Role of Unfolded Protein Response in the Apoptosis Induced by Alphaarterivirus: IRE1α as an Essential Pathway for In Vitro Replication
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture, Virus and Reagents
2.2. Viral Stock and Infection Experiments
2.3. RNA Extraction and Reverse Transcription Assay
2.4. qPCR Assay
2.5. PCR Assay
2.6. Western Blotting Analysis
2.7. UPR Inhibition and Viral Replication
2.8. Statistical Analysis
3. Results
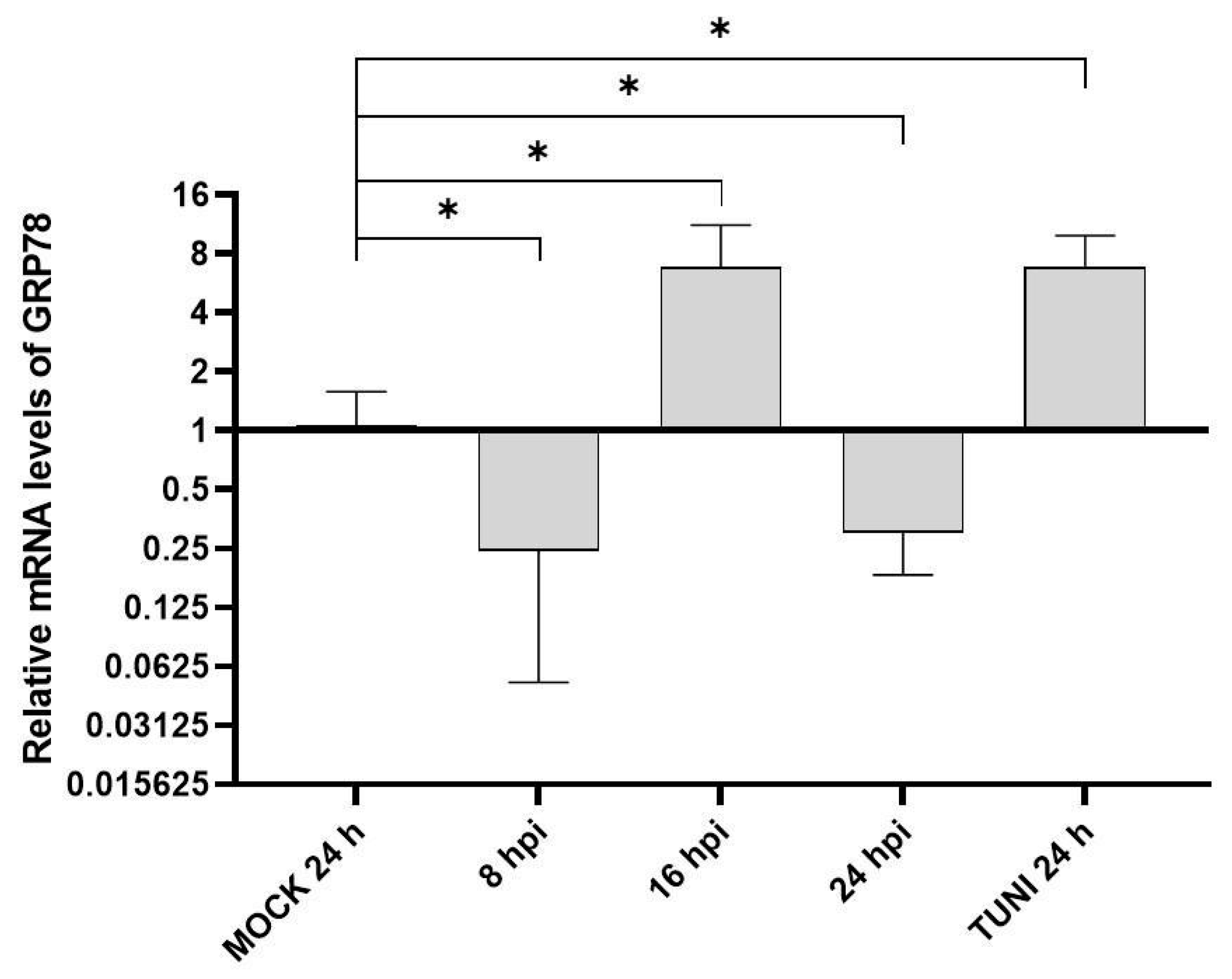
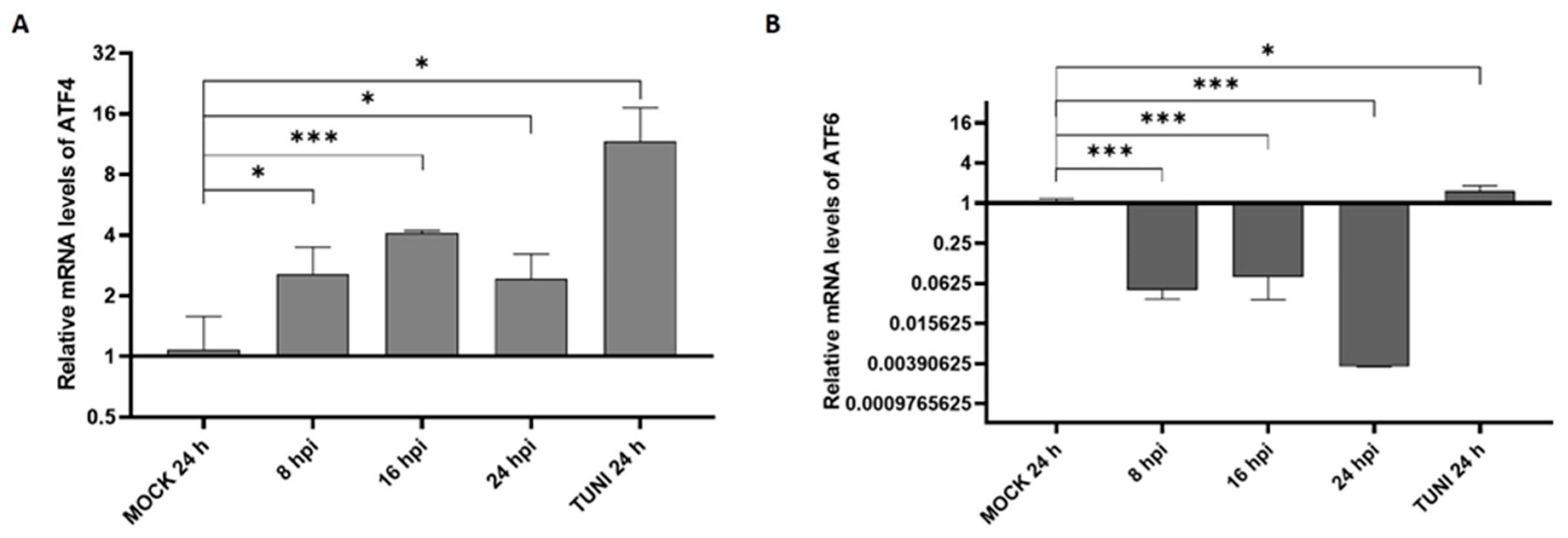
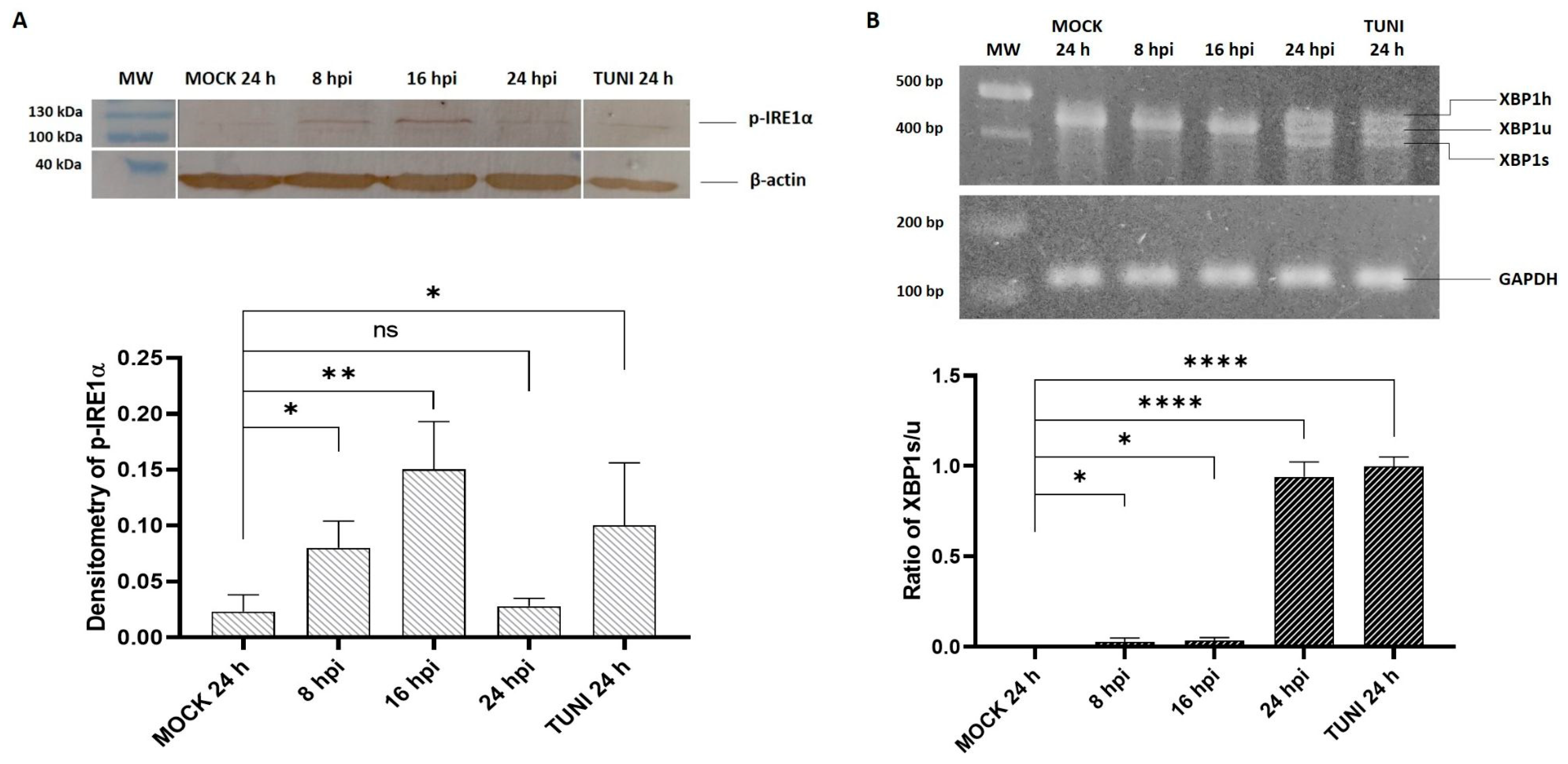
3.1. ER Stress Response Following EAV Infection
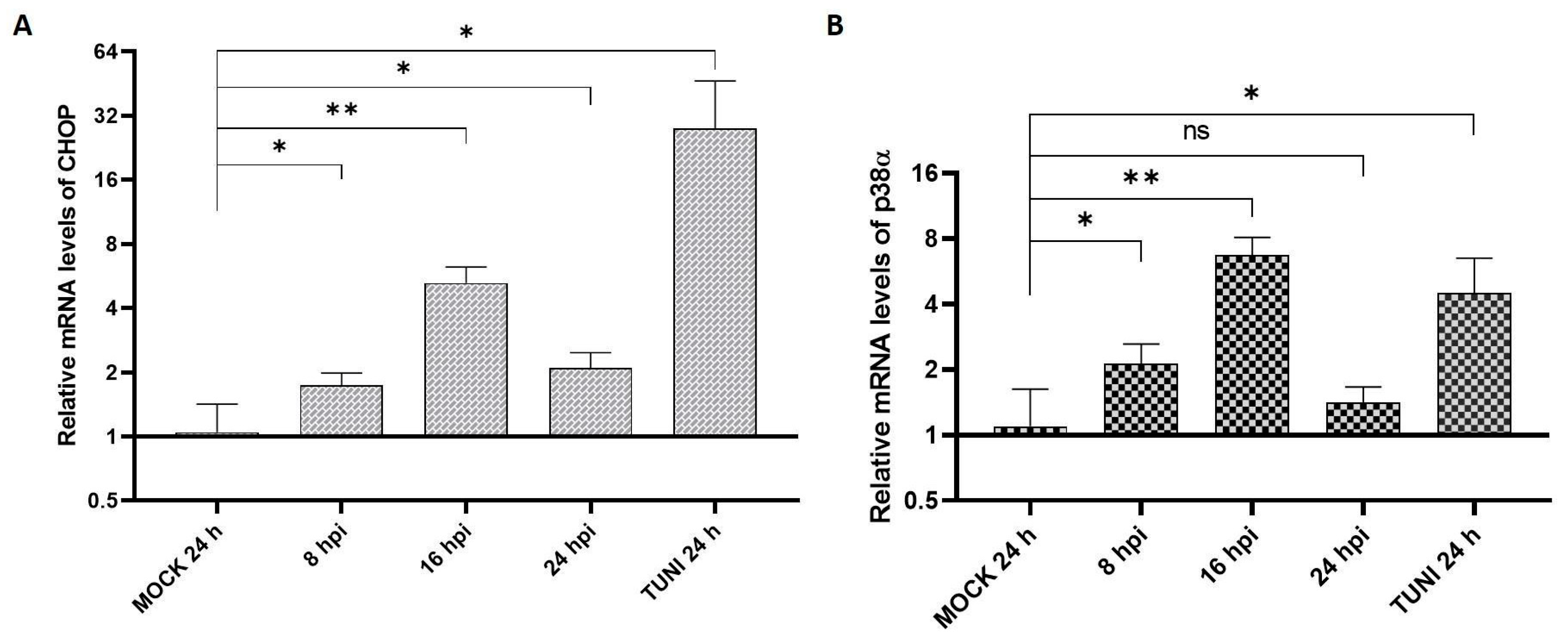
3.2. UPR Modulation
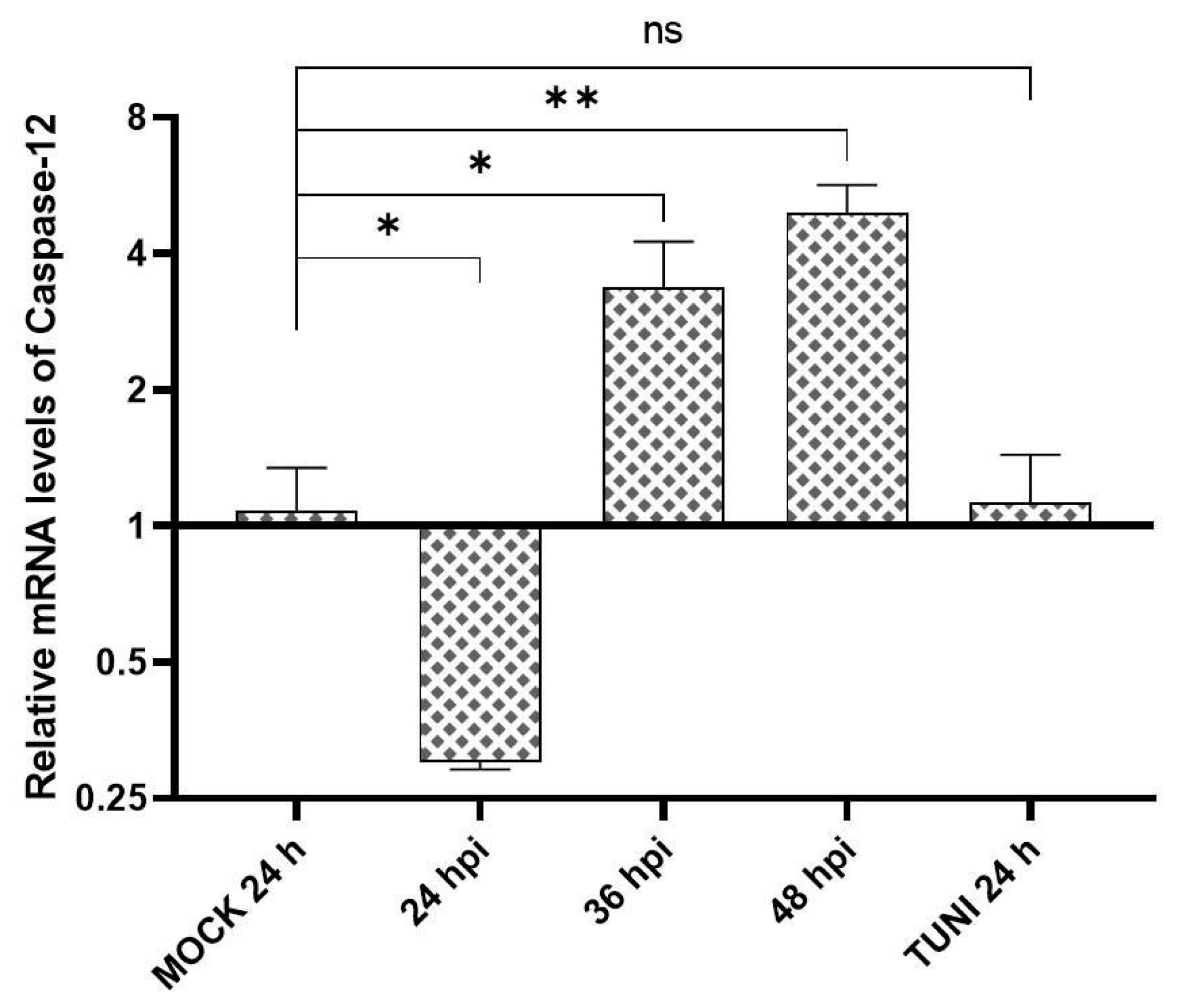
3.3. Apoptosis Indicators in EAV Infected Cells
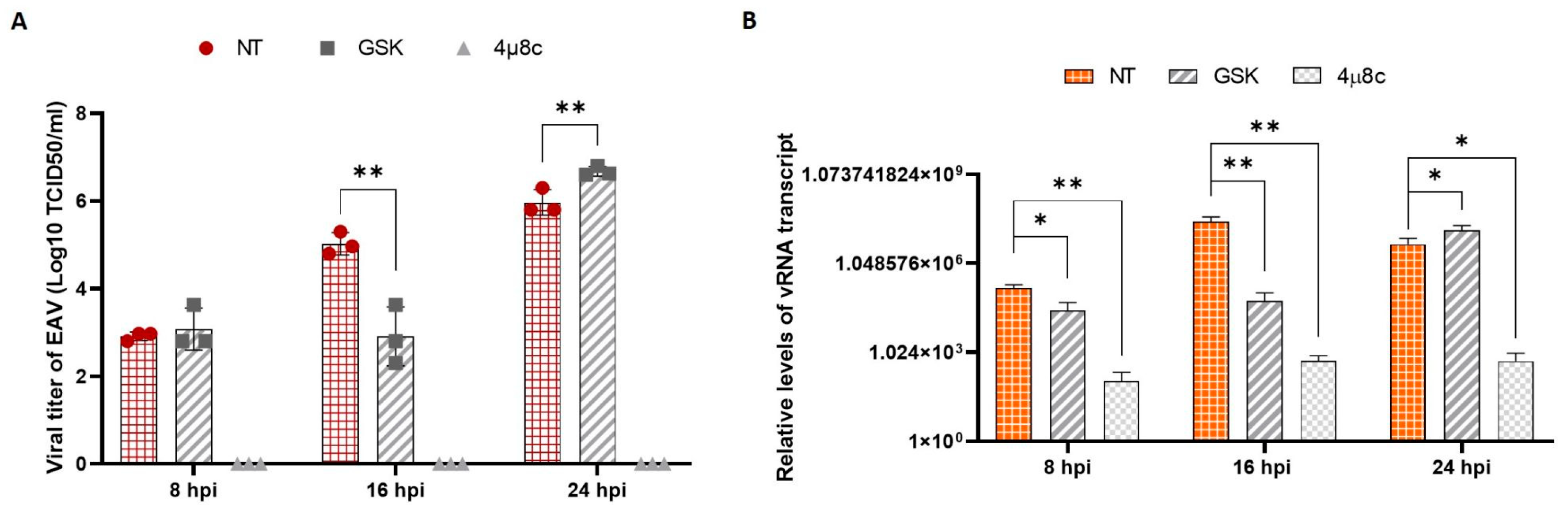
3.4. EAV Replication and UPR Inhibition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balasuriya, U.B.; Go, Y.Y.; MacLachlan, N.J. Equine arteritis virus. Vet. Microbiol. 2013, 167, 93–122. [Google Scholar] [CrossRef]
- Cavanagh, D. Nidovirales: A new order comprising Coronaviridae and Arteriviridae. Arch. Virol. 1997, 142, 629–633. [Google Scholar] [PubMed]
- Glaser, A.L.; Chirnside, E.D.; Horzinek, M.C.; de Vries, A.A. Equine arteritis virus. Theriogenology 1997, 47, 1275–1295. [Google Scholar] [CrossRef] [PubMed]
- van der Hoeven, B.; Oudshoorn, D.; Koster, A.J.; Snijder, E.J.; Kikkert, M.; Bárcena, M. Biogenesis and architecture of arterivirus replication organelles. Virus Res. 2016, 220, 70–90. [Google Scholar] [CrossRef]
- He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 2006, 13, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef]
- Szegezdi, E.; Logue, S.E.; Gorman, A.M.; Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006, 7, 880–885. [Google Scholar] [CrossRef]
- Wang, S.; Binder, P.; Fang, Q.; Wang, Z.; Xiao, W.; Liu, W.; Wang, X. Endoplasmic reticulum stress in the heart: Insights into mechanisms and drug targets. Br. J. Pharmacol. 2018, 175, 1293–1304. [Google Scholar] [CrossRef]
- Szegezdi, E.; Fitzgerald, U.; Samali, A. Caspase-12 and ER-stress-mediated apoptosis: The story so far. Ann. N. Y. Acad. Sci. 2003, 1010, 186–194. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, H. Novel Insight into the Role of Endoplasmic Reticulum Stress in the Pathogenesis of Myocardial Ischemia-Reperfusion Injury. Oxid Med. Cell. Longev. 2021, 2021, 5529810. [Google Scholar] [CrossRef]
- Read, A.; Schröder, M. The Unfolded Protein Response: An Overview. Biology 2021, 10, 384. [Google Scholar] [CrossRef]
- Hetz, C.; Papa, F.R. The Unfolded Protein Response and Cell Fate Control. Mol. Cell 2018, 69, 169–181. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Ryno, L.M.; Genereux, J.C.; Moresco, J.J.; Tu, P.G.; Wu, C.; Yates, J.R., 3rd; Su, A.I.; Kelly, J.W.; Wiseman, R.L. Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 2013, 3, 1279–1292. [Google Scholar] [CrossRef]
- Huo, Y.; Fan, L.; Yin, S.; Dong, Y.; Guo, X.; Yang, H.; Hu, H. Involvement of unfolded protein response, p53 and Akt in modulation of porcine reproductive and respiratory syndrome virus-mediated JNK activation. Virology 2013, 444, 233–240. [Google Scholar] [CrossRef]
- Chen, W.Y.; Schniztlein, W.M.; Calzada-Nova, G.; Zuckermann, F.A. Genotype 2 Strains of Porcine Reproductive and Respiratory Syndrome Virus Dysregulate Alveolar Macrophage Cytokine Production via the Unfolded Protein Response. J. Virol. 2018, 92, e01251-17. [Google Scholar] [CrossRef]
- Metz, G.E.; Galindo, I.; Abeyá, M.M.; Echeverría, M.G.; Alonso, C. Intrinsic, extrinsic and endoplasmic reticulum stress-induced apoptosis in RK13 cells infected with equine arteritis virus. Virus Res. 2016, 213, 219–223. [Google Scholar] [CrossRef]
- Shang, J.; Lehrman, M.A. Discordance of UPR signaling by ATF6 and Ire1p-XBP1 with levels of target transcripts. Biochem. Biophys. Res. Commun. 2004, 317, 390–396. [Google Scholar] [CrossRef]
- Mehrbod, P.; Ande, S.R.; Alizadeh, J.; Rahimizadeh, S.; Shariati, A.; Malek, H.; Hashemi, M.; Glover, K.K.M.; Sher, A.A.; Coombs, K.M.; et al. The roles of apoptosis, autophagy and unfolded protein response in arbovirus, influenza virus, and HIV infections. Virulence 2019, 10, 376–413. [Google Scholar] [CrossRef]
- Jheng, J.R.; Ho, J.Y.; Horng, J.T. ER stress, autophagy, and RNA viruses. Front. Microbiol. 2014, 5, 388. [Google Scholar] [CrossRef]
- Chen, Q.; Men, Y.; Wang, D.; Xu, D.; Liu, S.; Xiao, S.; Fang, L. Porcine reproductive and respiratory syndrome virus infection induces endoplasmic reticulum stress, facilitates virus replication, and contributes to autophagy and apoptosis. Sci. Rep. 2020, 10, 13131. [Google Scholar] [CrossRef]
- Diao, F.; Jiang, C.; Sun, Y.; Gao, Y.; Bai, J.; Nauwynck, H.; Wang, X.; Yang, Y.; Jiang, P.; Liu, X. Porcine reproductive and respiratory syndrome virus infection triggers autophagy via ER stress-induced calcium signaling to facilitate virus replication. PLoS Pathog. 2023, 19, e1011295. [Google Scholar] [CrossRef]
- Gao, P.; Chai, Y.; Song, J.; Liu, T.; Chen, P.; Zhou, L.; Ge, X.; Guo, X.; Han, J.; Yang, H. Reprogramming the unfolded protein response for replication by porcine reproductive and respiratory syndrome virus. PLoS Pathog. 2019, 15, e1008169. [Google Scholar] [CrossRef]
- Echavarría-Consuegra, L.; Cook, G.M.; Busnadiego, I.; Lefèvre, C.; Keep, S.; Brown, K.; Doyle, N.; Dowgier, G.; Franaszek, K.; Moore, N.A.; et al. Manipulation of the unfolded protein response: A pharmacological strategy against coronavirus infection. PLoS Pathog. 2021, 17, e1009644. [Google Scholar] [CrossRef]
- Colina, S.E.; Williman, M.M.; Tizzano, M.A.; Serena, M.S.; Echeverría, M.G.; Metz, G.E. Morbillivirus Canis Infection Induces Activation of Three Branches of Unfolded Protein Response, MAPK and Apoptosis. Viruses 2024, 16, 1846. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, N.; Meng, X.J. Induction of the unfolded protein response (UPR) suppresses porcine reproductive and respiratory syndrome virus (PRRSV) replication. Virus Res. 2020, 276, 197820. [Google Scholar] [CrossRef]
- Lee, S.M.; Kleiboeker, S.B. Porcine arterivirus activates the NF-kappaB pathway through IkappaB degradation. Virology 2005, 342, 47–59. [Google Scholar] [CrossRef]
- Mottahedin, A.; Paidikondala, M.; Cholleti, H.; Baule, C. NF-κB activation by equine arteritis virus is MyD88 dependent and promotes viral replication. Arch. Virol. 2013, 158, 701–705. [Google Scholar] [CrossRef]
- Archambault, D.; St-Laurent, G. Induction of apoptosis by equine arteritis virus infection. Virus Genes 2000, 20, 143–147. [Google Scholar] [CrossRef]
- Cholleti, H.; Paidikondala, M.; Munir, M.; Hakhverdyan, M.; Baule, C. Equine arteritis virus induced cell death is associated with activation of the intrinsic apoptotic signalling pathway. Virus Res. 2013, 171, 222–226. [Google Scholar] [CrossRef]
- Abeyá, M.M.; Metz, G.E.; Franco Cruz, R.; Correas, I.; Osorio, F.A.; Echeverria, M.G. Equine arteritis virus cytopathic effect: Caspase-dependent cell death as the major consequence observed. J. Microbiol. Exp. 2018, 6, 171–173. [Google Scholar] [CrossRef]
- Knoops, K.; Bárcena, M.; Limpens, R.W.; Koster, A.J.; Mommaas, A.M.; Snijder, E.J. Ultrastructural characterization of arterivirus replication structures: Reshaping the endoplasmic reticulum to accommodate viral RNA synthesis. J. Virol. 2012, 86, 2474–2487. [Google Scholar] [CrossRef]
- Kublicka, A.; Lorek, D.; Mikołajczyk-Martinez, A.; Chodaczek, G.; Chwirot, A.; Bażanów, B.; Matczuk, A.K. Imaging flow cytometry reveals the mechanism of equine arteritis virus entry and internalization. Sci. Rep. 2025, 15, 3246. [Google Scholar] [CrossRef]
- Fernández, J.J.; Marín, A.; Rosales, R.; Penrice-Randal, R.; Mlcochova, P.; Alvarez, Y.; Villalón-Letelier, F.; Yildiz, S.; Pérez, E.; Rathnasinghe, R.; et al. The IRE1α-XBP1 arm of the unfolded protein response is a host factor activated in SARS-CoV-2 infection. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167193. [Google Scholar] [CrossRef] [PubMed]
- Oda, J.M.; den Hartigh, A.B.; Jackson, S.M.; Tronco, A.R.; Fink, S.L. The unfolded protein response components IRE1α and XBP1 promote human coronavirus infection. mBio 2023, 14, e0054023. [Google Scholar] [CrossRef] [PubMed]
- Snijder, E.J.; Kikkert, M.; Fang, Y. Arterivirus molecular biology and pathogenesis. J. Gen. Virol. 2013, 94, 2141–2163. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colina, S.E.; Williman, M.M.; Serena, M.S.; Echeverría, M.G.; Metz, G.E. Role of Unfolded Protein Response in the Apoptosis Induced by Alphaarterivirus: IRE1α as an Essential Pathway for In Vitro Replication. Viruses 2025, 17, 1301. https://doi.org/10.3390/v17101301
Colina SE, Williman MM, Serena MS, Echeverría MG, Metz GE. Role of Unfolded Protein Response in the Apoptosis Induced by Alphaarterivirus: IRE1α as an Essential Pathway for In Vitro Replication. Viruses. 2025; 17(10):1301. https://doi.org/10.3390/v17101301
Chicago/Turabian StyleColina, Santiago Emanuel, Macarena Marta Williman, María Soledad Serena, María Gabriela Echeverría, and Germán Ernesto Metz. 2025. "Role of Unfolded Protein Response in the Apoptosis Induced by Alphaarterivirus: IRE1α as an Essential Pathway for In Vitro Replication" Viruses 17, no. 10: 1301. https://doi.org/10.3390/v17101301
APA StyleColina, S. E., Williman, M. M., Serena, M. S., Echeverría, M. G., & Metz, G. E. (2025). Role of Unfolded Protein Response in the Apoptosis Induced by Alphaarterivirus: IRE1α as an Essential Pathway for In Vitro Replication. Viruses, 17(10), 1301. https://doi.org/10.3390/v17101301







