Semen Quality in Rams Is Severely but Temporarily Affected by Bluetongue Virus Serotype 3 Infection
Abstract
1. Introduction
2. Materials and Methods
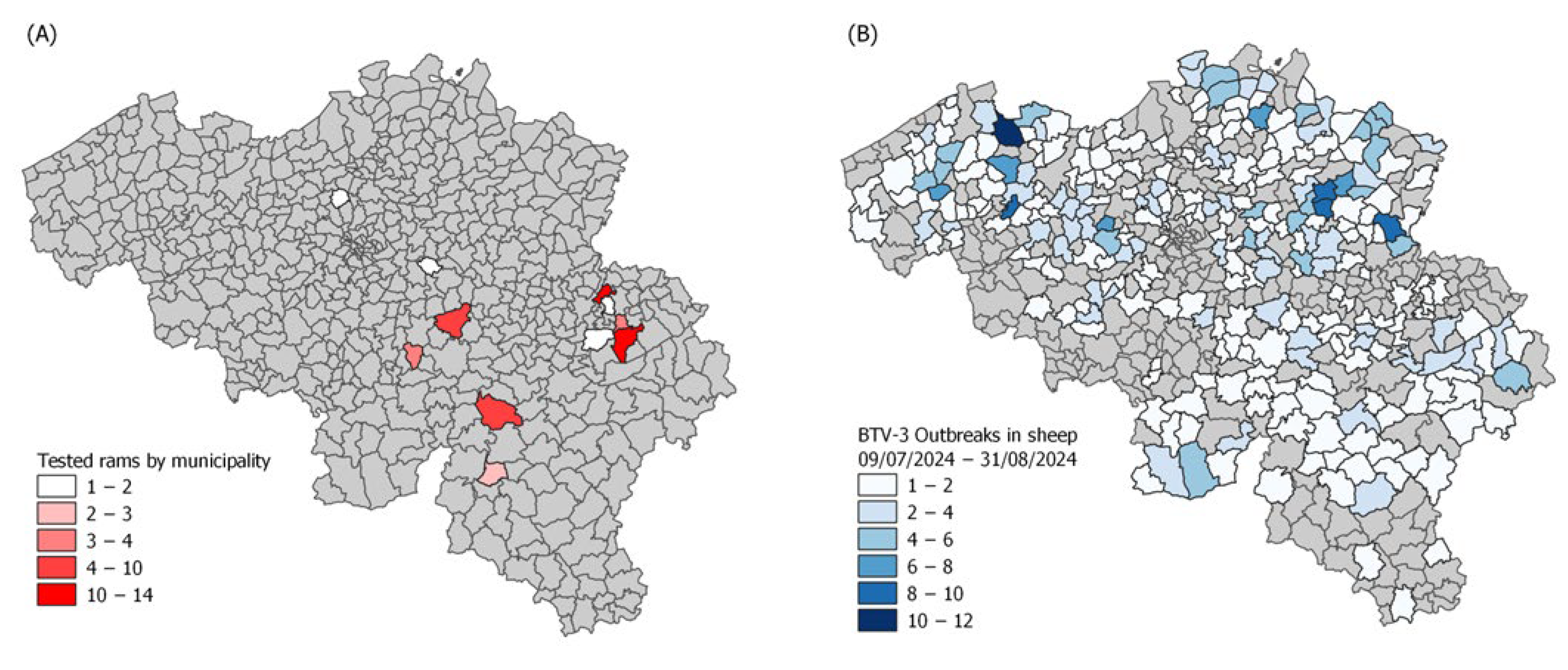
2.1. Animal Selection
2.2. Sample Collection
2.2.1. Blood Sampling
2.2.2. Semen Collection and Analysis
2.3. BTV RNA Detection
2.4. Anti-BTV Antibodies Detection
2.5. Data Analysis
3. Results
3.1. Animal Selection
3.2. BTV RNA Detection
3.3. Anti-BTV Antibodies Detection
3.4. Semen Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARSIA | Association Régionale de Santé et d’Identification Animale |
| BTV | Bluetongue Virus |
| CASA | Computer-Assisted Semen Analysis |
| Ct | Cycle Threshold |
| NRL | National Reference Laboratory |
| PN | Percentage of Negativity |
| SQS | Semen Quality Score |
| VP | Viral Protein |
References
- Matthijnssens, J.; Attoui, H.; Banyai, K.; Brussaard, C.P.D.; Danthi, P.; Del Vas, M.; Dermody, T.S.; Duncan, R.; Fang, Q.; Johne, R.; et al. ICTV Virus Taxonomy Profile: Sedoreoviridae 2022. J. Gen. Virol. 2022, 103, 001782. [Google Scholar] [CrossRef] [PubMed]
- Mellor, P.S.; Carpenter, S.; Harrup, L.; Baylis, M.; Mertens, P.P. Bluetongue in Europe and the Mediterranean Basin: History of occurrence prior to 2006. Prev. Vet. Med. 2008, 87, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Mayo, C.E. Potential strategies for control of bluetongue, a globally emerging, Culicoides-transmitted viral disease of ruminant livestock and wildlife. Antivir. Res. 2013, 99, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Drew, C.P.; Darpel, K.E.; Worwa, G. The pathology and pathogenesis of bluetongue. J. Comp. Pathol. 2009, 141, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Savini, G.; MacLachlan, N.J.; Sanchez-Vizcaino, J.M.; Zientara, S. Vaccines against bluetongue in Europe. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.; Vogtlin, A.; Hussy, D.; Jandt, T.; Gobet, H.; Hilbe, M.; Burgener, C.; Schweizer, L.; Hafliger-Speiser, S.; Beer, M.; et al. Putative Novel Atypical BTV Serotype ‘36’ Identified in Small Ruminants in Switzerland. Viruses 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, M.; Santman-Berends, I.; Harders, F.; Engelsma, M.; Vloet, R.P.M.; Dijkstra, E.; van Gennip, R.G.P.; Mars, M.H.; Spierenburg, M.; Roos, L.; et al. Emergence of Bluetongue Virus Serotype 3, the Netherlands, September 2023. Emerg. Infect. Dis. 2024, 30, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Van den Brink, K.; Santman-Berends, I.; Harkema, L.; Scherpenzeel, C.G.M.; Dijkstra, E.; Bisschop, P.I.H.; Peterson, K.; van de Burgwal, N.S.; Waldeck, H.W.F.; Dijkstra, T.; et al. Bluetongue virus serotype 3 in ruminants in the Netherlands: Clinical signs, seroprevalence and pathological findings. Vet. Rec. 2024, 195, e4533. [Google Scholar] [CrossRef] [PubMed]
- Leemans, J.; Raes, M.; Vanbinst, T.; De Clercq, K.; Saegerman, C.; Kirschvink, N. Viral RNA load in semen from bluetongue serotype 8-infected rams: Relationship with sperm quality. Vet. J. 2012, 192, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Marceau, A.; Madouasse, A.; Lehebel, A.; van Schaik, G.; Veldhuis, A.; Van der Stede, Y.; Fourichon, C. Can routinely recorded reproductive events be used as indicators of disease emergence in dairy cattle? An evaluation of 5 indicators during the emergence of bluetongue virus in France in 2007 and 2008. J. Dairy Sci. 2014, 97, 6135–6150. [Google Scholar] [CrossRef] [PubMed]
- Kirschvink, N.; Raes, M.; Saegerman, C. Impact of a natural bluetongue serotype 8 infection on semen quality of Belgian rams in 2007. Vet. J. 2009, 182, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Saegerman, C.; Bolkaerts, B.; Baricalla, C.; Raes, M.; Wiggers, L.; de Leeuw, I.; Vandenbussche, F.; Zimmer, J.Y.; Haubruge, E.; Cassart, D.; et al. The impact of naturally-occurring, trans-placental bluetongue virus serotype-8 infection on reproductive performance in sheep. Vet. J. 2011, 187, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Avelar, G.F.; Rathi, R.; Franca, L.R.; Dobrinski, I. The length of the spermatogenic cycle is conserved in porcine and ovine testis xenografts. J. Androl. 2006, 27, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Serna, M.; Miguel-Jimenez, S.; Perez-Pe, R.; Casao, A. Testicular Ultrasound Analysis as a Predictive Tool of Ram Sperm Quality. Biology 2022, 11, 261. [Google Scholar] [CrossRef] [PubMed]
- Toussaint, J.F.; Sailleau, C.; Breard, E.; Zientara, S.; De Clercq, K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 2007, 140, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Vandenbussche, F.; Vanbinst, T.; Verheyden, B.; Van Dessel, W.; Demeestere, L.; Houdart, P.; Bertels, G.; Praet, N.; Berkvens, D.; Mintiens, K.; et al. Evaluation of antibody-ELISA and real-time RT-PCR for the diagnosis and profiling of bluetongue virus serotype 8 during the epidemic in Belgium in 2006. Vet. Microbiol. 2008, 129, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H.; Navarro, D.; Pedersen, T.L. ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2023. [Google Scholar]
- Van Leeuw, V.; De Leeuw, I.; Degives, N.; Depoorter, P.; Dewulf, J.; Hanon, J.B.; Hooyberghs, J.; Linden, A.; Praet, L.; Raemaekers, M.; et al. Impact of BTV-3 Circulation in Belgium in 2024 and Current Knowledge Gaps Hindering an Evidence-Based Control Program. Viruses 2025, 17, 521. [Google Scholar] [CrossRef] [PubMed]
- Puggioni, G.; Pintus, D.; Melzi, E.; Meloni, G.; Rocchigiani, A.M.; Maestrale, C.; Manunta, D.; Savini, G.; Dattena, M.; Oggiano, A.; et al. Testicular Degeneration and Infertility following Arbovirus Infection. J. Virol. 2018, 92, e01131-18. [Google Scholar] [CrossRef] [PubMed]
- Muller, U.; Kemmerling, K.; Straet, D.; Janowitz, U.; Sauerwein, H. Effects of bluetongue virus infection on sperm quality in bulls: A preliminary report. Vet. J. 2010, 186, 402–403. [Google Scholar] [CrossRef] [PubMed]



| Sample_ID | Postal Code | Date First Case | Time to Test | Age (Year) | Vaccinated/Recovered | Ct in Semen | Ct in Blood | ELISA |
|---|---|---|---|---|---|---|---|---|
| 1 | 5190 | 12-08-24 | 19 | 1.5 | Vaccinated | 31.4 | 27.1 | Pos |
| 2 | 5190 | 12-08-24 | 19 | 0.5 | Vaccinated | Neg | Neg | Pos |
| 3 | 3640 | 19-07-24 | 43 | 1 | Vaccinated | Neg | Neg | Pos |
| 4 | 5190 | 12-08-24 | 19 | 0.5 | Vaccinated | Neg | Neg | Neg |
| 5 | 4650 | 19-07-24 | 43 | 1 | Vaccinated | Neg | Neg | Pos |
| 6 | 5190 | 12-08-24 | 19 | 1.5 | Recovered | NA | 26.6 | Pos |
| 7 | 3640 | 19-07-24 | 43 | 2 | Vaccinated | Neg | 34.3 | Pos |
| 8 | 6920 | 06-08-24 | 25 | 1.5 | Vaccinated | Neg | 31.4 | Pos |
| 9 | 1840 | 12-08-24 | 19 | 2 | Vaccinated | Neg | Neg | Pos |
| 10 | 1840 | 12-08-24 | 19 | 0.5 | Vaccinated | 26.7 | 28 | Pos |
| 11 | 6920 | 06-08-24 | 25 | 4.5 | Vaccinated | 30.9 | 28.7 | Pos |
| 12 | 6920 | 06-08-24 | 25 | 4.5 | Vaccinated | Neg | 25.2 | Pos |
| 13 | 5590 | 05-08-24 | 26 | 2 | Vaccinated | 26.6 | 28.9 | Pos |
| 14 | 4670 | 27-08-24 | 4 | 1 | Vaccinated | 34.9 | 34.3 | Pos |
| 15 | 5590 | 05-08-24 | 26 | 1 | Vaccinated | 31.2 | 32.9 | Pos |
| 16 | 4670 | 27-08-24 | 4 | 5 | Vaccinated | Neg | 29.7 | Pos |
| 17 | 4910 | 24-07-24 | 38 | 1.5 | Vaccinated | 34.1 | 28.4 | Pos |
| 18 | 4910 | 24-07-24 | 38 | 3.5 | Vaccinated | 31.1 | 30.2 | Pos |
| 19 | 4910 | 24-07-24 | 38 | 1.5 | Vaccinated | Neg | 25.3 | Pos |
| 20 | 4910 | 24-07-24 | 38 | 3 | Vaccinated | 29,8 | 28.3 | Pos |
| 21 | 4860 | NA | NA | 2 | Vaccinated | NA | Neg | Pos |
| 22 | 4910 | 24-07-24 | 38 | NA | Vaccinated | Neg | Neg | Pos |
| 23 | 4670 | 27-08-24 | 4 | 5 | Vaccinated | Neg | 28 | Pos |
| 24 | 4860 | NA | NA | 0.5 | Vaccinated | Neg | Neg | Pos |
| 25 | 4670 | 27-08-24 | 4 | 1 | Vaccinated | Neg | 29.3 | Pos |
| 26 | 4860 | NA | NA | 0.5 | Vaccinated | Neg | 28.9 | Pos |
| 27 | 4650 | 19-07-24 | 43 | 0.5 | Vaccinated | Neg | NA | Pos |
| 28 | 1367 | 01-08-24 | 30 | 2 | Recovered | NA | 23.5 | Pos |
| 29 | 5590 | 05-08-24 | 26 | 2 | Recovered | 30,9 | 31.1 | Pos |
| 30 | 5310 | 10-08-24 | 21 | 0.5 | Recovered | 31,9 | 25.5 | Pos |
| 31 | 5590 | 05-08-24 | 26 | 1 | Recovered | Neg | 31.7 | Pos |
| 32 | 5590 | 05-08-24 | 26 | 2 | Recovered | Neg | 29.2 | Pos |
| 33 | 5310 | 10-08-24 | 21 | 2.5 | Recovered | 35,1 | 26.9 | Pos |
| 34 | 1840 | 12-08-24 | 19 | 2 | Recovered | 24,9 | 28.5 | Pos |
| 35 | 4670 | 27-08-24 | 4 | 4 | Recovered | 32 | 28.9 | Pos |
| 36 | 5590 | 05-08-24 | 26 | 2 | Recovered | Neg | 29.8 | Pos |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinelle, L.; Egyptien, S.; Dechene, L.; Somville, M.; Derkenne, F.; Deleuze, S. Semen Quality in Rams Is Severely but Temporarily Affected by Bluetongue Virus Serotype 3 Infection. Viruses 2025, 17, 1371. https://doi.org/10.3390/v17101371
Martinelle L, Egyptien S, Dechene L, Somville M, Derkenne F, Deleuze S. Semen Quality in Rams Is Severely but Temporarily Affected by Bluetongue Virus Serotype 3 Infection. Viruses. 2025; 17(10):1371. https://doi.org/10.3390/v17101371
Chicago/Turabian StyleMartinelle, Ludovic, Sophie Egyptien, Lola Dechene, Marielle Somville, Frédéric Derkenne, and Stéfan Deleuze. 2025. "Semen Quality in Rams Is Severely but Temporarily Affected by Bluetongue Virus Serotype 3 Infection" Viruses 17, no. 10: 1371. https://doi.org/10.3390/v17101371
APA StyleMartinelle, L., Egyptien, S., Dechene, L., Somville, M., Derkenne, F., & Deleuze, S. (2025). Semen Quality in Rams Is Severely but Temporarily Affected by Bluetongue Virus Serotype 3 Infection. Viruses, 17(10), 1371. https://doi.org/10.3390/v17101371







