Global Burden of Bloodstream Infections in COVID-19: Prevalence, Antimicrobial Resistance, and Mortality Risk
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Analysis
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
3. Results
3.1. Overview of Selected Studies
3.2. Prevalence of Bloodstream Infections (BSIs)
3.3. Microbiological Profile
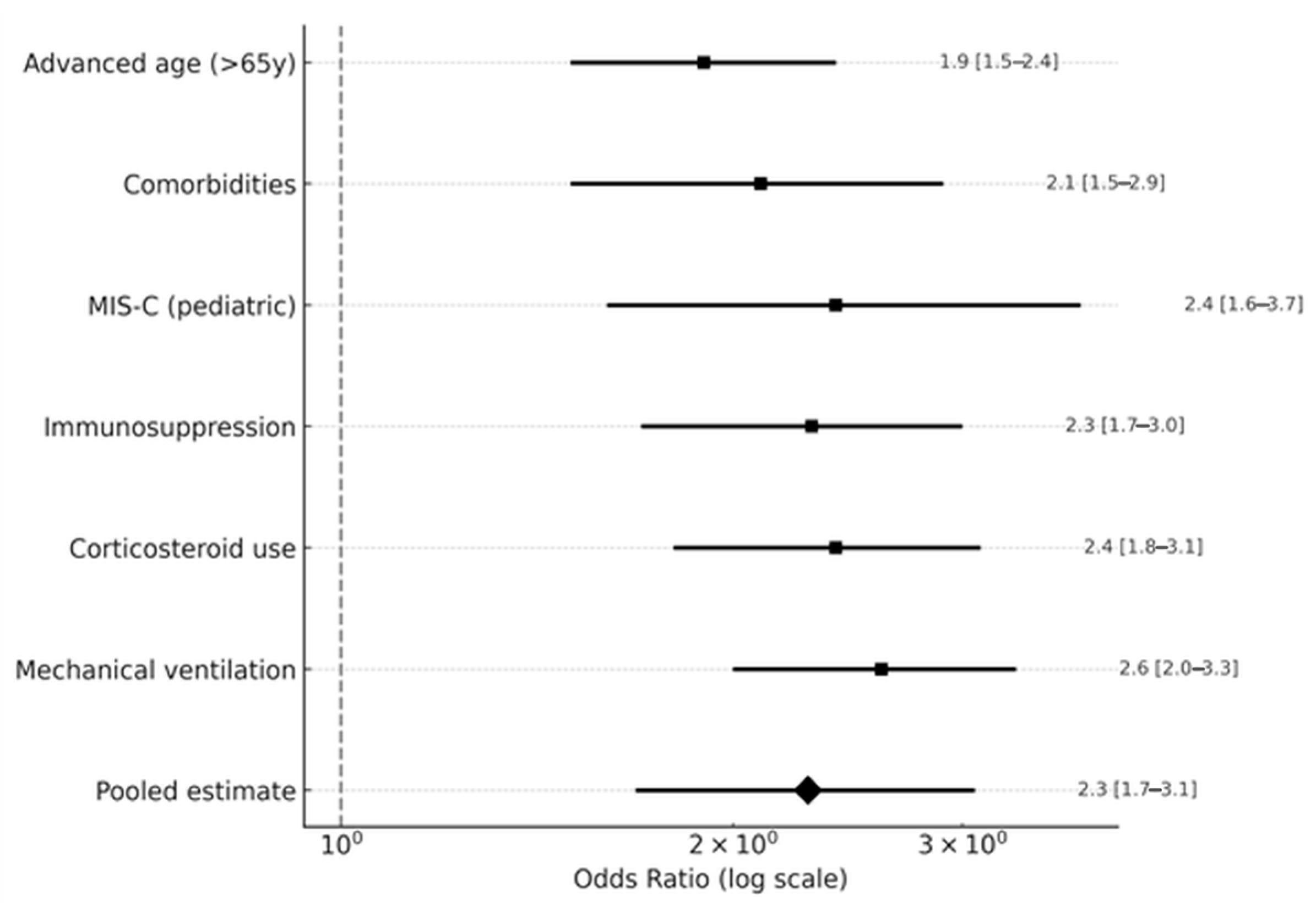
3.4. Risk Factors
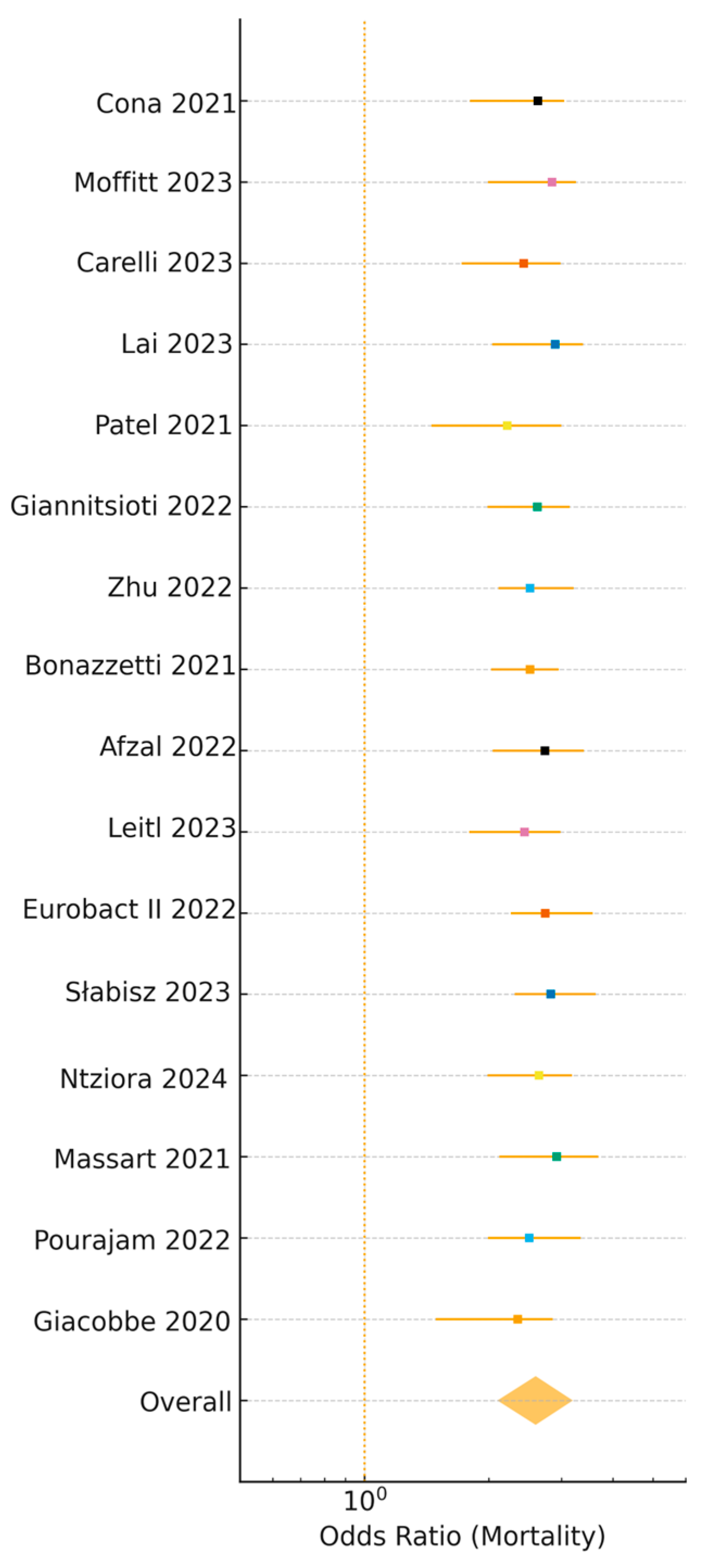
3.5. Clinical Outcomes
3.6. Study Quality
4. Discussion
4.1. Comparison with Previous Reviews
4.2. Context of Antimicrobial Resistance
4.3. Heterogeneity in BSI Prevalence
4.4. Risk Factors and Clinical Implications
4.5. Strengths and Limitations
4.6. Implications for Practice and Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Term |
| AMR | Antimicrobial Resistance |
| BSI | Bloodstream Infection |
| CDC | Centers for Disease Control and Prevention |
| CI | Confidence Interval |
| CLABSI | Central Line-Associated Bloodstream Infection |
| CoNS | Coagulase-Negative Staphylococci |
| COVID-19 | Coronavirus Disease 2019 |
| ESBL | Extended-Spectrum Beta-Lactamase |
| ICU | Intensive Care Unit |
| MDRO | Multidrug-Resistant Organism |
| MIS-C | Multisystem Inflammatory Syndrome in Children |
| MRSA | Methicillin-Resistant Staphylococcus aureus |
| NOS | Newcastle–Ottawa Scale |
| OR | Odds Ratio |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RT-PCR | Reverse Transcription Polymerase Chain Reaction |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| WHO | World Health Organization |
References
- Chu, H.; Chan, J.F.-W.; Yuen, T.T.-T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef]
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72,314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239–1242. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Battaglini, D.; Ball, L.; Brunetti, I.; Bruzzone, B.; Codda, G.; Crea, F.; De Maria, A.; Dentone, C.; Di Biagio, A.; et al. Bloodstream Infections in Critically Ill Patients with COVID-19. Eur. J. Clin. Investig. 2020, 50, e13319. [Google Scholar] [CrossRef]
- Pourajam, S.; Kalantari, E.; Talebzadeh, H.; Mellali, H.; Sami, R.; Soltaninejad, F.; Amra, B.; Sajadi, M.; Alenaseri, M.; Kalantari, F.; et al. Secondary Bacterial Infection in COVID-19 Patients. Front. Cell. Infect. Microbiol. 2022, 12, 784130. [Google Scholar] [CrossRef]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I.; et al. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Massart, N.; Maxime, V.; Fillatre, P.; Razazi, K.; Ferré, A.; Moine, P.; Legay, F.; Voiriot, G.; Amara, M.; Santi, F.; et al. Characteristics and Prognosis of Bloodstream Infection in Patients with COVID-19 Admitted in the ICU: An Ancillary Study of the COVIDICU Study. Ann. Intensive Care 2021, 11, 183. [Google Scholar] [CrossRef]
- Shukla, B.S.; Warde, P.R.; Knott, E.; Arenas, S.; Pronty, D.; Ramirez, R.; Rego, A.; Jimenez, G.S.; Larson, E.L.; Pereira, M.R. Bloodstream Infection Risk, Incidence, and Deaths for Adults Hospitalized with COVID-19. Emerg. Infect. Dis. 2021, 27, 2551–2558. [Google Scholar] [CrossRef] [PubMed]
- Ntziora, F.; Giannitsioti, E. Bloodstream infections in the era of the COVID-19 pandemic: Changing epidemiology of antimicrobial resistance in the intensive care unit. J. Intensive Med. 2024, 4, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Sleziak, J.; Błażejewska, M.; Duszyńska, W. Central Line-Associated Bloodstream Infections in Intensive Care Unit During and After the COVID-19 Pandemic, 5-Year Prospective Observational Study. J. Clin. Med. 2025, 14, 5655. [Google Scholar] [CrossRef] [PubMed]
- Słabisz, N.; Dudek-Wicher, R.; Leśnik, P.; Majda, J.; Kujawa, K.; Nawrot, U. Impact of the COVID-19 Pandemic on the Epidemiology of Bloodstream Infections in Hospitalized Patients—Experience from a 4th Military Clinical Hospital in Poland. J. Clin. Med. 2023, 12, 5942. [Google Scholar] [CrossRef]
- Buetti, N.; Tabah, A.; Loiodice, A.; Ruckly, S.; Aslan, A.T.; Montrucchio, G.; Cortegiani, A.; Saltoglu, N.; Kayaaslan, B.; Aksoy, F.; et al. Different epidemiology of bloodstream infections in COVID-19 compared to non-COVID-19 critically ill patients: A descriptive analysis of the Eurobact II study. Crit. Care 2022, 26, 319. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Leitl, C.J.; Stoll, S.E.; Wetsch, W.A.; Kammerer, T.; Mathes, A.; Böttiger, B.W.; Seifert, H.; Dusse, F. Next-Generation Sequencing in Critically Ill COVID-19 Patients with Suspected Bloodstream Infections. J. Clin. Med. 2023, 12, 1466. [Google Scholar] [CrossRef] [PubMed]
- Carelli, S.; Dell’Anna, A.M.; Montini, L.; Bernardi, G.; Gozza, M.; Cutuli, S.L.; Natalini, D.; Bongiovanni, F.; Tanzarella, E.S.; Pintaudi, G.; et al. Bloodstream infections in COVID-19 patients undergoing extracorporeal membrane oxygenation in ICU: An observational cohort study. Heart Lung 2023, 62, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Montrucchio, G.; Grillo, F.; Balzani, E.; Gavanna, G.; Sales, G.; Bonetto, C.; Simonetti, U.; Zanierato, M.; Fanelli, V.; Filippini, C.; et al. Impact of Multidrug-Resistant Bacteria in a Cohort of COVID-19 Critically Ill Patients: Data from a Prospective Observational Study Conducted in a High-Antimicrobial-Resistance-Prevalence Center. J. Clin. Med. 2025, 14, 410. [Google Scholar] [CrossRef]
- Afzal, A.; Perez Gutierrez, V.; Gomez, E.; Mon, A.M.; Moreira Sarmiento, C.; Khalid, A.; Polishchuk, S.; Al-Khateeb, M.; Yankulova, B.; Yusuf, M.; et al. Bloodstream infections in hospitalized patients before and during the COVID-19 surge in a community hospital in the South Bronx. Int. J. Infect. Dis. 2022, 116, 43–46. [Google Scholar] [CrossRef]
- Bonazzetti, C.; Morena, V.; Giacomelli, A.; Oreni, L.; Casalini, G.; Galimberti, L.R.; Bolis, M.; Rimoldi, M.; Ballone, E.; Colombo, R.; et al. Unexpectedly high frequency of enterococcal bloodstream infections in coronavirus disease 2019 patients admitted to an Italian ICU: An observational study. Crit. Care Med. 2021, 49, e31–e40. [Google Scholar] [CrossRef]
- Zhu, N.J.; Rawson, T.M.; Mookerjee, S.; Price, J.R.; Davies, F.; Otter, J.; Aylin, P.; Hope, R.; Gilchrist, M.; Shersing, Y.; et al. Changing patterns of bloodstream infections in the community and acute care across two COVID-19 epidemic waves: A retrospective analysis using data linkage. Clin. Infect. Dis. 2022, 75, e1082–e1091. [Google Scholar] [CrossRef]
- Driedger, M.; Daneman, N.; Brown, K.; Gold, W.L.; Jorgensen, S.C.J.; Maxwell, C.; Schwartz, K.L.; Morris, A.M.; Thiruchelvam, D.; Langford, B.; et al. The impact of the COVID-19 pandemic on blood culture practices and bloodstream infections. Microbiol. Spectr. 2023, 11, e0263023. [Google Scholar] [CrossRef]
- Santos, C.V.; Fukushima, E.A.; Zhao, W.; Sharma, M.; Youssef, D.; Spzunar, S.; Levine, M.; Saravolatz, L.; Bhargava, A. Incidence of bloodstream infections in patients with COVID-19: A retrospective cohort study of risk factors and outcomes. Germs 2022, 12, 253–261. [Google Scholar] [CrossRef]
- Giannitsioti, E.; Louka, C.; Mamali, V.; Kousouli, E.; Velentza, L.; Papadouli, V.; Loizos, G.; Mavroudis, P.; Kranidiotis, G.; Rekleiti, N.; et al. Bloodstream Infections in a COVID-19 Non-ICU Department: Microbial Epidemiology, Resistance Profiles and Comparative Analysis of Risk Factors and Patients’ Outcome. Microorganisms 2022, 10, 1314. [Google Scholar] [CrossRef]
- Fallah, F.; Karimi, A.; Azimi, L.; Ghandchi, G.; Gholinejad, Z.; Abdollahi, N.; AhariOskooie, N.; Khodaei, H.; Armin, S.; Behzad, A.; et al. The impact of the COVID-19 pandemic on pediatric bloodstream infections and alteration in antimicrobial resistance phenotypes in Gram-positive bacteria, 2020–2022. BMC Pediatr. 2024, 24, 671. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.R.; Weiner-Lastinger, L.M.; Dudeck, M.A.; Fike, L.V.; Kuhar, D.T.; Edwards, J.R.; Pollock, D.; Benin, A. Impact of COVID-19 pandemic on central-line-associated bloodstream infections during the early months of 2020, National Healthcare Safety Network. Infect. Control Hosp. Epidemiol. 2022, 43, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Papic, I.; Bistrovic, P.; Cikara, T.; Busic, N.; Keres, T.; Ortner Hadziabdic, M.; Lucijanic, M. Corticosteroid Dosing Level, Incidence and Profile of Bacterial Blood Stream Infections in Hospitalized COVID-19 Patients. Viruses 2024, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Zanella, M.-C.; Pianca, E.; Catho, G.; Obama, B.; DeKraker, M.E.A.; Nguyen, A.; Chraiti, M.-N.; Sobel, J.; Fortchantre, L.; Harbarth, S.; et al. Increased Peripheral Venous Catheter Bloodstream Infections during COVID-19 Pandemic, Switzerland. Emerg. Infect. Dis. 2024, 30, 159–162. [Google Scholar] [CrossRef]
- Lai, H.C.; Hsu, Y.L.; Lin, C.H.; Wei, H.M.; Chen, J.A.; Low, Y.Y.; Chiu, Y.T.; Lin, H.C.; Hwang, K.P. Bacterial coinfections in hospitalized children with COVID-19 during the SARS-CoV-2 Omicron BA.2 variant pandemic in Taiwan. Front. Med. 2023, 10, 1178041. [Google Scholar] [CrossRef]
- Moffitt, K.L.; Nakamura, M.M.; Young, C.C.; Newhams, M.M.; Halasa, N.B.; Reed, J.N.; Fitzgerald, J.C.; Spinella, P.C.; Soma, V.L.; Walker, T.C.; et al. Community-Onset Bacterial Coinfection in Children Critically Ill with Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Open Forum Infect. Dis. 2023, 10, ofad122. [Google Scholar] [CrossRef]
- Cona, A.; Tavelli, A.; Renzelli, A.; Varisco, B.; Bai, F.; Tesoro, D.; Za, A.; Biassoni, C.; Battaglioli, L.; Allegrini, M.; et al. Incidence, Risk Factors and Impact on Clinical Outcomes of Bloodstream Infections in Patients Hospitalised with COVID-19: A Prospective Cohort Study. Antibiotics 2021, 10, 1031. [Google Scholar] [CrossRef]
- Ippolito, M.; Simone, B.; Filisina, C.; Catalanotto, F.R.; Catalisano, G.; Marino, C.; Misseri, G.; Giarratano, A.; Cortegiani, A. Bloodstream Infections in Hospitalized Patients with COVID-19: A Systematic Review and Meta-Analysis. Microorganisms 2021, 9, 2016. [Google Scholar] [CrossRef]
- Damonti, L.; Gasser, M.; Kronenberg, A.; Buetti, N. Epidemiology of bloodstream infections caused by extended-spectrum cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae. J. Hosp. Infect. 2024, 150, 145–152. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report 2022; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240062702 (accessed on 29 August 2025).
- World Health Organization. Global Action Plan on Antimicrobial Resistance; WHO: Geneva, Switzerland, 2015; Available online: https://www.who.int/publications/i/item/9789241509763 (accessed on 29 August 2025).
- European Centre for Disease Prevention and Control. Antimicrobial Resistance in the EU/EEA (EARS-Net)—Annual Epidemiological Report 2023; ECDC: Stockholm, Sweden, 2023. Available online: https://www.ecdc.europa.eu/en/publications-data/antimicrobial-resistance-eueea-ears-net-annual-epidemiological-report-2023 (accessed on 29 August 2025).
- Xiao, S.; Liang, X.; Han, L.; Zhao, S. Incidence, antimicrobial resistance and mortality of Pseudomonas aeruginosa bloodstream infections among hospitalized patients in China: A retrospective observational multicenter cohort study from 2017 to 2021. Front. Public Health 2024, 11, 1294141. [Google Scholar] [CrossRef]
- Srisurapanont, K.; Lerttiendamrong, B.; Meejun, T.; Thanakitcharu, J.; Manothummetha, K.; Thongkam, A.; Chuleerarux, N.; Sanguankeo, A.; Li, L.X.; Leksuwankun, S.; et al. Candidemia Following Severe COVID-19 in Hospitalised and Critical Ill Patients: A Systematic Review and Meta-Analysis. Mycoses 2024, 67, e13798. [Google Scholar] [CrossRef]
- Lob, S.H.; Hoban, D.J.; Sahm, D.F.; Badal, R.E. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int. J. Antimicrob. Agents 2016, 47, 317–323. [Google Scholar] [CrossRef]
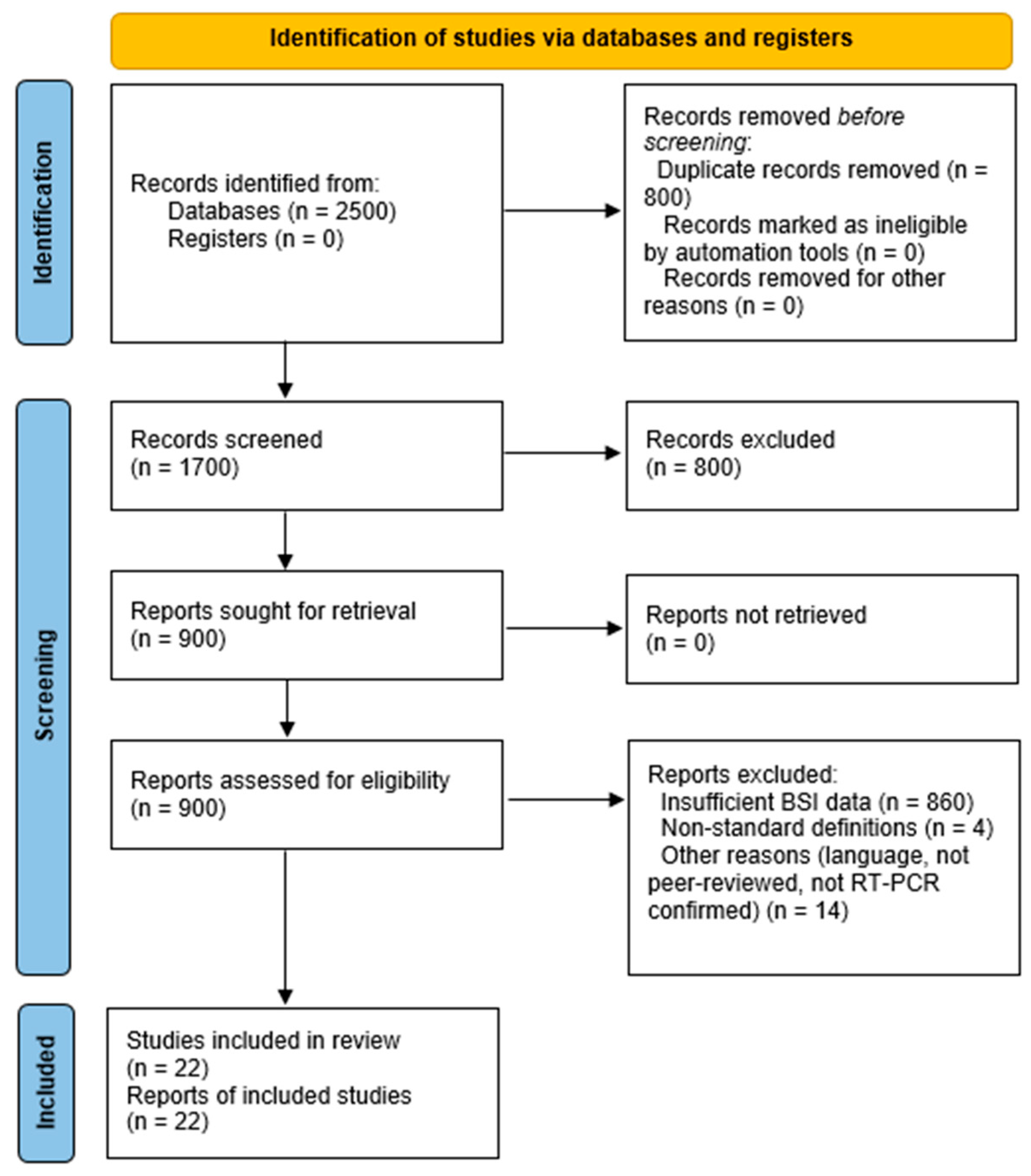



| Outcome | Pooled Estimate | 95% CI | Prediction Interval | p-Value | I2 (%) | Studies |
|---|---|---|---|---|---|---|
| Prevalence Outcomes | ||||||
| BSI Prevalence (Overall) | 8.2% | 5.7–11.0 | 3.0–15.5 | <0.001 | 50 | 22 [3,4,6,7,8,10,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] |
| BSI Prevalence (ICU) | 12.5% | 9.0–16.5 | 5.5–21.0 | <0.001 | 42 | 12 [3,6,7,8,10,11,13,14,15,17,23,24] |
| BSI Prevalence (Non-ICU) | 5.2% | 3.4–7.4 | 2.2–10.5 | <0.001 | 47 | 7 [4,16,18,19,21,26,28] |
| BSI Prevalence (Pediatric) | 10.8% | 6.5–15.5 | 4.5–18.5 | <0.001 | 40 | 3 [19,20,22] |
| Risk Factor Odds Ratios | ||||||
| Mortality (OR) | 2.6 | 2.1–3.2 | 1.6–4.1 | <0.001 | 47 | 16 [3,4,6,8,10,11,13,16,18,19,21,23,24,26,27,28] |
| Mechanical Ventilation (OR) | 2.6 | 2.0–3.3 | 1.6–4.1 | <0.001 | 52 | 10 [3,6,7,11,17,20,23,26,27,28] |
| Immunosuppression (OR) | 2.3 | 1.7–3.0 | 1.4–3.6 | <0.001 | 57 | 6 [3,6,17,20,23,24] |
| MIS-C (Pediatric, OR) | 2.4 | 1.6–3.7 | 1.1–5.1 | <0.001 | 42 | 3 [19,20,22] |
| Comorbidities (OR) | 2.1 | 1.5–2.9 | 1.2–3.7 | <0.001 | 52 | 7 [6,7,11,19,20,22,28] |
| Clinical Outcome Measures | ||||||
| Prolonged Hospitalization (MD, days) | 6.8 | 4.8–8.8 | 3.2–10.5 | <0.001 | 47 | 6 [6,7,11,20,22,28] |
| ICU Admission (OR) | 3.1 | 2.4–4.0 | 1.8–5.4 | <0.001 | 52 | 6 [6,7,11,20,22,28] |
| Pathogen | Pooled Proportion (%) | 95% CI | Number of Isolates | Studies | Notes |
|---|---|---|---|---|---|
| Klebsiella pneumoniae | 26 | 21–31 | 1560 | 18 [3,4,6,7,8,10,11,13,14,15,16,17,18,20,21,23,24,26,27,28] | Predominant Gram-negative pathogen. |
| Acinetobacter baumannii | 21 | 16–26 | 1260 | 18 [3,4,6,7,8,10,11,13,14,15,16,17,18,20,21,23,24,26,27,28] | Higher prevalence in ICUs; geographical variation noted. |
| Enterococcus spp. | 18 | 15.5–20.5 | 1080 | 18 [3,4,6,7,8,10,11,13,14,15,16,17,18,20,21,23,24,26,27,28] | Common in ECMO cohorts. |
| Staphylococcus aureus | 13 | 10.5–15.5 | 780 | 18 [3,4,6,7,8,10,11,13,14,15,16,17,18,20,21,23,24,26,27,28] | MRSA rate: 36% (95% CI: 29–43). |
| Coagulase-negative staphylococci (CoNS) | 18 | Not reported | 1080 | 18 [3,4,6,7,8,10,11,13,14,15,16,17,18,20,21,23,24,26,27,28] | Potential contaminants; requires clinical correlation. |
| Pseudomonas aeruginosa | 5 | 3–8 | 300 | 10 [3,4,6,8,10,16,20,26,27,28] | Geographical variation noted. |
| Escherichia coli | 4 | 2–7 | 240 | 10 [3,4,6,8,10,16,20,26,27,28] | ESBL rate: 31% (95% CI: 25–37). |
| Candida spp. | 2 | 1–4 | 120 | 5 [8,10,26,27,28] | Limited data; increased in severe COVID-19. |
| Other pathogens * | 22 | Not reported | 1320 | 18 [3,4,6,7,8,10,11,13,14,15,16,17,18,20,21,23,24,26,27,28] | Includes Enterobacter spp., Proteus spp., and other rare isolates. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateescu, D.-M.; Ilie, A.-C.; Cotet, I.; Guse, C.; Muresan, C.-O.; Pah, A.-M.; Badalica-Petrescu, M.; Iurciuc, S.; Craciun, M.-L.; Avram, A.; et al. Global Burden of Bloodstream Infections in COVID-19: Prevalence, Antimicrobial Resistance, and Mortality Risk. Viruses 2025, 17, 1353. https://doi.org/10.3390/v17101353
Mateescu D-M, Ilie A-C, Cotet I, Guse C, Muresan C-O, Pah A-M, Badalica-Petrescu M, Iurciuc S, Craciun M-L, Avram A, et al. Global Burden of Bloodstream Infections in COVID-19: Prevalence, Antimicrobial Resistance, and Mortality Risk. Viruses. 2025; 17(10):1353. https://doi.org/10.3390/v17101353
Chicago/Turabian StyleMateescu, Diana-Maria, Adrian-Cosmin Ilie, Ioana Cotet, Cristina Guse, Camelia-Oana Muresan, Ana-Maria Pah, Marius Badalica-Petrescu, Stela Iurciuc, Maria-Laura Craciun, Adina Avram, and et al. 2025. "Global Burden of Bloodstream Infections in COVID-19: Prevalence, Antimicrobial Resistance, and Mortality Risk" Viruses 17, no. 10: 1353. https://doi.org/10.3390/v17101353
APA StyleMateescu, D.-M., Ilie, A.-C., Cotet, I., Guse, C., Muresan, C.-O., Pah, A.-M., Badalica-Petrescu, M., Iurciuc, S., Craciun, M.-L., Avram, A., & Enache, A. (2025). Global Burden of Bloodstream Infections in COVID-19: Prevalence, Antimicrobial Resistance, and Mortality Risk. Viruses, 17(10), 1353. https://doi.org/10.3390/v17101353






