Standardization of Quantitative Plaque-Based Viral Assays for Orthoflavivirus Cacipacoré
Abstract
1. Introduction
2. Materials and Methods
2.1. Safety Practices
2.2. Virus Strains and Cell Lines
2.3. Titrations
2.4. Focus-Forming Unit Assay
2.5. Plaque-Forming Unit Assay
2.6. Image Acquisition
3. Results
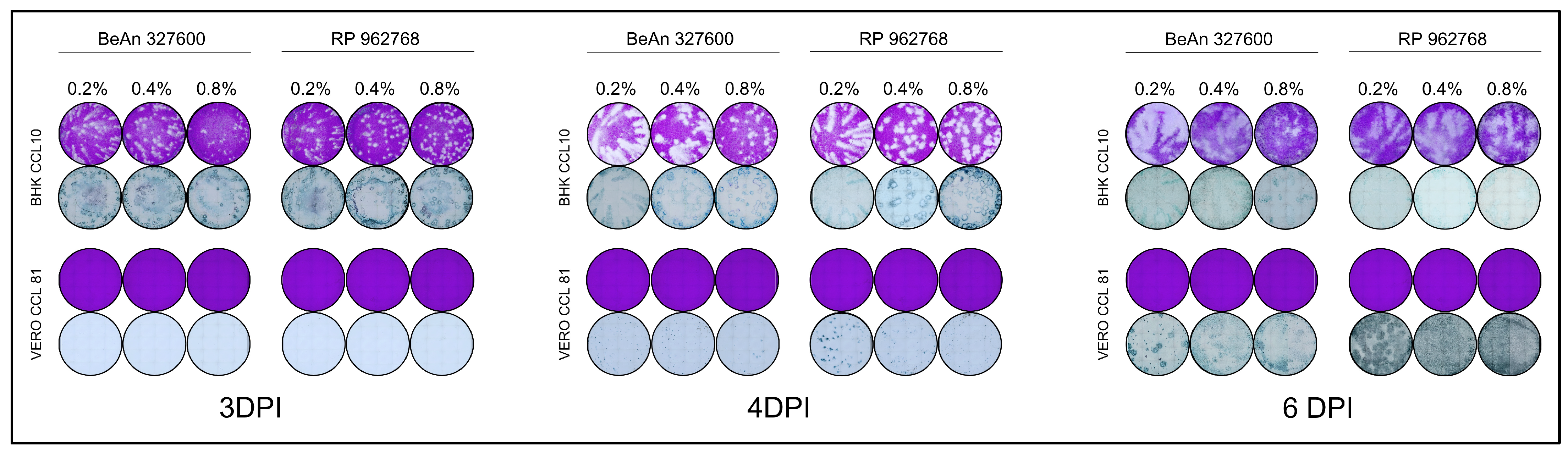
3.1. Comparison of Methylcelulose Overlays
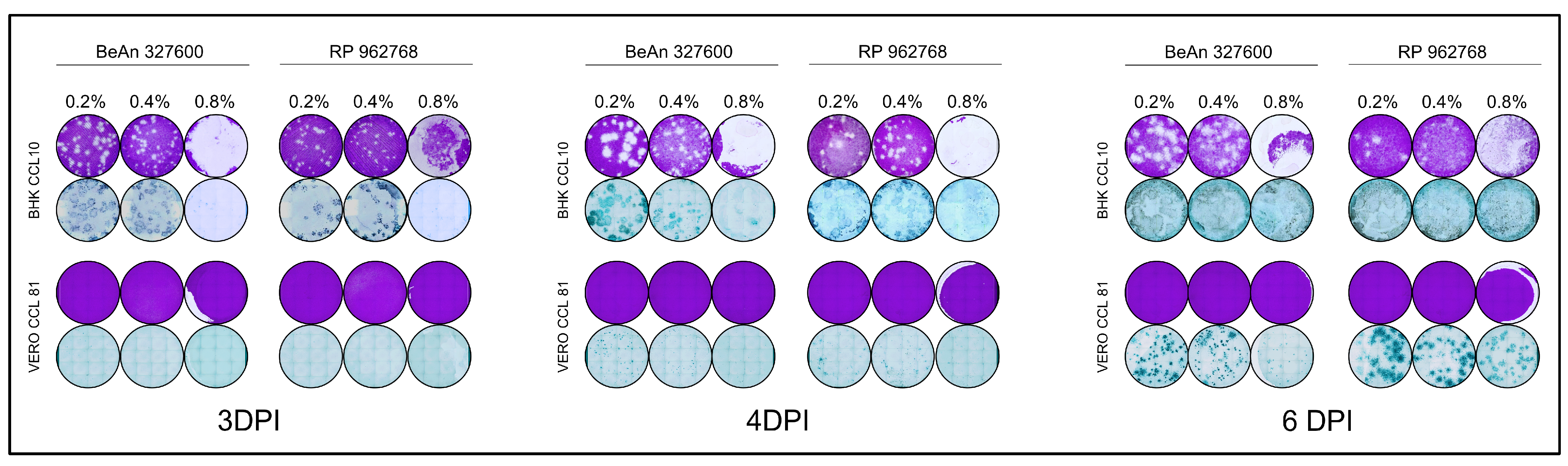
3.2. Comparison of Low Melting Agarose Overlays
3.3. Final Overlay Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travassos da Rosa, J.F.; Travassos da Rosa, A.; Vasconcelos, P.F.; Pinheiro, F.; Travassos da Rosa, E.S.; Dias, L.B.; Cruz, A.C. Arboviruses Isolated in the Evandro Chagas Institute, Including Some Described for the First Time in the Brazilian Amazon Region, Their Known Hosts, and Their Pathology for Man. In An Overview of Arbovirology in Brazil and Neighbouring Countries; Instituto Evandro Chagas: Ananindeua, Brazil, 1998; pp. 18–31. Available online: https://patuaback.iec.gov.br/server/api/core/bitstreams/87aca0a2-1a1d-4b9d-8c57-eece2fecfcd1/content (accessed on 30 November 2024).
- Figueiredo, M.L.G.D.; Amarilla, A.A.; Figueiredo, G.G.D.; Alfonso, H.L.; Lippi, V.; Maia, F.G.M.; Morais, F.A.; Costa, C.A.D.; Henriques, D.A.; Durigon, E.L.; et al. Cacipacore Virus as an Emergent Mosquito-Borne Flavivirus. Rev. Soc. Bras. Med. Trop. 2017, 50, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Karabatsos, N. The International Catalog of Arboviruses—ArboCat Virus: Cacipacore Virus (CPCV). Available online: https://wwwn.cdc.gov/arbocat/VirusDetails.aspx?ID=89 (accessed on 30 November 2023).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Biomas e Sistema Costeiro-Marinho Do Brasil—PGI. Available online: https://www.ibge.gov.br/apps/biomas/#/home (accessed on 26 January 2025).
- Saivish, M.V.; Nogueira, M.L.; Rossi, S.L.; Vasilakis, N. Beyond Borders: Investigating the Mysteries of Cacipacoré, a Lesser-Studied Arbovirus in Brazil. Viruses 2024, 16, 336. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.C.; Tavares, G.D.S.B.; Vieira, D.S.; Honda, E.R.; Pereira, S.S.; Tada, M.S. Notification of the First Isolation of Cacipacore Virus in a Human in the State of Rondônia, Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Oliva, O.; Araujo, F.; Martins, L.; Chiang, J.; Henriques, D.; Silva, E.; Rodrigues, D.; Prazeres, A.; Tavares-Neto, J.; et al. Epidemiology of Saint Louis Encephalitis Virus in the Brazilian Amazon Region and in the State of Mato Grosso Do Sul, Brazil: Elevated Prevalence of Antibodies in Horses. Rev. Pan-Amaz. Saúde 2010, 1, 81–85. [Google Scholar] [CrossRef][Green Version]
- Monteiro, H.A.O. Avaliação da Diversidade de Insetos Hematófagos da Subordem Nematocera e de Vertebrados Silvestres: Transmissão de Arbovírus na área de Influência do Projeto Salobo, Carajás e Pará. Masters Dissertation, Universidade Federal do Pará, Belém, Brazil, 2009. [Google Scholar][Green Version]
- Casseb, A.R.; Cruz, A.V.; Jesus, I.S.; Chiang, J.O.; Martins, L.C.; Silva, S.P.; Henriques, D.F.; Casseb, L.M.; Vasconcelos, P.F.C. Seroprevalence of Flaviviruses Antibodies in Water Buffaloes (Bubalus bubalis) in Brazilian Amazon. J. Venom. Anim. Toxins Trop. Dis. 2014, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.A.A. Inquéritos Sorológicos Em Equídeos E Aves Silvestres Para Detecção De Anticorpos Anti-Arbovírus De Importância Em Saúde Pública No Brasil. Ph.D. Thesis, Universidade Federal de Goiás, Goiânia, Brazil, 2011. [Google Scholar]
- de Oliveira-Filho, E.F.; Fischer, C.; Berneck, B.S.; Carneiro, I.O.; Kühne, A.; de Almeida Campos, A.C.; Ribas, J.R.L.; Netto, E.M.; Franke, C.R.; Ulbert, S.; et al. Ecologic Determinants of West Nile Virus Seroprevalence among Equids, Brazil. Emerg. Infect. Dis. 2021, 27, 2466–2470. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, A.P. Investigação para a circulação do vírus do oeste do Nilo e outros flavivírus no Pantanal de Mato Grosso do Sul. Ph.D. Thesis, Instituto Oswaldo Cruz, Rio de Janeiro, Brazil, 2012. [Google Scholar]
- Pauvolid-Corrêa, A.; Campos, Z.; Juliano, R.; Velez, J.; Nogueira, R.M.R.; Komar, N. Serological Evidence of Widespread Circulation of West Nile Virus and Other Flaviviruses in Equines of the Pantanal, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e2706. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.M.; Andreotti, R.; de Almeida, P.S.; Marques, A.C.; Rodrigues, S.G.; Chiang, J.O.; Vasconcelos, P.F.D.C. Detection of Arboviruses of Public Health Interest in Free-Living New World Primates (Sapajus spp.; Alouatta Caraya) Captured in Mato Grosso Do Sul, Brazil. Rev. Soc. Bras. Med. Trop. 2013, 46, 684–690. [Google Scholar] [CrossRef] [PubMed]
- de Figueiredo, G.G.; Amarilla, A.A.; de Souza, W.M.; Fumagalli, M.J.; de Figueiredo, M.L.G.; Szabó, M.P.J.; Badra, S.J.; Setoh, Y.X.; Khromykh, A.A.; Aquino, V.H.; et al. Genetic Characterization of Cacipacoré Virus from Ticks Collected in São Paulo State, Brazil. Arch. Virol. 2017, 162, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Batista, W.C. Mapeamento de arboviroses no Estado de Rondônia. Ph.D. Thesis, Universidade Federal do Amazonas, Manaus, Brazil, 2007. [Google Scholar]
- Guimarães, M.D.C.N.; Freitas, M.N.O.; Sousa, A.W.D.; Cunha, M.A.C.R.D.; Almada, G.L.; Romano, A.P.M.; Santos, M.G.D.P.; Rodrigues, G.A.P.; Martins, L.C.; Chiang, J.O.; et al. Serological Evidence of Arboviruses in Horses During West Nile Fever Monitoring Surveillance in Southeastern Brazil. Front. Trop. Dis. 2022, 3, 881710. [Google Scholar] [CrossRef]
- Maeda, A.; Maeda, J. Review of Diagnostic Plaque Reduction Neutralization Tests for Flavivirus Infection. Vet. J. 2013, 195, 33–40. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Plaque Reduction Neutralization Testing of Human Antibodies to Dengue Viruses; World Health Organization: Geneva, Switzerland, 2007.
- Azar, S.R.; Rossi, S.L.; Haller, S.H.; Yun, R.; Huang, J.H.; Plante, J.A.; Zhou, J.; Olano, J.P.; Roundy, C.M.; Hanley, K.A.; et al. ZIKV Demonstrates Minimal Pathologic Effects and Mosquito Infectivity in Viremic Cynomolgus Macaques. Viruses 2018, 10, 661. [Google Scholar] [CrossRef] [PubMed]
- Plante, K.S.; Plante, J.A.; Azar, S.R.; Shinde, D.P.; Scharton, D.; Versiani, A.F.; da Silva, N.I.O.; Strange, T.; Sacchetto, L.; Fokam, E.B.; et al. Potential of Ilhéus Virus to Emerge. Heliyon 2024, 10, e27934. [Google Scholar] [CrossRef] [PubMed]
- Nawa, M.; Takasaki, T.; Yamada, K.-I.; Akatsuka, T.; Kurane, I. Development of Dengue IgM-Capture Enzyme-Linked Immunosorbent Assay with Higher Sensitivity Using Monoclonal Detection Antibody. J. Virol. Methods 2001, 92, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Saivish, M.V.; Menezes, G.D.L.; da Silva, R.A.; Fontoura, M.A.; Shimizu, J.F.; da Silva, G.C.D.; Teixeira, I.D.S.; Mistrão, N.F.B.; Hernandes, V.M.; Rahal, P.; et al. Antiviral Activity of Quercetin Hydrate against Zika Virus. Int. J. Mol. Sci. 2023, 24, 7504. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Fukasawa, M.; Shirasago, Y.; Suzuki, R.; Osada, N.; Yamaji, T.; Wakita, T.; Konishi, E.; Hanada, K. Comparative Characterization of Flavivirus Production in Two Cell Lines: Human Hepatoma-Derived Huh7.5.1-8 and African Green Monkey Kidney-Derived Vero. PLoS ONE 2020, 15, e0232274. [Google Scholar] [CrossRef] [PubMed]
- Mahid, M.B.A.; Bist, P.; Sigmundsson, K.; Mazlan, M.D.B.M.; Watanabe, S.; Choy, M.M.; Vasudevan, S.G.; Chan, K.W.K. An Improved Focus-Forming Assay for Determination of the Dengue Virus Titer. Bio-Protocol 2024, 14, e5084. [Google Scholar] [CrossRef] [PubMed]
- Neupane, B.; Bai, F. Quantification of West Nile Virus by Plaque-Forming Assay. Methods Mol. Biol. 2023, 2585, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Fitzmeyer, E.A.; Williams, L.; Spangler, M.C.; Bosco-Lauth, A.M.; Ebel, G.D. WNV and SLEV Coinfection in Avian and Mosquito Hosts: Impact on Viremia, Antibody Responses, and Vector Competence. J. Virol. 2024, 98, e01041-24. [Google Scholar] [CrossRef] [PubMed]
- Baer, A.; Kehn-Hall, K. Viral Concentration Determination Through Plaque Assays: Using Traditional and Novel Overlay Systems. J. Vis. Exp. JoVE 2014, 93, 52065. [Google Scholar] [CrossRef]
| Overlay Condition | Concentration |
|---|---|
| Methylcellulose | 0.2%, 0.4% or 0.8% |
| Low melting Agarose |
| BHK | Vero | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain BeAn 327600 | ||||||||||||
| PFU | FFU | PFU | FFU | |||||||||
| 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | |
| 3 d.p.i. | 1.10–2.58 | 0.87–2.53 | 0.56–2.50 | 1.43–2.93 | 1.66–2.95 | 1.45–3.22 | n/o | n/o | n/o | n/o | n/o | n/o |
| 4 d.p.i. | n/m | 1.61–5.14 | 0.75–3.14 | n/m | 2.27–4.85 | 1.41–3.44 | n/o | n/o | n/o | 0.29–0.49 | 0.26–0.43 | 0.22–1.03 |
| 6 d.p.i. | n/m | n/m | n/m | n/m | n/m | n/m | n/o | n/o | n/o | 0.36–5.23 | n/m | n/m |
| Strain RP 962768 | ||||||||||||
| PFU | FFU | PFU | FFU | |||||||||
| 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | |
| 3 d.p.i. | 0.77–2.57 | 1.17–2.21 | 0.54–2.18 | 1.51–4.31 | 1.35–3.32 | 1.84–3.03 | n/o | n/o | n/o | n/o | n/o | n/o |
| 4 d.p.i. | n/m | 1.44–3.95 | 1.43–3.58 | n/m | 1.60–4.30 | 1.40–5.27 | n/o | n/o | n/o | 0.36–3.28 | 0.26–0.58 | 0.22–0.36 |
| 6 d.p.i. | n/m | n/m | n/m | n/m | n/m | n/m | n/o | n/o | n/o | 3.35–4.70 | n/m | n/m |
| BHK | Vero | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain BeAn 327600 | ||||||||||||
| PFU | FFU | PFU | FFU | |||||||||
| 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | |
| 3 d.p.i. | 1.53–3.87 | 1.57–3.29 | 1.44–2.23 | 1.83–5.31 | 1.94–3.45 | n/m | n/o | n/o | n/o | n/o | n/o | n/o |
| 4 d.p.i. | 1.64–5.69 | 1.74–4.72 | 1.82–4.00 | 2.91–5.57 | 2.51–5.57 | n/m | n/o | n/o | n/o | 0.52–3.80 | 0.46–3.71 | n/m |
| 6 d.p.i. | 4.48–5.70 | 1.71–4.92 | n/m | n/m | n/m | n/m | n/o | n/o | n/o | 0.52–4.11 | 0.46–4.41 | n/m |
| Strain RP 962768 | ||||||||||||
| PFU | FFU | PFU | FFU | |||||||||
| 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | 0.2% | 0.4% | 0.8% | |
| 3 d.p.i. | 0.95–3.05 | 1.01–3.40 | 1.52–2.51 | 2.07–4.30 | 1.67–3.01 | n/m | n/o | n/o | n/o | n/o | n/o | n/o |
| 4 d.p.i. | 0.83–5.28 | 1.71–4.24 | 1.05–3.58 | 3.56–5.12 | 2.10–4.78 | n/m | n/o | n/o | n/o | 1.95–4.97 | 0.75–5.48 | n/m |
| 6 d.p.i. | 1.43–4.48 | 1.25–4.34 | n/m | n/m | n/m | n/m | n/o | n/o | n/o | 1.95–4.22 | 0.77–5.30 | n/m |
| Overlay Matrix | Concentration (%) | Cell Line | D.P.I. | PFU Assay Rating * | FFU Assay Rating * |
|---|---|---|---|---|---|
| Agarose | 0.2 | BHK | 3 | +++ | ++ |
| 4 | +++ | ++ | |||
| 6 | ++ | ns | |||
| 0.4 | 3 | +++ | ++ | ||
| 4 | +++ | ++ | |||
| 6 | ++ | ns | |||
| 0.8 | 3 | ns | ns | ||
| 4 | ns | ns | |||
| 6 | ns | ns | |||
| Methylcellulose | 0.2 | 3 | ++ | + | |
| 4 | ns | ns | |||
| 6 | ns | ns | |||
| 0.4 | 3 | +++ | ++ | ||
| 4 | ++ | + | |||
| 6 | ns | ns | |||
| 0.8 | 3 | +++ | ++ | ||
| 4 | +++ | ++ | |||
| 6 | ns | ns | |||
| Agarose | 0.2 | Vero | 3 | ns | ns |
| 4 | ns | + | |||
| 6 | ns | +++ | |||
| 0.4 | 3 | ns | ns | ||
| 4 | ns | + | |||
| 6 | ns | +++ | |||
| 0.8 | 3 | ns | ns | ||
| 4 | ns | + | |||
| 6 | ns | +++ | |||
| Methylcellulose | 0.2 | 3 | ns | ns | |
| 4 | ns | + | |||
| 6 | ns | + | |||
| 0.4 | 3 | ns | ns | ||
| 4 | ns | + | |||
| 6 | ns | ns | |||
| 0.8 | 3 | ns | ns | ||
| 4 | ns | + | |||
| 6 | ns | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saivish, M.V.; da Silva, N.I.O.; Steck, M.R.; Marques, R.E.; Nogueira, M.L.; Rossi, S.L.; Vasilakis, N. Standardization of Quantitative Plaque-Based Viral Assays for Orthoflavivirus Cacipacoré. Viruses 2025, 17, 1355. https://doi.org/10.3390/v17101355
Saivish MV, da Silva NIO, Steck MR, Marques RE, Nogueira ML, Rossi SL, Vasilakis N. Standardization of Quantitative Plaque-Based Viral Assays for Orthoflavivirus Cacipacoré. Viruses. 2025; 17(10):1355. https://doi.org/10.3390/v17101355
Chicago/Turabian StyleSaivish, Marielena Vogel, Natalia I. O. da Silva, Madeline R. Steck, Rafael E. Marques, Mauricio L. Nogueira, Shannan L. Rossi, and Nikos Vasilakis. 2025. "Standardization of Quantitative Plaque-Based Viral Assays for Orthoflavivirus Cacipacoré" Viruses 17, no. 10: 1355. https://doi.org/10.3390/v17101355
APA StyleSaivish, M. V., da Silva, N. I. O., Steck, M. R., Marques, R. E., Nogueira, M. L., Rossi, S. L., & Vasilakis, N. (2025). Standardization of Quantitative Plaque-Based Viral Assays for Orthoflavivirus Cacipacoré. Viruses, 17(10), 1355. https://doi.org/10.3390/v17101355









