | EACMV DNA-B (BC1) | 800 pb | [15] |
| WAVE-EB 2694R | TGCTCTCGCCTCTCTCTTCT | |||
| VNF031F | GGATACAGATAGGGTTCCCAC | EACMCMV DNA-A (AC2/AC3) | ≈560 pb | [36] |
| VNF032R | GACGAGGACAAGAATTCCAAT |
| Severity Level | Total Sample | Virus Detected | Negatives (%) | ||
|---|---|---|---|---|---|
| ACMV (%) | EACMCMV (%) | ACMV + EACMCMV (%) | |||
| Asymptomatic (score 1) | 911 | 34 (3.73) | 6 (0.66) | 8 (0.88) | 863 (94.73) |
| Moderate (score 2–3) | 54 | 24 (44.44) | 0 (0) | 9 (16.67) | 21 (38.89) |
| Severe (score 4) | 41 | 22 (53.66) | 0 (0) | 11 (26.83) | 8 (19.51) |
| Very severe (score 5) | 5 | 2 (40) | 0 (0) | 3 (60) | 0 (0) |
| Total | 1011 | 82 (8.11) | 6 (0.59) | 31 (3.07) | 892 (88.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seka, J.S.S.; Pita, J.S.; Kouassi, M.K.; Amoakon, W.J.-L.; Kouakou, B.S.M.; Combala, M.; Otron, D.H.; Essis, B.S.; Dibi, K.E.B.; Eni, A.O.; et al. Reinfection Dynamics of Disease-Free Cassava Plants in Three Agroecological Regions of Côte d’Ivoire. Viruses 2025, 17, 1393. https://doi.org/10.3390/v17101393
Seka JSS, Pita JS, Kouassi MK, Amoakon WJ-L, Kouakou BSM, Combala M, Otron DH, Essis BS, Dibi KEB, Eni AO, et al. Reinfection Dynamics of Disease-Free Cassava Plants in Three Agroecological Regions of Côte d’Ivoire. Viruses. 2025; 17(10):1393. https://doi.org/10.3390/v17101393
Chicago/Turabian StyleSeka, John Steven S., Justin S. Pita, Modeste K. Kouassi, William J. -L. Amoakon, Bekanvié S. M. Kouakou, Mariam Combala, Daniel H. Otron, Brice Sidoine Essis, Konan Evrard B. Dibi, Angela O. Eni, and et al. 2025. "Reinfection Dynamics of Disease-Free Cassava Plants in Three Agroecological Regions of Côte d’Ivoire" Viruses 17, no. 10: 1393. https://doi.org/10.3390/v17101393
APA StyleSeka, J. S. S., Pita, J. S., Kouassi, M. K., Amoakon, W. J.-L., Kouakou, B. S. M., Combala, M., Otron, D. H., Essis, B. S., Dibi, K. E. B., Eni, A. O., Kouassi, N. K., & Tiendrébéogo, F. (2025). Reinfection Dynamics of Disease-Free Cassava Plants in Three Agroecological Regions of Côte d’Ivoire. Viruses, 17(10), 1393. https://doi.org/10.3390/v17101393








