Refractory CMV Enteritis in Small Bowel Transplantation: A Case Highlighting the Challenges of Balancing Immunosuppression and Novel Antiviral Therapies
Abstract
1. Introduction
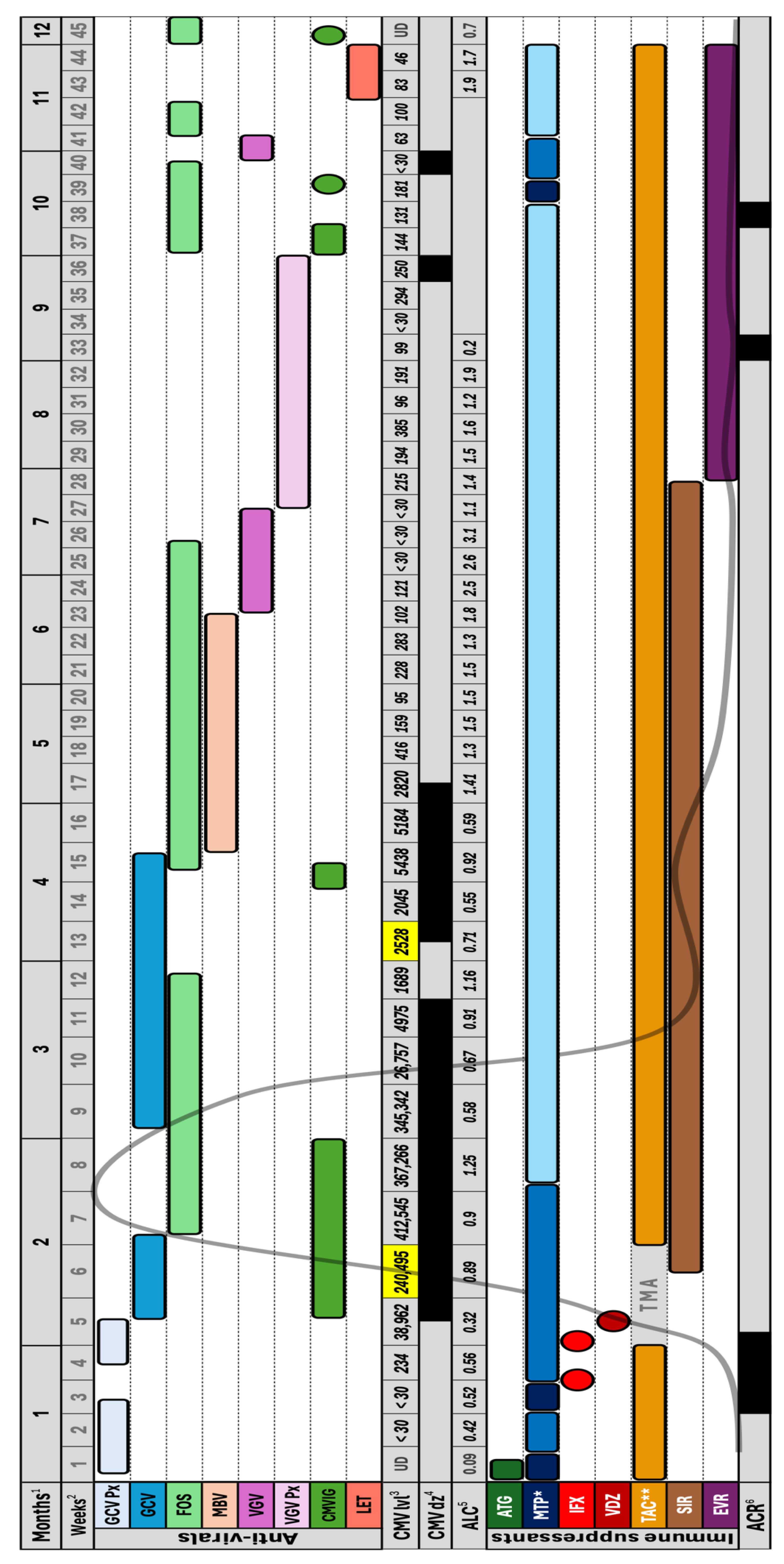
2. Clinical Case
3. Discussion
3.1. Host Risk Factors and Induction Immunosuppression
3.2. Biologic Therapy and the Risk of CMV Reactivation
3.3. The Effect of Maintenance Immunosuppression on CMV Reactivation
3.4. CMV Prophlyaxis and the Onset of Infection
3.5. Refractory CMV Disease
3.6. Novel Antiviral Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kotton, C.N.; Kumar, D.; Manuel, O.; Chou, S.; Hayden, R.T.; Danziger-Isakov, L.; Asberg, A.; Tedesco-Silva, H.; Humar, A. The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation 2025, 109, 1066–1110. [Google Scholar] [CrossRef] [PubMed]
- Zuhair, M.; Smit, G.S.A.; Wallis, G.; Jabbar, F.; Smith, C.; Devleesschauwer, B.; Griffiths, P. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev. Med. Virol. 2019, 29, e2034. [Google Scholar] [CrossRef] [PubMed]
- Alsanea, M.S.; Al-Qahtani, A.A.; Almaghrabi, R.S.; AlAbdulkareem, M.A.; Alahideb, B.M.; Obeid, D.; Alsuwairi, F.A.; Alhamlan, F.S. Diagnosis of Human Cytomegalovirus Drug Resistance Mutations in Solid Organ Transplant Recipients-A Review. Diagnostics 2024, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Razonable, R.R.; Humar, A. Cytomegalovirus in solid organ transplant recipients-Guidelines of the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13512. [Google Scholar] [CrossRef]
- Carbone, J. The Immunology of Posttransplant CMV Infection: Potential Effect of CMV Immunoglobulins on Distinct Components of the Immune Response to CMV. Transplantation 2016, 100 (Suppl. S3), S11–S18. [Google Scholar] [CrossRef]
- Crough, T.; Khanna, R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin. Microbiol. Rev. 2009, 22, 76–98. [Google Scholar] [CrossRef]
- Currier, E.E.; Ichkanian, Y.; Dabaja, M.; Segovia, M.C.; Patel, Y.; Nagai, S.; Sudan, D.L.; Jafri, S.M. Cytomegalovirus Infection Management in Multivisceral and Intestinal Transplant: A Dual Institution Study. Transplant. Proc. 2023, 55, 413–416. [Google Scholar] [CrossRef]
- Mañez, R.; Kusne, S.; Green, M.; Abu-Elmagd, K.; Irish, W.; Reyes, J.; Furukawa, H.; Tzakis, A.; Fung, J.J.; Todo, S.; et al. Incidence and risk factors associated with the development of cytomegalovirus disease after intestinal transplantation. Transplantation 1995, 59, 1010–1014. [Google Scholar] [CrossRef]
- Silva, J.T.; San-Juan, R.; Fernández-Caamaño, B.; Prieto-Bozano, G.; Fernández-Ruiz, M.; Lumbreras, C.; Calvo-Pulido, J.; Jiménez-Romero, C.; Resino-Foz, E.; López-Medrano, F.; et al. Infectious Complications Following Small Bowel Transplantation. Am. J. Transplant. 2016, 16, 951–959. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, J.; Li, J.; Zhou, J.; Mao, X.; Qiu, T. Risk factors for cytomegalovirus infection and disease after kidney transplantation: A meta-analysis. Transpl. Immunol. 2022, 74, 101677. [Google Scholar] [CrossRef]
- Kotton, C.N.; Kamar, N. New Insights on CMV Management in Solid Organ Transplant Patients: Prevention, Treatment, and Management of Resistant/Refractory Disease. Infect. Dis. Ther. 2023, 12, 333–342. [Google Scholar] [CrossRef]
- Saleem, A.; Samad, M.; Khaliq, I.; Ilyas, O.; Farah, B.; Alomari, A.; Faisal, M.; Abusuliman, M.; Omeish, H.; Jafri, S. Infectious Morbidity and Mortality in Small Intestine Transplantation: A Decade of Experience. Am. J. Transplant. 2025, 25 (Suppl. S1), S845. [Google Scholar] [CrossRef]
- Fernandez, A.A.; Simkins, J.; Anjan, S.; Abbo, L.; Selvaggi, G.; Venkatasamy, V.; Miyashiro, R.; Martin, E.; Turkeltaub, J.; Arosemena, L.; et al. Clinical characteristics and outcomes in CMV infection in intestinal transplant recipients: A single-center experience. Transpl. Infect. Dis. 2023, 25, e14071. [Google Scholar] [CrossRef] [PubMed]
- Henrique Pinto, C.; Tedesco-Silva Jr, H.; Rosso Felipe, C.; Nicolau Ferreira, A.; Cristelli, M.; Almeida Viana, L.; Aguiar, W.; Medina-Pestana, J. Targeted preemptive therapy according to perceived risk of CMV infection after kidney transplantation. Braz. J. Infect. Dis. 2016, 20, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Axelrod, D.; Leventhal, J.R.; Gallon, L.G.; Parker, M.A.; Kaufman, D.B. Reduction of CMV disease with steroid-free immunosuppresssion in simultaneous pancreas-kidney transplant recipients. Am. J. Transplant. 2005, 5, 1423–1429. [Google Scholar] [CrossRef]
- Kroemer, A.; Belyayev, L.; Khan, K.; Loh, K.; Kang, J.; Duttargi, A.; Dhani, H.; Sadat, M.; Aguirre, O.; Gusev, Y.; et al. Rejection of intestinal allotransplants is driven by memory T helper type 17 immunity and responds to infliximab. Am. J. Transplant. 2021, 21, 1238–1254. [Google Scholar] [CrossRef]
- Beduschi, T.; Garcia, J.; Jebrock, J.; Tekin, A.; Selvaggi, G.; Fan, J.; Nishida, S.; Ruiz, P.; Vianna, R. Vedolizumab for the Treatment of Refractory Severe Rejection in Intestinal Transplantation. Transplantation 2017, 101, S59. [Google Scholar] [CrossRef]
- Trentadue, G.; Kats-Ugurlu, G.; Blokzijl, T.; Diercks, G.F.; Haveman, J.W.; Faber, K.N.; Dijkstra, G. Safe and Successful Treatment of Acute Cellular Rejection of an Intestine and Abdominal Wall Transplant With Vedolizumab. Transplant. Direct 2020, 6, e527. [Google Scholar] [CrossRef]
- Meroni, P.L.; Valentini, G.; Ayala, F.; Cattaneo, A.; Valesini, G. New strategies to address the pharmacodynamics and pharmacokinetics of tumor necrosis factor (TNF) inhibitors: A systematic analysis. Autoimmun. Rev. 2015, 14, 812–829. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, G.; Kong, D.; Li, G.; Wang, H.; Qin, H.; Wang, H. Risk Factors of Cytomegalovirus Reactivation in Ulcerative Colitis Patients: A Meta-Analysis. Diagnostics 2021, 11, 1952. [Google Scholar] [CrossRef]
- Meyer, F.; Weil-Verhoeven, D.; Prati, C.; Wendling, D.; Verhoeven, F. Safety of biologic treatments in solid organ transplant recipients: A systematic review. Semin. Arthritis Rheum. 2021, 51, 1263–1273. [Google Scholar] [CrossRef]
- Avsar, Y.; Cicinnati, V.R.; Kabar, I.; Wolters, H.; Anthoni, C.; Schmidt, H.H.J.; Beckebaum, S. Small bowel transplantation complicated by cytomegalovirus tissue invasive disease without viremia. J. Clin. Virol. 2014, 60, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Wyant, T.; Fedyk, E.; Abhyankar, B. An Overview of the Mechanism of Action of the Monoclonal Antibody Vedolizumab. J. Crohn’s Colitis 2016, 10, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Hommel, C.; Roblin, X.; Brichet, L.; Bihin, B.; Pillet, S.; Rahier, J.-F. P579 Risk of CMV reactivation in UC patients with previous history of CMV infection following infliximab or vedolizumab treatments. J. Crohn’s Colitis 2018, 12 (Suppl. S1), S400. [Google Scholar] [CrossRef]
- Meeralam, Y.; Al Qurashi, B.; Al Masoudi, A.; Alhejaili, T.L.; Khayat, M.; Aljoaid, A.M.; Al Harthi, W.; Hafiz, W.A.; Shariff, M.K. Cytomegalovirus Colitis in a Patient With Ulcerative Colitis on Vedolizumab Monotherapy. Cureus 2023, 15, e35473. [Google Scholar] [CrossRef]
- Taneja, V.; Anand, R.S.; El-Dallal, M.; Dong, J.; Desai, N.; Taneja, I.; Feuerstein, J.D. Safety of Biologic and Small Molecule Therapy for Inflammatory Bowel Disease Among Solid Organ Transplant Recipients: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2023, 30, 585–593. [Google Scholar] [CrossRef]
- Thomson, A.W.; Bonham, C.A.; Zeevi, A. Mode of action of tacrolimus (FK506): Molecular and cellular mechanisms. Ther. Drug Monit. 1995, 17, 584–591. [Google Scholar] [CrossRef]
- Formisano, L.; Napolitano, F.; Rosa, R.; D’Amato, V.; Servetto, A.; Marciano, R.; De Placido, P.; Bianco, C.; Bianco, R. Mechanisms of resistance to mTOR inhibitors. Crit. Rev. Oncol./Hematol. 2020, 147, 102886. [Google Scholar] [CrossRef]
- Tedesco- Silva, H.; Felipe, C.; Ferreira, A.; Cristelli, M.; Oliveira, N.; Sandes-Freitas, T.; Aguiar, W.; Campos, E.; Gerbase-DeLima, M.; Franco, M.; et al. Reduced Incidence of Cytomegalovirus Infection in Kidney Transplant Recipients Receiving Everolimus and Reduced Tacrolimus Doses. Am. J. Transplant. 2015, 15, 2655–2664. [Google Scholar] [CrossRef]
- Wolf, S.; Hoffmann, V.S.; Sommer, F.; Schrempf, M.; Li, M.; Ryll, M.; Wirth, U.; Ilmer, M.; Werner, J.; Andrassy, J. Effect of Sirolimus vs. Everolimus on CMV-Infections after Kidney Transplantation-A Network Meta-Analysis. J. Clin. Med. 2022, 11, 4216. [Google Scholar] [CrossRef]
- Rao, B.; Segovia, M.C.; Kazimi, M.; Parekh, R.; Raoufi, M.; Jafri, S.M. Use of Everolimus After Multivisceral Transplantation: A Report of Two Cases. Transplant. Proc. 2016, 48, 485–488. [Google Scholar] [CrossRef]
- Avery, R.K.; Arav-Boger, R.; Marr, K.A.; Kraus, E.; Shoham, S.; Lees, L.; Trollinger, B.; Shah, P.; Ambinder, R.; Neofytos, D.; et al. Outcomes in Transplant Recipients Treated With Foscarnet for Ganciclovir-Resistant or Refractory Cytomegalovirus Infection. Transplantation 2016, 100, e74–e80. [Google Scholar] [CrossRef]
- Aguado, J.M.; Navarro, D.; Montoto, C.; Yébenes, M.; de Castro-Orós, I. Incidence of refractory CMV infection with or without antiviral resistance in Spain: A systematic literature review. Transplant. Rev. 2024, 38, 100804. [Google Scholar] [CrossRef] [PubMed]
- Yamani, M.H.; Avery, R.; Mawhorter, S.D.; McNeill, A.; Cook, D.; Ratliff, N.B.; Pelegrin, D.; Colosimo, P.; Kiefer, K.; Ludrosky, K.; et al. The impact of CytoGam on cardiac transplant recipients with moderate hypogammaglobulinemia: A randomized single-center study. J. Heart Lung Transplant. 2005, 24, 1766–1769. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Fournier, M.; Sundberg, A.K.; Song, I.H. Maribavir: Mechanism of action, clinical, and translational science. Clin. Transl. Sci. 2024, 17, e13696. [Google Scholar] [CrossRef] [PubMed]
- Avery, R.K.; Alain, S.; Alexander, B.D.; Blumberg, E.A.; Chemaly, R.F.; Cordonnier, C.; Duarte, R.F.; Florescu, D.F.; Kamar, N.; Kumar, D.; et al. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results from a Phase 3 Randomized Clinical Trial. Clin. Infect. Dis. 2022, 75, 690–701. [Google Scholar] [CrossRef]
- Liu, H.M.; Tsai, Y.H.; Chen, Y. Successful Management of Refractory Cytomegalovirus Infection After Intestinal Transplantation Using Maribavir. Transpl. Infect. Dis. 2025, 27, e14427. [Google Scholar] [CrossRef]
- Razonable, R.R. Current Perspectives on Letermovir and Maribavir for the Management of Cytomegalovirus Infection in Solid Organ Transplant Recipients. Drug Des. Dev. Ther. 2024, 18, 3987–4001. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saud, A.A.; Abufarhaneh, E.H.; Alsanea, M.S.; Alameer, R.M.; Yamani, A.H.; Alhamlan, F.S.; Almaghrabi, R.S. Refractory CMV Enteritis in Small Bowel Transplantation: A Case Highlighting the Challenges of Balancing Immunosuppression and Novel Antiviral Therapies. Viruses 2025, 17, 1379. https://doi.org/10.3390/v17101379
Al-Saud AA, Abufarhaneh EH, Alsanea MS, Alameer RM, Yamani AH, Alhamlan FS, Almaghrabi RS. Refractory CMV Enteritis in Small Bowel Transplantation: A Case Highlighting the Challenges of Balancing Immunosuppression and Novel Antiviral Therapies. Viruses. 2025; 17(10):1379. https://doi.org/10.3390/v17101379
Chicago/Turabian StyleAl-Saud, Abdulrahman A., Ehab H. Abufarhaneh, Madain S. Alsanea, Reem M. Alameer, Amani H. Yamani, Fatimah S. Alhamlan, and Reem S. Almaghrabi. 2025. "Refractory CMV Enteritis in Small Bowel Transplantation: A Case Highlighting the Challenges of Balancing Immunosuppression and Novel Antiviral Therapies" Viruses 17, no. 10: 1379. https://doi.org/10.3390/v17101379
APA StyleAl-Saud, A. A., Abufarhaneh, E. H., Alsanea, M. S., Alameer, R. M., Yamani, A. H., Alhamlan, F. S., & Almaghrabi, R. S. (2025). Refractory CMV Enteritis in Small Bowel Transplantation: A Case Highlighting the Challenges of Balancing Immunosuppression and Novel Antiviral Therapies. Viruses, 17(10), 1379. https://doi.org/10.3390/v17101379






