Different Susceptibility of Mammalian Cell Lines to Severe Fever with Thrombocytopenia Syndrome Virus Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. FFA of SFTSV
2.3. SFTSV Infections in Cell Lines
2.4. PFA of SFTSV
2.5. Real-Time RT-PCR
3. Results
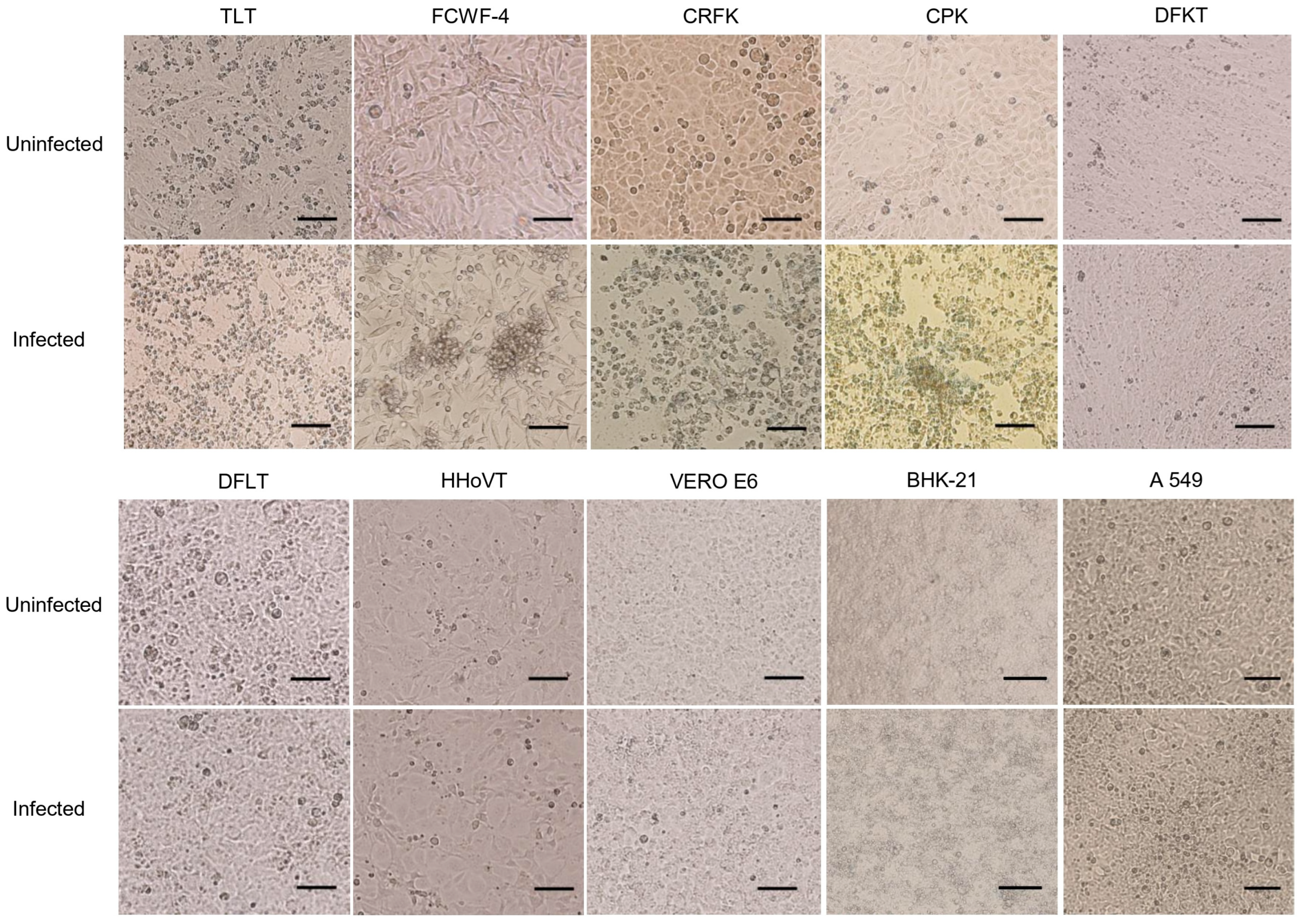
3.1. CPE Formation in SFTSV-Infected Cell Lines
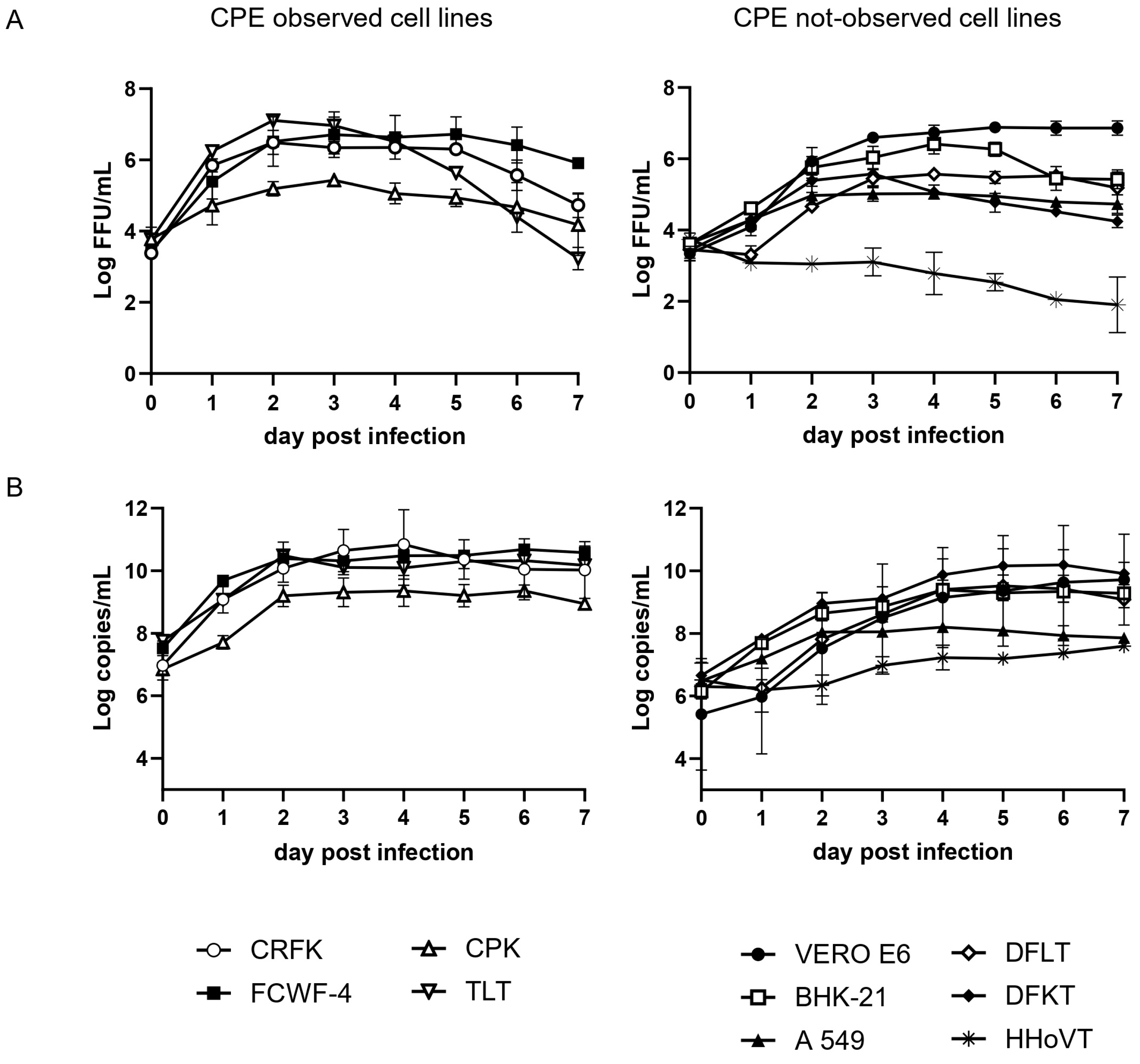
3.2. Growth Kinetics of SFTSV in Cell Lines
3.3. PFA of SFTSV
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ICTV. ICTV Taxonomy History: SFTS Virus. Available online: https://ictv.global/report/chapter/phenuiviridae/phenuiviridae/bandavirus (accessed on 14 August 2025).
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Q.; He, B.; Huang, S.Y.; Wei, F.; Zhu, X.Q. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect. Dis. 2014, 14, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.H.; Choe, P.G.; Kim, N.J.; Lee, J.K.; Oh, M.D. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg. Infect. Dis. 2013, 19, 1892–1894. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ongkittikul, S.; Watanawong, R.; Rompho, P. Severe fever with thrombocytopenia syndrome virus: The first case report in Thailand, Bangkok. Bangk. Med. J. 2020, 16, 204. [Google Scholar] [CrossRef]
- Tran, X.C.; Yun, Y.; Van, A.L.; Kim, S.H.; Thao, N.T.P.; Man, P.K.C.; Yoo, J.R.; Heo, S.T.; Cho, N.H.; Lee, K.H. Endemic severe fever with thrombocytopenia syndrome, Vietnam. Emerg. Infect. Dis. 2019, 25, 1029–1031. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Win, A.M.; Nguyen, Y.T.H.; Kim, Y.; Ha, N.Y.; Kang, J.G.; Kim, H.; San, B.; Kyaw, O.; Htike, W.W.; Choi, D.O.; et al. Genotypic Heterogeneity of Orientia tsutsugamushi in Scrub Typhus Patients and Thrombocytopenia Syndrome Co-infection, Myanmar. Emerg. Infect. Dis. 2020, 26, 1878–1881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zohaib, A.; Zhang, J.; Saqib, M.; Athar, M.A.; Hussain, M.H.; Chen, J.; Sial, A.U.; Tayyab, M.H.; Batool, M.; Khan, S.; et al. Serologic Evidence of Severe Fever with Thrombocytopenia Syndrome Virus and Related Viruses in Pakistan. Emerg. Infect. Dis. 2020, 26, 1513–1516. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, L.; Li, J.; Cui, X.; Jia, N.; Wei, J.; Xia, L.; Wang, H.; Zhou, Y.; Wang, Q.; Liu, X.; et al. Distribution of Haemaphysalis longicornis and associated pathogens: Analysis of pooled data from a China field survey and global published data. Lancet Planet. Health 2020, 4, e320–e329. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.M.; Lee, W.G.; Ryou, J.; Yang, S.C.; Park, S.W.; Roh, J.Y.; Lee, Y.J.; Park, C.; Han, M.G. Severe fever with thrombocytopenia syndrome virus in ticks collected from humans, South Korea, 2013. Emerg. Infect. Dis. 2014, 20, 1358–1361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fang, X.; Hu, J.; Peng, Z.; Dai, Q.; Liu, W.; Liang, S.; Li, Z.; Zhang, N.; Bao, C. Epidemiological and clinical characteristics of severe fever with thrombocytopenia syndrome bunyavirus human-to-human transmission. PLoS Negl. Trop. Dis. 2021, 15, e0009037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gai, Z.; Liang, M.; Zhang, Y.; Zhang, S.; Jin, C.; Wang, S.W.; Sun, L.; Zhou, N.; Zhang, Q.; Sun, Y.; et al. Person-to-Person Transmission of Severe Fever with Thrombocytopenia Syndrome Bunyavirus Through Blood Contact. Clin. Infect. Dis. 2012, 54, 249–252. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seo, J.W.; Kim, D.; Yun, N.; Kim, D.M. Clinical Update of Severe Fever with Thrombocytopenia Syndrome. Viruses 2021, 13, 1213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, J.G. Severe fever with thrombocytopenia syndrome presenting as acute hepatic failure. Clin. Exp. Emerg. Med. 2015, 2, 137–140. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Zhao, C.; Zhang, X.; Si, X.; Ye, L.; Lawrence, K.; Lu, Y.; Du, C.; Xu, H.; Yang, Q.; Xia, Q.; et al. Hedgehogs as Amplifying Hosts of Severe Fever with Thrombocytopenia Syndrome Virus, China. Emerg. Infect. Dis. 2022, 28, 2491–2499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.W.; Wen, H.L.; Fang, L.Z.; Zhang, Z.T.; He, S.T.; Xue, Z.F.; Ma, D.Q.; Zhang, X.S.; Wang, T.; Yu, H.; et al. Prevalence of SFTSV among Asian house shrews and rodents, China, January-August 2013. Emerg. Infect. Dis. 2014, 20, 2126–2128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.; Li, P.; Li, K.-F.; Wang, H.-L.; Dai, Y.-X.; Cheng, X.; Yan, J.-B. Animals as amplification hosts in the spread of severe fever with thrombocytopenia syndrome virus: A systematic review and meta-analysis. Int. J. Infect. Dis. 2019, 79, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Kang, J.-G.; Oh, S.-S.; Chae, J.-B.; Cho, Y.-K.; Cho, Y.-S.; Lee, H.; Chae, J.-S. Molecular detection of severe fever with thrombocytopenia syndrome virus (SFTSV) in feral cats from Seoul, Korea. Ticks Tick Borne Dis. 2017, 8, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Kida, K.; Matsuoka, Y.; Shimoda, T.; Matsuoka, H.; Yamada, H.; Saito, T.; Imataki, O.; Kadowaki, N.; Noguchi, K.; Maeda, K.; et al. A Case of Cat-to-Human Transmission of Severe Fever with Thrombocytopenia Syndrome Virus. Jpn. J. Infect. Dis. 2019, 72, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-M.; Yu, M.-A.; Park, S.-J.; Kim, Y.-I.; Robles, N.J.; Kwon, H.-I.; Kim, E.-H.; Si, Y.-J.; Nguyen, H.D.; Choi, Y.K. Seroprevalence and genetic characterization of severe fever with thrombocytopenia syndrome virus in domestic goats in South Korea. Ticks Tick Borne Dis. 2018, 9, 1202–1206. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Fukuma, A.; Shimojima, M.; Yamashita, Y.; Mizota, F.; Yamashita, M.; Otsuka, Y.; Kan, M.; Fukushi, S.; Tani, H.; et al. Seroprevalence of severe fever with thrombocytopenia syndrome (SFTS) virus antibodies in humans and animals in Ehime prefecture Japan, an endemic region of SFTS. J. Infect. Chemother. 2018, 24, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Tabara, K.; Fujita, H.; Hirata, A.; Hayasaka, D. Investigation of Severe Fever with Thrombocytopenia Syndrome Virus Antibody among Domestic Bovines Transported to Slaughterhouse in Shimane Prefecture, Japan. Jpn. J. Infect. Dis. 2016, 69, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Matsuu, A.; Momoi, Y.; Nishiguchi, A.; Noguchi, K.; Yabuki, M.; Hamakubo, E.; Take, M.; Maeda, K. Natural severe fever with thrombocytopenia syndrome virus infection in domestic cats in Japan. Vet. Microbiol. 2019, 236, 108346. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nabeshima, T.; Inoue, S.; Tun, M.M.N.; Obata, M.; Hu, W.; Shimoda, H.; Kurihara, S.; Izumikawa, K.; Morita, K.; et al. Severe Fever with Thrombocytopenia Syndrome in Cats and Its Prevalence among Veterinarian Staff Members in Nagasaki, Japan. Viruses 2021, 13, 1142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Irie, M.; Miyoshi, T.; Hiramoto, A.; Hirata, M.; Takanosu, M.; Park, E.-S.; Maeda, K. Diagnosis of severe fever with thrombocytopenia syndrome (SFTS) in a cat with clinical findings resembling lymphoma. J. Vet. Med. Sci. 2022, 84, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Bao, C.; Zhou, M.; Hu, J.; Tang, F.; Guo, X.; Jiao, Y.; Zhang, W.; Luo, P.; Li, L.; et al. Seroprevalence and Risk Factors for Severe Fever with Thrombocytopenia Syndrome Virus Infection in Jiangsu Province, China, 2011. Am. J. Trop. Med. Hyg. 2014, 90, 256–259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miyauchi, A.; Sada, K.-E.; Yamamoto, H.; Iriyoshi, H.; Touyama, Y.; Hashimoto, D.; Nojima, S.; Yamanaka, S.; Ishijima, K.; Maeda, K.; et al. Suspected Transmission of Severe Fever with Thrombocytopenia Syndrome Virus from a Cat to a Veterinarian by a Single Contact: A Case Report. Viruses 2022, 14, 223. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamanaka, A.; Kirino, Y.; Fujimoto, S.; Ueda, N.; Himeji, D.; Miura, M.; Sudaryatma, P.E.; Sato, Y.; Tanaka, H.; Mekata, H.; et al. Direct Transmission of Severe Fever with Thrombocytopenia Syndrome Virus from Domestic Cat to Veterinary Personnel. Emerg. Infect. Dis. 2020, 26, 2994–2998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, J.; Du, S.; Liu, Q. The cellular host factors for severe fever with thrombocytopenia syndrome virus infection. Anim. Zoonoes 2025, 1, 146–163. [Google Scholar] [CrossRef]
- Hofmann, H.; Li, X.; Zhang, X.; Liu, W.; Kühl, A.; Kaup, F.; Soldan, S.S.; González-Scarano, F.; Weber, F.; He, Y.; et al. Severe Fever with Thrombocytopenia Virus Glycoproteins Are Targeted by Neutralizing Antibodies and Can Use DC-SIGN as a Receptor for pH-Dependent Entry into Human and Animal Cell Lines. J. Virol. 2013, 87, 4384–4394. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tani, H.; Shimojima, M.; Fukushi, S.; Yoshikawa, T.; Fukuma, A.; Taniguchi, S.; Morikawa, S.; Saijo, M. Characterization of Glycoprotein-Mediated Entry of Severe Fever with Thrombocytopenia Syndrome Virus. J. Virol. 2016, 90, 5292–5301. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sun, Q.; Jin, C.; Zhu, L.; Liang, M.; Lu, C.; Cardona, C.J.; Li, D.; Xing, Z. Host Responses and Regulation by NFκB Signaling in the Liver and Liver Epithelial Cells Infected with A Novel Tick-borne Bunyavirus. Sci. Rep. 2015, 5, 11816. [Google Scholar] [CrossRef]
- Keita, M.; Yasuko, O.; Kimberly, M.-W.; Dana, S.; Friederike, F.; Mifang, L.; Hideki, E. Animal Models of Emerging Tick-Borne Phleboviruses: Determining Target Cells in a Lethal Model of SFTSV Infection. Front. Microbiol. 2017, 8, 104. [Google Scholar] [CrossRef]
- Hayasaka, D.; Shimada, S.; Aoki, K.; Takamatsu, Y.; Uchida, L.; Horio, M.; Fuxun, Y.; Morita, K. Epidemiological Survey of Severe Fever with Thrombocytopenia Syndrome Virus in Ticks in Nagasaki, Japan. Trop. Med. Health 2015, 43, 159–164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Taniguchi, S.; Fukuma, A.; Tani, H.; Fukushi, S.; Saijo, M.; Shimojima, M. A neutralization assay with a severe fever with thrombocytopenia syndrome virus strain that makes plaques in inoculated cells. J. Virol. Methods 2017, 244, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Fukushi, S.; Tani, H.; Fukuma, A.; Taniguchi, S.; Toda, S.; Shimazu, Y.; Yano, K.; Morimitsu, T.; Ando, K.; et al. Sensitive and specific PCR systems for detection of both Chinese and Japanese severe fever with thrombocytopenia syndrome virus strains and prediction of patient survival based on viral load. J. Clin. Microbiol. 2014, 52, 3325–3333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayasaka, D.; Fuxun, Y.; Yoshikawa, A.; Posadas-Herrera, G.; Shimada, S.; Tun, M.M.; Agoh, M.; Morita, K. Seroepidemiological evidence of severe fever with thrombocytopenia syndrome virus infections in wild boars in Nagasaki, Japan. Trop. Med. Health 2016, 44, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cooper, P.D. The plaque assay of animal viruses. Adv. Virus Res. 1961, 8, 319–378. [Google Scholar] [CrossRef] [PubMed]
- Stone, E.T.; Hirsch, A.J.; Smith, J.L.; Brien, J.D.; Pinto, A.K. Titration and neutralizing antibody quantification by focus forming assay for Powassan virus. STAR Protoc. 2022, 3, 101473. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, J.; Lu, G.; Wen, L.; Luo, P.; Huang, Y.; Liang, R.; Tang, K.; Qin, Z.; Chan, C.C.; Chik, K.K.; et al. Severe fever with thrombocytopenia syndrome virus (SFTSV)-host interactome screen identifies viral nucleoprotein-associated host factors as potential antiviral targets. Comput. Struct. Biotechnol. J. 2021, 19, 5568–5577. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dighe, H.; Sarkale, P.; Patil, D.Y.; Mohandas, S.; Shete, A.M.; Sahay, R.R.; Lakra, R.; Patil, S.; Majumdar, T.; Gawande, P.; et al. Differential Cell Line Susceptibility to the SARS-CoV-2 Omicron BA.1.1 Variant of Concern. Vaccines 2022, 10, 1962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shimoda, H.; Nishizato, M.; Nochide, K.; Hayasaka, D. Establishment of Cell Lines from Wild Animals; Joint Graduate School of Veterinary Medicine, Yamaguchi University: Yamaguchi, Japan, 2025; manuscript in preparation. [Google Scholar]
- Wulandari, S.; Nyampong, S.; Beránková, M.; Lokupathirage, S.M.W.; Yoshimatsu, K.; Shimoda, H.; Hayasaka, D. Two amino acid pairs in the Gc glycoprotein of severe fever with thrombocytopenia syndrome virus responsible for the enhanced virulence. J. Virol. 2025, 601, 110294. [Google Scholar] [CrossRef]
- Zhang, M.; Du, Y.; Yang, L.; Zhan, L.; Yang, B.; Huang, X.; Xu, B.; Morita, K.; Yu, F. Development of Monoclonal Antibody Based IgG and IgM ELISA for Diagnosis of Severe Fever with Thrombocytopenia Syndrome Virus Infection. Braz. J. Infect. Dis. 2022, 26, 102386. [Google Scholar] [CrossRef]
- Nyampong, S.; Shimoda, H.; Hayasaka, D. Stability and UV Irradiation Effects on Severe Fever with Thrombocytopenia Syndrome Virus; Joint Graduate School of Veterinary Medicine, Yamaguchi University: Yamaguchi, Japan, 2025; manuscript in preparation. [Google Scholar]
- Turnell, A.S.; Grand, R.J. DNA viruses and the cellular DNA-damage response. J. Gen. Virol. 2012, 93, 2076–2097. [Google Scholar] [CrossRef] [PubMed]
- McKinney, C.C.; Hussmann, K.L.; McBride, A.A. The Role of the DNA Damage Response throughout the Papillomavirus Life Cycle. Viruses 2015, 7, 2450–2469. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Li, Q.; Kang, L.; Li, B.; Ye, H.; Duan, X.; Xie, H.; Jiang, M.; Li, S.; Zhu, Y.; et al. Mitophagy Activation Targeting PINK1 Is an Effective Treatment to Inhibit Zika Virus Replication. ACS Infect. Dis. 2023, 9, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, Y.; Meng, X.; Wang, Z.; Younis, M.; Liu, Y.; Wang, P.; Huang, X. SARS-CoV-2 membrane protein causes the mitochondrial apoptosis and pulmonary edema via targeting BOK. Cell Death Differ. 2022, 29, 1395–1408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Matsuno, K.; Nonoue, N.; Noda, A.; Kasajima, N.; Noguchi, K.; Takano, A.; Shimoda, H.; Orba, Y.; Muramatsu, M.; Sakoda, Y.; et al. Fatal Tickborne Phlebovirus Infection in Captive Cheetahs, Japan. Emerg. Infect. Dis. 2018, 24, 1726–1729. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Park, E.S.; Shimojima, M.; Nagata, N.; Ami, Y.; Yoshikawa, T.; Iwata-Yoshikawa, N.; Fukushi, S.; Watanabe, S.; Kurosu, T.; Kataoka, M.; et al. Severe Fever with Thrombocytopenia Syndrome Phlebovirus causes lethal viral hemorrhagic fever in cats. Sci. Rep. 2019, 9, 11990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshikawa, R.; Sakabe, S.; Urata, S.; Yasuda, J. Species-Specific Pathogenicity of Severe Fever with Thrombocytopenia Syndrome Virus Is Determined by Anti-STAT2 Activity of NSs. J. Virol. 2019, 93, e02226-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Duncan, C.J.A.; Hambleton, S. Human Disease Phenotypes Associated with Loss and Gain of Function Mutations in STAT2: Viral Susceptibility and Type I Interferonopathy. J. Clin. Immunol. 2021, 41, 1446–1456. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoshikawa, R.; Kawakami, M.; Yasuda, J. The NSs protein of severe fever with thrombocytopenia syndrome virus differentially inhibits the type 1 interferon response among animal species. J. Biol. Chem. 2023, 299, 104819. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yahiro, T.; Yamada, K.; Kimitsuki, K.; Okuyama, M.W.; Honda, A.; Kato, M.; Narimatsu, H.; Hiramatsu, K.; Nishizono, A. Distribution of Severe Fever with Thrombocytopenia Syndrome Virus and Antiviral Antibodies in Wild and Domestic Animals in Oita Prefecture, Japan. Am. J. Trop. Med. Hyg. 2022, 106, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.S.; Kim, J.; Son, K.; Kim, Y.; Hwang, J.; Jeong, H.; Ahn, T.Y.; Jheong, W.H. Phylogenetic analysis of severe fever with thrombocytopenia syndrome virus in Korean water deer (Hydropotes inermis argyropus) in the Republic of Korea. Ticks Tick Borne Dis. 2020, 11, 101221. [Google Scholar] [CrossRef] [PubMed]
- Schönbächler, K.; Hatt, J.-M.; Silaghi, N.; Merz, C.; Fraefel, C.; Bachofen, C. Frühsommer-Meningoenzephalitis-Virus Nachweis beim Europäischen Igel (Erinaceus europaeus). Schweiz. Arch. Tierheilkd. 2019, 161, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Domanska-Blicharz, K.; Lisowska, A.; Opolska, J.; Ruszkowski, J.J.; Gogulski, M.; Pomorska-Mól, M. Whole genome characteristics of hedgehog coronaviruses from Poland and analysis of the evolution of the Spike protein for its interspecies transmission potential. BMC Vet. Res. 2024, 20, 424. [Google Scholar] [CrossRef] [PubMed]
- Saldanha, I.F.; Lawson, B.; Goharriz, H.; Fernandez, J.R.-R.; John, S.K.; Fooks, A.R.; Cunningham, A.A.; Johnson, N.; Horton, D.L. Extension of the known distribution of a novel clade C betacoronavirus in a wildlife host. Epidemiol. Infect. 2019, 147, e169. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, M.M.; Lei, X.Y.; Yu, X.J. SFTS phlebovirus promotes LC3-II accumulation and nonstructural protein of SFTS phlebovirus co-localizes with autophagy proteins. Sci. Rep. 2018, 8, 5287. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fenner, F.; Bachmann, P.A.; Gibbs, E.P.J.; Murphy, F.A.; Studdert, M.J.; White, D.O. Cultivation and Assay of Viruses. Vet. Virol. 2014, 1987, 39–53. [Google Scholar] [CrossRef] [PubMed Central]
| Origin | CPE Observed | Viral Titers (FFU/mL) | ||||
|---|---|---|---|---|---|---|
| Species | Tissue | |||||
| CPE cells | dpi | 0 dpi | 2 dpi | |||
| TLT | Tiger | Liver | established | 3 | 103.8 | 107.1 |
| FCWF-4 | Cat | Macrophage | commercial | 5 | 103.4 | 106.5 |
| CRFK | Cat | Kidney | commercial | 5 | 103.4 | 106.5 |
| CPK | Pig | Kidney | commercial | 4 | 103.8 | 105.2 |
| Non-CPE cells | 0 dpi | 4 dpi | ||||
| DFKT | Wild deer | Kidney | established | - | 103.4 | 105.2 |
| DFLT | Wild deer | Liver | established | - | 103.4 | 105.6 |
| HHoVT | Hedgehog | Ovary | established | - | 103.4 | - |
| Vero E6 | Monkey | Kidney | commercial | - | 103.4 | 106.7 |
| BHK-21 | Hamster | Kidney | commercial | - | 103.4 | 106.4 |
| A549 | Human | Lung | commercial | - | 103.4 | 105.0 |
| SFTSV Strain | FFA (FFU/mL) | PFA (PFU/mL) |
|---|---|---|
| Vero E6 | TLT | |
| Tk-F123 | 4.3 × 107 | 7.3 × 107 |
| Ng-F264 | 4.8 × 107 | 1.3 × 108 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anggita, M.; Nyampong, S.; Hu, W.; Shimoda, H.; Hayasaka, D. Different Susceptibility of Mammalian Cell Lines to Severe Fever with Thrombocytopenia Syndrome Virus Infection. Viruses 2025, 17, 1380. https://doi.org/10.3390/v17101380
Anggita M, Nyampong S, Hu W, Shimoda H, Hayasaka D. Different Susceptibility of Mammalian Cell Lines to Severe Fever with Thrombocytopenia Syndrome Virus Infection. Viruses. 2025; 17(10):1380. https://doi.org/10.3390/v17101380
Chicago/Turabian StyleAnggita, Marla, Samuel Nyampong, Weiyin Hu, Hiroshi Shimoda, and Daisuke Hayasaka. 2025. "Different Susceptibility of Mammalian Cell Lines to Severe Fever with Thrombocytopenia Syndrome Virus Infection" Viruses 17, no. 10: 1380. https://doi.org/10.3390/v17101380
APA StyleAnggita, M., Nyampong, S., Hu, W., Shimoda, H., & Hayasaka, D. (2025). Different Susceptibility of Mammalian Cell Lines to Severe Fever with Thrombocytopenia Syndrome Virus Infection. Viruses, 17(10), 1380. https://doi.org/10.3390/v17101380







