Differentiation of West Nile and Usutu Virus Infections by Antibodies Directed to the Non-Structural Protein 1
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Serum Samples
2.2. PCR and Neutralization Tests (NTs)
2.3. NS1 Protein Production and Purification
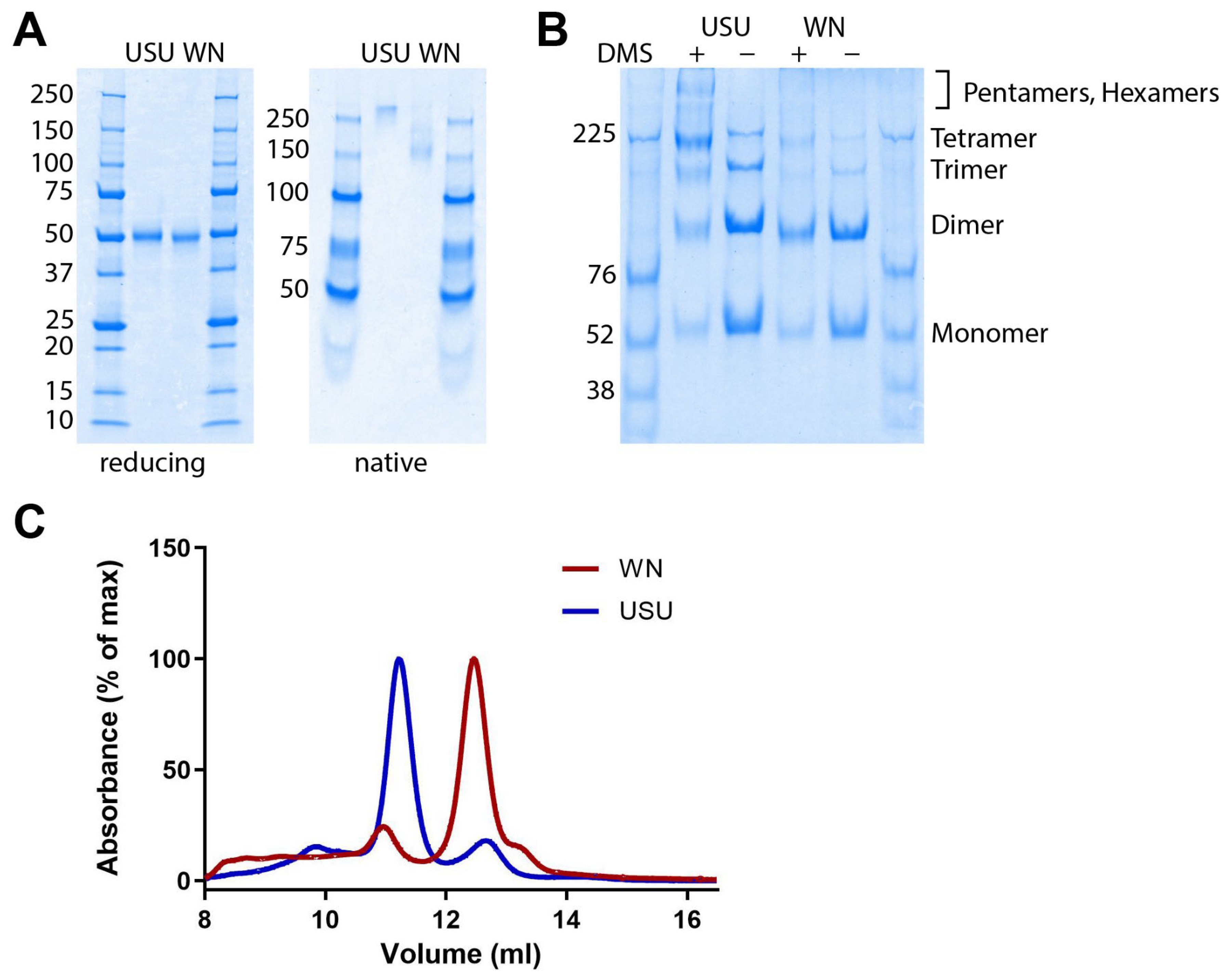
2.4. Determination of the Oligomeric State of NS1
2.5. NS1 IgM ELISA
2.6. NS1 IgG ELISA
2.7. Data Analysis and Statistics
3. Results
3.1. Characterization of Recombinant WN and USU NS1 Proteins
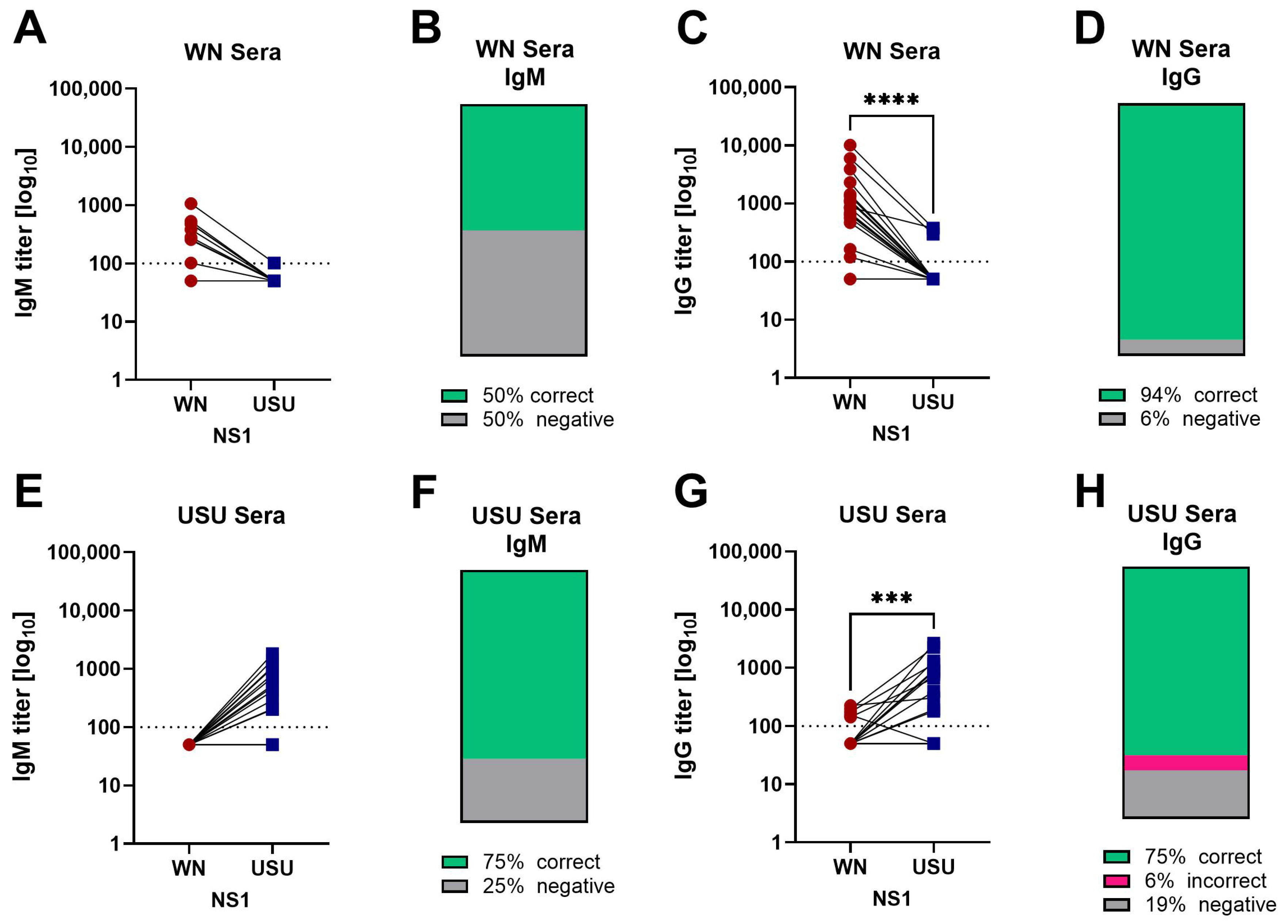
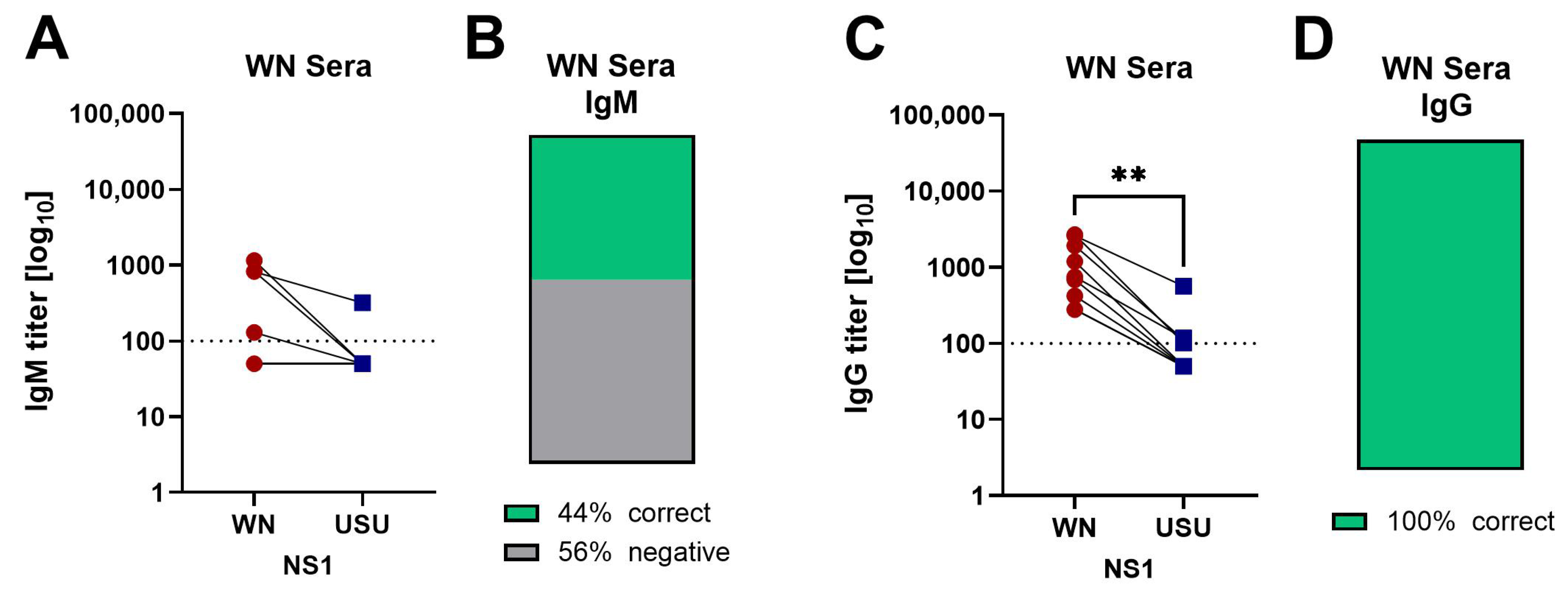
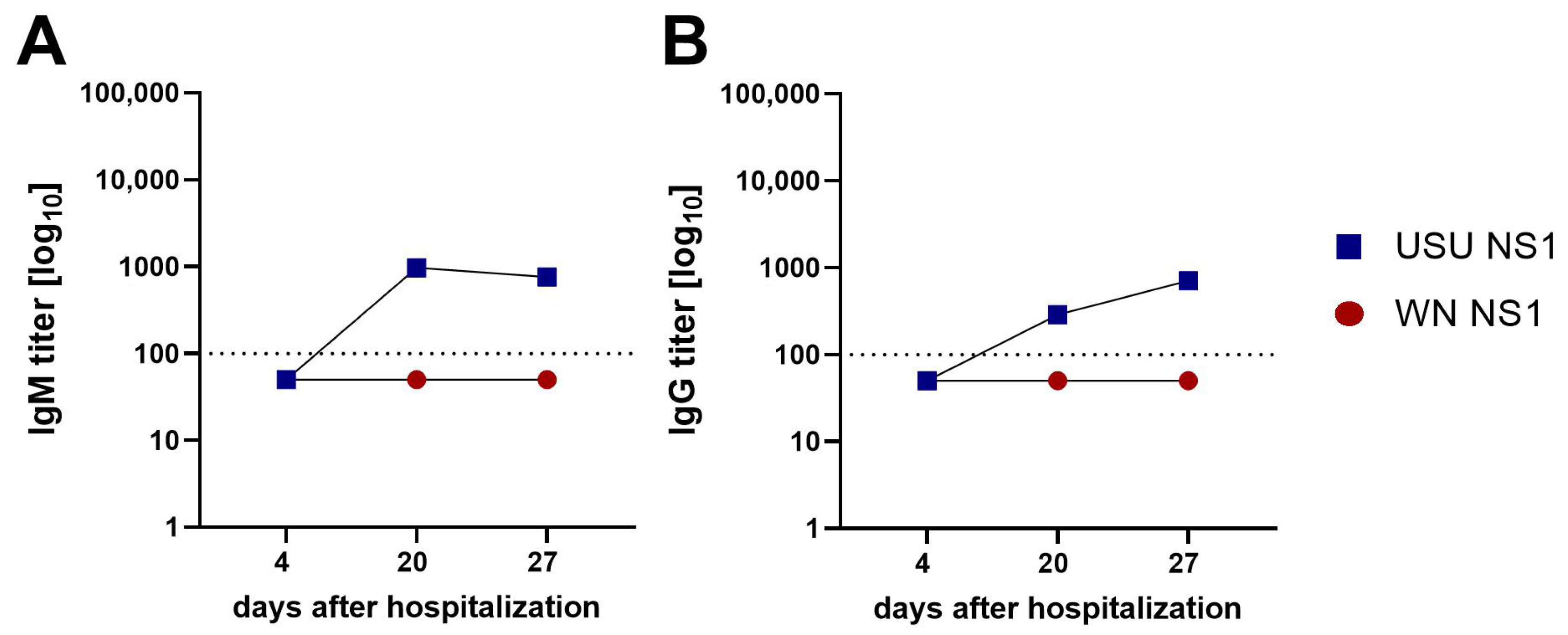
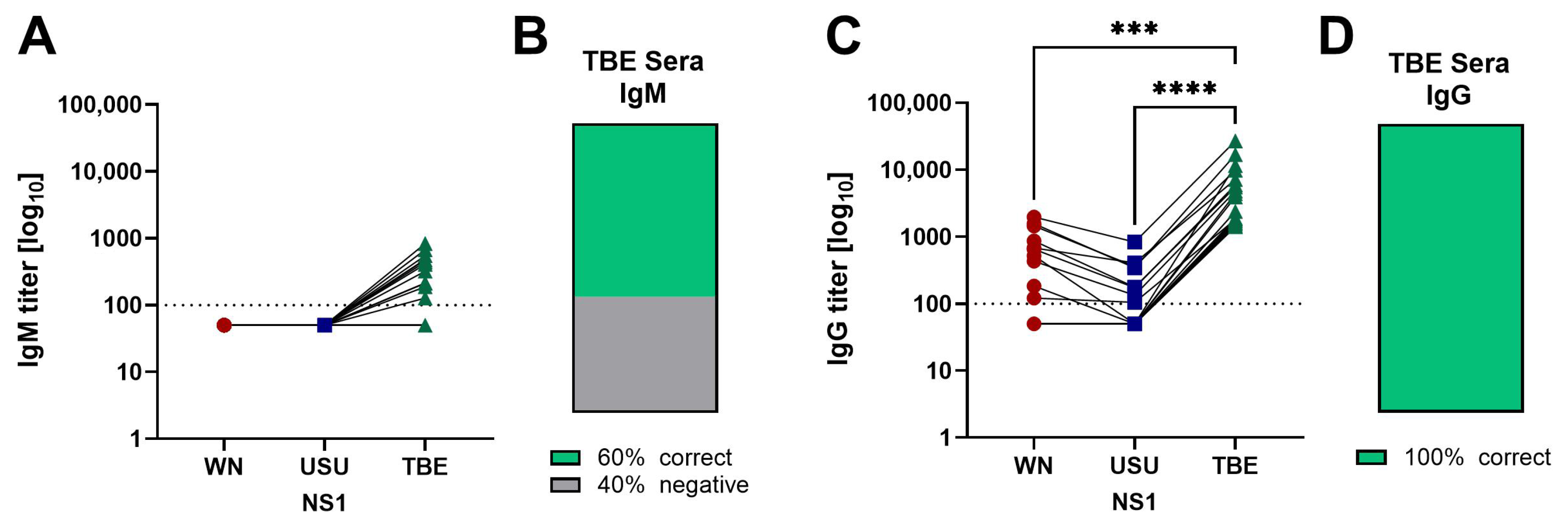
3.2. Detection of NS1 IgM and IgG Antibodies in Serum Samples of WN and USU Cases
3.3. Comparison of WN and USU NS1 IgG and NT Titers
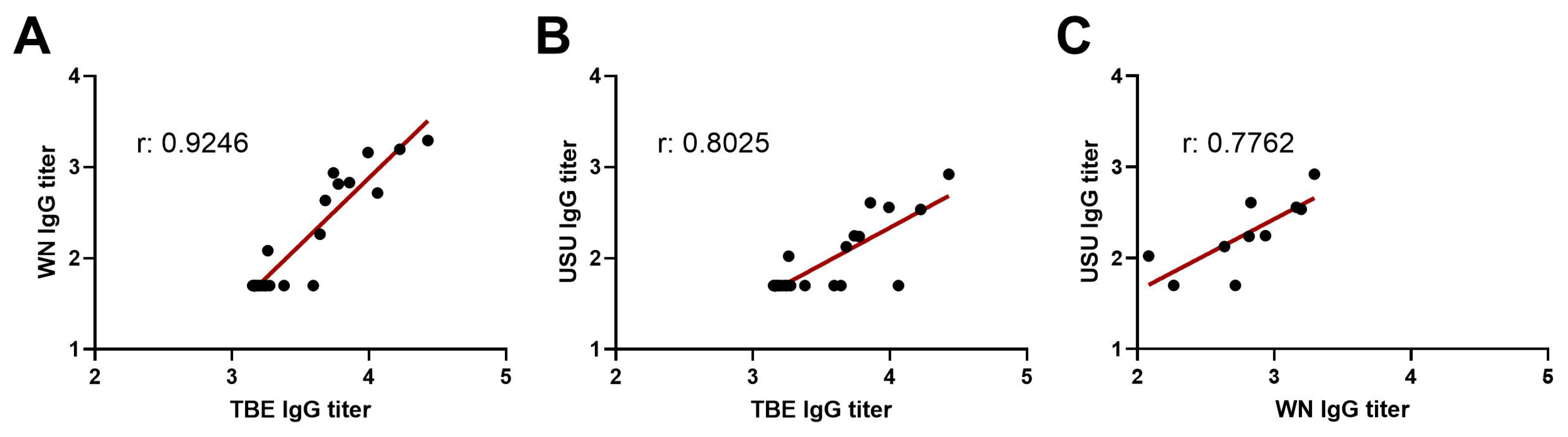
3.4. Detection of Broadly Cross-Reactive Antibodies in WN and USU ELISAs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| NS1 | Non-structural protein 1 |
| SEC | Size-exclusion chromatgography |
| TBE(V) | Tick-borne encephalitis (virus) |
| USU(V) | Usutu (virus) |
| WN(V) | West Nile (virus) |
References
- Calisher, C.H.; Karabatsos, N.; Dalrymple, J.M.; Shope, R.E.; Porterfield, J.S.; Westaway, E.G.; Brandt, W.E. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 1989, 70 Pt 1, 37–43. [Google Scholar] [CrossRef]
- Smithburn, K.; Hughes, T.; Burke, A.; Paul, J. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. 1940, 20, 471–472. [Google Scholar] [CrossRef]
- Woodall, J. The viruses isolated from arthropods at the East African Virus Research Institute in the 26 years ending December 1963. Proc. E Afr. Acad. 1964, 2, 141–146. [Google Scholar]
- Weissenbock, H.; Kolodziejek, J.; Url, A.; Lussy, H.; Rebel-Bauder, B.; Nowotny, N. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg. Infect. Dis. 2002, 8, 652–656. [Google Scholar] [CrossRef]
- Zeller, H.G.; Schuffenecker, I. West Nile virus: An overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Aberle, S.W.; Kolodziejek, J.; Jungbauer, C.; Stiasny, K.; Aberle, J.H.; Zoufaly, A.; Hourfar, M.K.; Weidner, L.; Nowotny, N. Increase in human West Nile and Usutu virus infections, Austria, 2018. Eurosurveillance 2018, 23, 1800545. [Google Scholar] [CrossRef]
- Wodak, E.; Richter, S.; Bago, Z.; Revilla-Fernandez, S.; Weissenbock, H.; Nowotny, N.; Winter, P. Detection and molecular analysis of West Nile virus infections in birds of prey in the eastern part of Austria in 2008 and 2009. Vet Microbiol. 2011, 149, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Stiasny, K.; Aberle, S.W.; Heinz, F.X. Retrospective identification of human cases of West Nile virus infection in Austria (2009 to 2010) by serological differentiation from Usutu and other flavivirus infections. Eurosurveillance 2013, 18, 20614. [Google Scholar] [CrossRef]
- Bakonyi, T.; Jungbauer, C.; Aberle, S.W.; Kolodziejek, J.; Dimmel, K.; Stiasny, K.; Allerberger, F.; Nowotny, N. Usutu virus infections among blood donors, Austria, July and August 2017—Raising awareness for diagnostic challenges. Eurosurveillance 2017, 22, 17-00644. [Google Scholar] [CrossRef]
- Domanović, D.; Gossner, C.M.; Lieshout-Krikke, R.; Mayr, W.; Baroti-Toth, K.; Dobrota, A.M.; Escoval, M.A.; Henseler, O.; Jungbauer, C.; Liumbruno, G.; et al. West Nile and Usutu virus infections and challenges to blood safety in the European Union. Emerg. Infect. Dis. 2019, 25, 1050–1057. [Google Scholar] [CrossRef]
- Agliani, G.; Giglia, G.; Marshall, E.M.; Gröne, A.; Rockx, B.H.G.; van den Brand, J.M.A. Pathological features of West Nile and Usutu virus natural infections in wild and domestic animals and in humans: A comparative review. One Health 2023, 16, 100525. [Google Scholar] [CrossRef]
- Tyler, K.L. Current developments in understanding of West Nile virus central nervous system disease. Curr. Opin. Neurol. 2014, 27, 342–348. [Google Scholar] [CrossRef]
- Cle, M.; Beck, C.; Salinas, S.; Lecollinet, S.; Gutierrez, S.; Van de Perre, P.; Baldet, T.; Foulongne, V.; Simonin, Y. Usutu virus: A new threat? Epidemiol. Infect. 2019, 147, e232. [Google Scholar] [CrossRef]
- Sejvar James, J. West Nile virus infection. Microbiol. Spectr. 2016, 4, 175–199. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, G.; Bertola, M.; Lazzaro, E.; Morini, M.; Masi, G.; Sinigaglia, A.; Trevisan, M.; Gossner, C.M.; Haussig, J.M.; Bakonyi, T.; et al. Epidemiology, surveillance and diagnosis of Usutu virus infection in the EU/EEA, 2012 to 2021. Eurosurveillance 2023, 28, 2200929. [Google Scholar] [CrossRef]
- Kerkhof, K.; Falconi-Agapito, F.; Van Esbroeck, M.; Talledo, M.; Arien, K.K. Reliable serological diagnostic tests for arboviruses: Feasible or utopia? Trends Microbiol. 2020, 28, 276–292. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis. Microbiol. Mol. Biol. Rev. 2017, 81, e00055-16. [Google Scholar] [CrossRef]
- Berneck, B.S.; Rockstroh, A.; Barzon, L.; Sinigaglia, A.; Vocale, C.; Landini, M.P.; Rabenau, H.F.; Schmidt-Chanasit, J.; Ulbert, S. Serological differentiation of West Nile virus- and Usutu virus-induced antibodies by envelope proteins with modified cross-reactive epitopes. Transbound. Emerg. Dis. 2022, 69, 2779–2787. [Google Scholar] [CrossRef]
- Chan, K.R.; Ismail, A.A.; Thergarajan, G.; Raju, C.S.; Yam, H.C.; Rishya, M.; Sekaran, S.D. Serological cross-reactivity among common flaviviruses. Front. Cell Infect. Microbiol. 2022, 12, 975398. [Google Scholar] [CrossRef]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Rey, F.A.; Stiasny, K.; Vaney, M.C.; Dellarole, M.; Heinz, F.X. The bright and the dark side of human antibody responses to flaviviruses: Lessons for vaccine design. EMBO Rep. 2018, 19, 206–224. [Google Scholar] [CrossRef]
- Rossbacher, L.; Malafa, S.; Huber, K.; Thaler, M.; Aberle, S.W.; Aberle, J.H.; Heinz, F.X.; Stiasny, K. Effect of previous heterologous flavivirus vaccinations on human antibody responses in tick-borne encephalitis and dengue virus infections. J. Med. Virol. 2023, 95, e29245. [Google Scholar] [CrossRef]
- Ceconi, M.; Ariën, K.K.; Delputte, P. Diagnosing arthropod-borne flaviviruses: Non-structural protein 1 (NS1) as a biomarker. Trends Microbiol. 2024, 32, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Chew, B.L.A.; Pan, Q.; Hu, H.; Luo, D. Structural biology of flavivirus NS1 protein and its antibody complexes. Antivir. Res. 2024, 227, 105915. [Google Scholar] [CrossRef] [PubMed]
- Akey, D.L.; Brown, W.C.; Jose, J.; Kuhn, R.J.; Smith, J.L. Structure-guided insights on the role of NS1 in flavivirus infection. Bioessays 2015, 37, 489–494. [Google Scholar] [CrossRef]
- Glasner, D.R.; Puerta-Guardo, H.; Beatty, P.R.; Harris, E. The good, the bad, and the shocking: The multiple roles of dengue virus nonstructural protein 1 in protection and pathogenesis. Annu. Rev. Virol. 2018, 5, 227–253. [Google Scholar] [CrossRef]
- Carpio, K.L.; Barrett, A.D.T. Flavivirus NS1 and its potential in vaccine development. Vaccines 2021, 9, 622. [Google Scholar] [CrossRef] [PubMed]
- Graninger, M.; Hubmer, S.; Riederer, F.; Kettner, S.; Hauk, M.; Auf, T.; Aberle, J.H.; Stiasny, K.; Aberle, S.W.; Camp, J.V. The first case of Usutu virus neuroinvasive disease in Austria, 2021. Open Forum Infect. Dis. 2022, 9, ofac255. [Google Scholar] [CrossRef]
- Stiasny, K.; Leitner, A.; Holzmann, H.; Heinz, F.X. Dynamics and extent of non-structural protein 1-antibody responses in tick-borne encephalitis vaccination breakthroughs and unvaccinated patients. Viruses 2021, 13, 1007. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Maizel, J.V., Jr. Polyacrylamide gel electrophoresis of viral proteins. Methods Virol. 1971, 5, 179–246. [Google Scholar]
- Malafa, S.; Medits, I.; Aberle, J.H.; Aberle, S.W.; Haslwanter, D.; Tsouchnikas, G.; Wolfel, S.; Huber, K.L.; Percivalle, E.; Cherpillod, P.; et al. Impact of flavivirus vaccine-induced immunity on primary Zika virus antibody response in humans. PLoS Negl. Trop. Dis. 2020, 14, e0008034. [Google Scholar] [CrossRef]
- Cabanova, V.; Kerlik, J.; Kirschner, P.; Rosochova, J.; Klempa, B.; Slavikova, M.; Lickova, M. Co-Circulation of West Nile, Usutu, and Tick-Borne Encephalitis Viruses in the Same Area: A Great Challenge for Diagnostic and Blood and Organ Safety. Viruses 2023, 15, 366. [Google Scholar] [CrossRef]
- Fisher, R.; Lustig, Y.; Sklan, E.H.; Schwartz, E. The role of NS1 protein in the diagnosis of flavivirus infections. Viruses 2023, 15, 572. [Google Scholar] [CrossRef]
- Chao, D.Y.; Galula, J.U.; Shen, W.F.; Davis, B.S.; Chang, G.J. Nonstructural protein 1-specific immunoglobulin m and g antibody capture enzyme-linked immunosorbent assays in diagnosis of flaviviral infections in humans. J. Clin. Microbiol. 2015, 53, 557–566. [Google Scholar] [CrossRef]
- Steinhagen, K.; Probst, C.; Radzimski, C.; Schmidt-Chanasit, J.; Emmerich, P.; van Esbroeck, M.; Schinkel, J.; Grobusch, M.P.; Goorhuis, A.; Warnecke, J.M.; et al. Serodiagnosis of Zika virus (ZIKV) infections by a novel NS1-based ELISA devoid of cross-reactivity with dengue virus antibodies: A multicohort study of assay performance, 2015 to 2016. Eurosurveillance 2016, 21, 30426. [Google Scholar] [CrossRef] [PubMed]
- Mora-Cardenas, E.; Aloise, C.; Faoro, V.; Knap Gasper, N.; Korva, M.; Caracciolo, I.; D’Agaro, P.; Avsic-Zupanc, T.; Marcello, A. Comparative specificity and sensitivity of NS1-based serological assays for the detection of flavivirus immune response. PLoS Negl. Trop. Dis. 2020, 14, e0008039. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Choo, J.J.Y.; McElnea, C.; Duyen, H.T.L.; Wills, B.; Young, P.R. Kinetics of NS1 and anti-NS1 IgG following dengue infection reveals likely early formation of immune complexes in secondary infected patients. Sci. Rep. 2025, 15, 6684. [Google Scholar] [CrossRef]
- Könenkamp, L.; Ziegler, U.; Naucke, T.; Groschup, M.H.; Steffen, I. Antibody ratios against NS1 antigens of tick-borne encephalitis and West Nile viruses support differential flavivirus serology in dogs. Transbound. Emerg. Dis. 2022, 69, e2789–e2799. [Google Scholar] [CrossRef]
- Caracciolo, I.; Mora-Cardenas, E.; Aloise, C.; Carletti, T.; Segat, L.; Burali, M.S.; Chiarvesio, A.; Totis, V.; Avsic-Zupanc, T.; Mastrangelo, E.; et al. Comprehensive response to Usutu virus following first isolation in blood donors in the Friuli Venezia Giulia region of Italy: Development of recombinant NS1-based serology and sensitivity to antiviral drugs. PLoS Negl. Trop. Dis. 2020, 14, e0008156. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.-Y.; Youn, H.H.; Brites, C.; Tsai, J.-J.; Tyson, J.; Pedroso, C.; Drexler, J.F.; Stone, M.; Simmons, G.; Busch, M.P.; et al. Distinguishing secondary dengue virus infection from Zika virus infection with previous dengue by a combination of 3 simple serological tests. Clin. Infect. Dis. 2017, 65, 1829–1836. [Google Scholar] [CrossRef] [PubMed]
- Girl, P.; Euringer, K.; Coroian, M.; Mihalca, A.D.; Borde, J.P.; Dobler, G. Comparison of five serological methods for the detection of West Nile virus antibodies. Viruses 2024, 16, 788. [Google Scholar] [CrossRef] [PubMed]
- Medina, F.A.; Vila, F.; Adams, L.E.; Cardona, J.; Carrion, J.; Lamirande, E.; Acosta, L.N.; De León-Rodríguez, C.M.; Beltran, M.; Grau, D.; et al. Comparison of the sensitivity and specificity of commercial anti-dengue virus IgG tests to identify persons eligible for dengue vaccination. J. Clin. Microbiol. 2024, 62, e0059324. [Google Scholar] [CrossRef]
- Ackermann-Gäumann, R.; Brêchet, A.; Smetana, J.; Salát, J.; Lienhard, R.; Croxatto, A.; Polcarová, P.; Chlíbek, R.; Růžek, D. Vaccination against tick-borne encephalitis elicits a detectable NS1 IgG antibody response. J. Virol. Methods 2023, 322, 114831. [Google Scholar] [CrossRef]
- Girl, P.; Bestehorn-Willmann, M.; Zange, S.; Borde, J.P.; Dobler, G.; von Buttlar, H. Tick-borne encephalitis virus nonstructural protein 1 IgG enzyme-linked immunosorbent assay for differentiating infection versus vaccination antibody responses. J. Clin. Microbiol. 2020, 58, e01783-19. [Google Scholar] [CrossRef]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A.; Jaconi, S.; Mele, F.; et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef]
- Shu, B.; Ooi, J.S.G.; Tan, A.W.K.; Ng, T.S.; Dejnirattisai, W.; Mongkolsapaya, J.; Fibriansah, G.; Shi, J.; Kostyuchenko, V.A.; Screaton, G.R.; et al. CryoEM structures of the multimeric secreted NS1, a major factor for dengue hemorrhagic fever. Nat. Commun. 2022, 13, 6756. [Google Scholar] [CrossRef]
- Chew, B.L.A.; Ngoh, A.Q.; Phoo, W.W.; Weng, M.J.G.; Sheng, H.J.; Chan, K.W.K.; Tan, E.Y.J.; Gelbart, T.; Xu, C.; Tan, G.S.; et al. Structural basis of Zika virus NS1 multimerization and human antibody recognition. Npj Viruses 2024, 2, 14. [Google Scholar] [CrossRef]
- Chew, B.L.A.; Ngoh, A.N.Q.; Phoo, W.W.; Chan, K.W.K.; Ser, Z.; Tulsian, N.K.; Lim, S.S.; Weng, M.J.G.; Watanabe, S.; Choy, M.M.; et al. Secreted dengue virus NS1 from infection is predominantly dimeric and in complex with high-density lipoprotein. eLife 2024, 12, RP90762. [Google Scholar] [CrossRef]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi-Jorge, T.; Bordignon, J.; Koishi, A.; Zanluca, C.; Mosimann, A.L.; Duarte dos Santos, C.N. Development of a quantitative NS1-capture enzyme-linked immunosorbent assay for early detection of yellow fever virus infection. Sci. Rep. 2017, 7, 16229. [Google Scholar] [CrossRef] [PubMed]
| Cases | n (Cases) | Patient/Blood Donor | Age (Median/Years) | Age (Range/Years) | Sex (f/m) |
|---|---|---|---|---|---|
| WN | 18 | 13/5 | 57 | 36–77 | 5/13 |
| USU | 17 | 1/16 | 57 | 23–81 | 2/15 |
| Cases | n (Cases) | Patient | Age (Median/Years) | Age (Range/Years) | Sex (f/m) |
|---|---|---|---|---|---|
| WN | 9 | 9 | 67 | 35–87 | 5/4 |
| Cases | n | WN NT | USU NT | WN NS1 IgM | USU NS1 IgM | WN NS1 IgG | USU NS1 IgG |
|---|---|---|---|---|---|---|---|
| Number of Positive Cases/Total Number of Cases (% Positive Cases) | |||||||
| WN | 27 | 27/27 (100%) | 22/26 (85%) * | 13/27 (48%) | 2/27 (7%) | 26/27 (96%) | 7/27 (26%) |
| USU | 17 | 11/17 (65%) | 17/17 (100%) | 0/17 (0%) | 13/17 (76%) | 6/17 (35%) | 13/17 (76%) |
| Cases * | Days After Positive PCR | WN NT | USU NT | WN NS1 IgM | USU NS1 IgM | WN NS1 IgG | USU NS1 IgG |
|---|---|---|---|---|---|---|---|
| Titers | |||||||
| WN 18 | 80 | 80 | 120 | 1062 | 101 | 1434 | neg |
| USU 7 | 120 | 120 | 120 | neg | 197 | 1080 | 2247 |
| USU 15 | 80 | 80 | 80 | neg | neg | 201 | 862 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roßbacher, L.; Taschler, S.; Cecchettin, E.; Popovitsch, A.; Aberle, S.W.; Aberle, J.H.; Medits-Weiss, I.; Stiasny, K. Differentiation of West Nile and Usutu Virus Infections by Antibodies Directed to the Non-Structural Protein 1. Viruses 2025, 17, 1357. https://doi.org/10.3390/v17101357
Roßbacher L, Taschler S, Cecchettin E, Popovitsch A, Aberle SW, Aberle JH, Medits-Weiss I, Stiasny K. Differentiation of West Nile and Usutu Virus Infections by Antibodies Directed to the Non-Structural Protein 1. Viruses. 2025; 17(10):1357. https://doi.org/10.3390/v17101357
Chicago/Turabian StyleRoßbacher, Lena, Samuel Taschler, Elena Cecchettin, Amelie Popovitsch, Stephan W. Aberle, Judith H. Aberle, Iris Medits-Weiss, and Karin Stiasny. 2025. "Differentiation of West Nile and Usutu Virus Infections by Antibodies Directed to the Non-Structural Protein 1" Viruses 17, no. 10: 1357. https://doi.org/10.3390/v17101357
APA StyleRoßbacher, L., Taschler, S., Cecchettin, E., Popovitsch, A., Aberle, S. W., Aberle, J. H., Medits-Weiss, I., & Stiasny, K. (2025). Differentiation of West Nile and Usutu Virus Infections by Antibodies Directed to the Non-Structural Protein 1. Viruses, 17(10), 1357. https://doi.org/10.3390/v17101357






