Abstract
The increasing challenges posed by plant viral diseases demand innovative and sustainable management strategies to minimize agricultural losses. Exogenous double-stranded RNA (dsRNA)-mediated RNA interference (RNAi) represents a transformative approach to combat plant viral pathogens without the need for genetic transformation. This review explores the mechanisms underlying dsRNA-induced RNAi, highlighting its ability to silence specific viral genes through small interfering RNAs (siRNAs). Key advancements in dsRNA production, including cost-effective microbial synthesis and in vitro methods, are examined alongside delivery techniques such as spray-induced gene silencing (SIGS) and nanocarrier-based systems. Strategies for enhancing dsRNA stability, including the use of nanomaterials like layered double hydroxide nanosheets and carbon dots, are discussed to address environmental degradation challenges. Practical applications of this technology against various plant viruses and its potential to ensure food security are emphasized. The review also delves into regulatory considerations, risk assessments, and the challenges associated with off-target effects and pathogen resistance. By evaluating both opportunities and limitations, this review underscores the role of exogenous dsRNA as a sustainable solution for achieving viral disease resistance in plants.
1. Introduction
Food production must meet a dual challenge by 2050: to increase by 60% to feed a projected global population of 10 billion while minimizing food losses caused by pests and pathogens, which currently range between 17% and 30% depending on the crop species [1]. In India alone, annual crop losses due to pests and diseases account for approximately 30% of production, translating into an economic loss of around INR 90,000 crore [2]. Viral diseases in plants significantly contribute to these losses, with global yield reductions of 10–15%, amounting to an economic impact exceeding USD 60 billion annually [1]. The emergence of new viral diseases further underscores the urgent need for innovative control strategies [3].
Recent advancements in molecular biology and genetics have yielded innovative strategies for plant protection [4]. One such groundbreaking development is the exogenous application of double-stranded RNA (dsRNA) to induce RNA interference (RNAi). This approach represents a transformative step in crop protection, offering a novel method to mitigate pathogen attacks [5,6,7,8]. RNAi, first demonstrated as a mechanism for gene silencing in Caenorhabditis elegans in 1998, relies on dsRNA to target specific genes [9]. Although earlier studies observed RNAi-like effects in plants, fungi, and nematodes, this discovery identified dsRNA as the fundamental trigger [10]. Subsequent research established RNAi pathways as conserved across eukaryotic organisms, playing critical roles in antiviral defense (via small interfering RNAs, or siRNAs), gene regulation (through microRNAs, or miRNAs), and genome protection against transposons (via PIWI-interacting RNAs, or piRNAs) [11].
Over the past two decades, RNAi technology has revolutionized diverse biological fields, including human medicine and agriculture [8]. In agriculture, RNAi has emerged as a promising tool for managing viral diseases, with the exogenous application of dsRNA gaining attention as an alternative to genetic transformation [5,6]. This technique offers several advantages, including greater flexibility, ease of use, and environmental safety, making it a viable solution for sustainable plant protection. Studies have consistently demonstrated the efficacy of exogenous dsRNA against plant viruses [12]. The mechanism of exogenous dsRNA-induced RNAi-based viral resistance is mentioned in Figure 1.
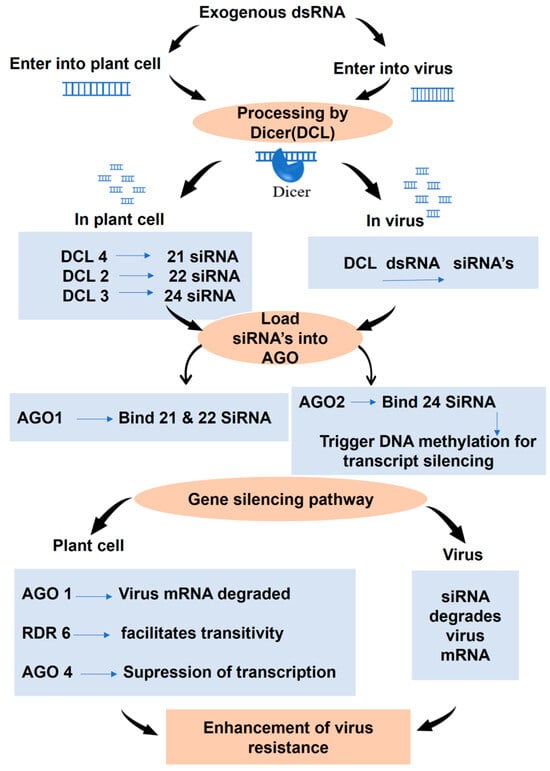
Figure 1.
Mechanism of exogenous dsRNA-induced RNAi-based viral disease resistance in plants.
This review focuses on the potential of exogenous dsRNA-based RNAi technology for achieving viral disease resistance in crops. We explore the mechanisms underlying dsRNA-mediated resistance, including nucleic acid recognition and uptake in plants. Additionally, we address the production, scalability, and cost implications of dsRNA for large-scale agricultural applications. Various delivery methods, such as spray-induced gene silencing (SIGS), host-induced gene silencing (HIGS), and direct application techniques, are discussed in detail. Strategies to enhance the stability and efficacy of dsRNA are also examined, along with practical considerations for implementing this technology in viral disease management. Finally, we delve into the challenges, risk assessments, and regulatory frameworks associated with RNAi technology. By evaluating both the potential and limitations of dsRNA-based approaches, this review underscores their critical role in advancing sustainable viral disease resistance in plants and contributing to global food security.
2. Inducers of RNAi Pathway
Antiviral RNA silencing is a key plant defense mechanism against viruses, initiated by virus-derived double-stranded RNAs (dsRNAs) produced by RNA-dependent RNA polymerases (RDRs). These dsRNAs are processed into 21–24 nt viral small interfering RNAs (vsiRNAs) by Dicer-like enzymes (DCLs), with assistance from RNA-binding proteins like DRB4. The process is amplified by host RDRs (RDR1 and RDR6), generating secondary vsiRNAs that guide Argonaute (AGO)-containing RNA-induced silencing complexes (RISCs) to suppress viral replication. However, viruses counteract this defense by producing viral suppressors of RNA silencing (VSRs), which interfere with various steps of the pathway [13].
RNAi pathway inducers, such as mild cross-protective viral strains, hpRNAs, dsRNAs, artificial miRNAs (amiRNAs), and sRNAs, can induce viral resistance in plants through exogenous RNAi (exoRNAi), which is particularly useful in non-transgenic regions, transgenesis-recalcitrant crops, or during virus epidemics. This RNA vaccination method allows rapid screening for RNAi-efficient molecules without off-target effects, offering a novel tool to enhance plant resistance against viruses, pathogens, and pests [14].
2.1. Leveraging Mild Strains
Cross-protection, or mild strain cross-protection, involves inoculating plants with a mild virus variant (e.g., T36 strain variants of citrus tristeza virus, CTV) to protect against more virulent strains [15,16,17]. This method has been effectively used for managing CTV and pepino mosaic virus (PepMV) [18]. It is considered more socially acceptable than transgenic approaches but requires evaluation of durability and potential impacts on other host plants [19]. While effective against related viral isolates, rare cases of protection against phylogenetically distinct viruses have been reported [20,21].
RNA interference (RNAi) plays a key role, potentially through localized RNAi or competition for host resources [22]. Another model suggests mild strains block virion uncoating of severe strains, possibly through CP recoating (e.g., TMV) [23]. Superinfection exclusion (SIE), observed in CTV, prevents severe strains from infecting plants preimmunized with mild isolates, requiring sequence identity and suggesting nucleic acid-based mechanisms [20]. Protein-mediated SIE, linked to the CTV-encoded p33 protein, regulates viral virulence and mediates SIE [24,25].
2.2. Hairpin RNA
The discovery of RNAi has transformed crop improvement by enabling resistance against various viruses [7]. Initially, independent expression of sense and antisense RNA strands provided about 20% resistance [26]. The hpRNA strategy was developed, where linked sense and antisense strands expressed from a single promoter efficiently produce dsRNA, triggering RNAi and generating small RNAs [26,27]. Unlike earlier methods, hpRNA bypasses the need for plant-encoded RDRs for dsRNA synthesis [28].
The hpRNA transgene targeting viral genes provides strong resistance against homologous viruses [29,30] and can target multiple viruses using chimeric constructs [31]. Due to challenges in stable transformation [32], agro-infiltration is used for transient expression and screening of hpRNA constructs [33]. This method uses recombinant Agrobacterium tumefaciens to transiently express dsRNA, triggering systemic silencing via siRNAs [34]. These methods allow studying initiation and maintenance of PTGS, unlike transgenic approaches that primarily study PTGS maintenance [26,35,36]. Transient agro-infiltration enables comparative RNAi construct analysis without positional effects seen in stable transgenics [37], but resistance lasts only 2–4 weeks and requires repeated application. It has been successfully used to study resistance against African cassava mosaic virus and to inhibit PVY transmission by aphids [38].
2.3. Biogenesis and Functionality of Small Interfering RNAs (siRNAs)
Several siRNA populations, including miRNAs, phasiRNAs, tasiRNAs, and vasiRNAs, play critical roles in plant development and immunity. miRNAs, transcribed from miRNA genes, form secondary structures processed by DCL1 into mature 21–22 nt miRNAs [39,40]. These bind to AGO1-RISC to target RNA for degradation or translation inhibition [41,42]. miRNAs contribute to antiviral defense directly, such as miR168, miR395a, and miR395d in cotton, which target cotton leaf curl Burewala virus genes. Overexpression of miR398 and miR2950 suppresses symptoms of cotton leaf curl Multan virus (CLCuMuV) and its betasatellite [43,44]. In rice, miR444 indirectly enhances resistance to rice stripe virus by suppressing MADS-box proteins, activating RDR1, and amplifying RNAi signaling via secondary vsiRNA biosynthesis [45]. RDR1 also produces virus-activated siRNAs (vasiRNAs), expanding its antiviral role [39].
PhasiRNAs and tasiRNAs originate from miRNA-mediated cleavage of PHAS and TAS transcripts. These are processed by RDR6 and DCL4 into 21-nt phased siRNAs. PhasiRNAs act in cis to target source or related genes (e.g., NB-LRR), while tasiRNAs act in trans to repress unrelated genes, fine-tuning gene expression and environmental adaptation [40]. Virus entry disrupts host RNA metabolism, triggering vasiRNA production, which targets ribosomal RNA and host gene exons as an innate antiviral defense [39]. This has been observed in Arabidopsis thaliana with cucumber mosaic virus (CMV) 2b and turnip mosaic virus.
2.4. Nucleic Acid Uptake in Plants
Externally applied dsRNAs likely mimic extracellular nucleic acids from pathogens, acting as MAMPs or PAMPs to activate plant immunity via PRRs [46]. For instance, viral dsRNA [47] reduces disease symptoms by triggering PTI in Arabidopsis and tobacco. PTI responses include MPK activation, ROS production, and defense gene expression. Fragmented self-DNA also serves as a DAMP, eliciting defense responses [48]. Arabidopsis recognizes viral dsRNA through SERK1, independent of RNA silencing pathways [47]. However, the molecular mechanisms behind exogenous nucleic acid recognition, uptake, and the specific receptors involved remain unclear.
3. Exogenous dsRNA: Production and Cost Optimization
3.1. Strategies for Efficient Large-Scale Production
Efficient dsRNA or sRNA production is vital for RNAi-based crop protection. Large-scale production for biopesticides requires cost-effective methods, unlike HIGS and MIGS. Although RNAi mechanisms are absent in prokaryotes, rhizobial tRNA-derived sRNA fragments can hijack host RNAi machinery in legumes [49]. Microbial fermentation, using organisms like E. coli and Pseudomonas syringae, (Figure 2) [50] offers scalable alternatives, though challenges in yield and purity remain a concern [51,52,53]. RNAgri’s microbial technology reduces costs to $2/g compared to $60/g for in vitro synthesis using bacteriophage T7 DdRP, which is effective but expensive and suited for small-scale production [54,55,56]. Typically, 2–10 g of dsRNA per hectare is needed. Emerging technologies like bacterial minicells promise enhanced dsRNA protection and controlled release in agriculture.
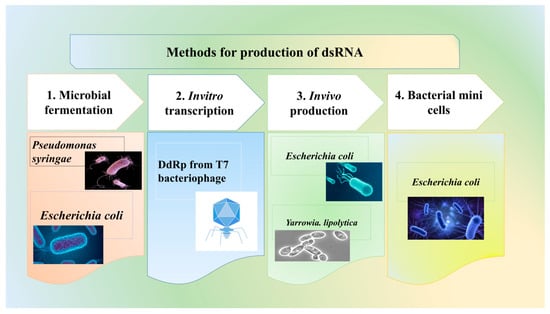
Figure 2.
Methods for production of dsRNA.
3.2. Assessing Cost and Feasibility for Agricultural Applications
In vitro and in vivo methods using DNA-dependent RNA polymerase (DdRP) from bacteriophage T7 are widely used for dsRNA production [55]. In vitro transcription, though high-quality, is costly and limited to small-scale production, while in vivo methods utilizing microorganisms like E. coli and Y. lipolytica offer scalable alternatives [50,55]. Microbial fermentation technologies, such as those by RNAgri, enhance dsRNA stability using protective proteins, making them safer and more affordable for large-scale use.
Demand for dsRNA ranges from 2–10 g per hectare, with microbial methods reducing production costs to $2/g compared to $60/g for in vitro systems [56,57,58]. Companies like GreenLight Biosciences and Eupheria Biotech now produce large-scale dsRNA at around $1/g [59]. Innovations, including bacterial minicells, promise improved dsRNA stability and controlled release, addressing contamination and cost challenges [60,61,62].
4. Targeted Delivery of dsRNA
RNAi-based crop protection relies on effective dsRNA delivery, yet the mechanisms of sRNA transfer between donor and recipient organisms remain unclear. Exogenous RNAs can induce gene silencing, but the SIGS strategy faces delivery challenges. Artificial nanomaterials enhance dsRNA stability [63,64,65], although their behavior under dynamic field conditions requires further investigation [64].
4.1. Nanocarrier-Driven Approaches to dsRNA Delivery
Nanomaterials show promise in enhancing the efficiency of SIGS, but concerns remain regarding their biosafety, including effects on crop growth, soil biodiversity, nontarget organisms, and human health [63,66]. Studies on silver nanoparticles (AgNPs) revealed reduced seed germination and seedling growth in rice [67]. By contrast, carbon dots, with low toxicity, have shown potential for RNA delivery in plants and animals [68,69]. Carbon dots can alter the cell cycle and exhibit biotoxic effects on microbial communities. These effects include reduced enzyme activities and nitrogen removal efficiency, leading to decreased microbial diversity and abundance [63,70]. The observed impacts of nanomaterials on ecosystems highlight the need for comprehensive environmental safety evaluations for RNA biopesticides that utilize nanomaterial carriers.
4.2. Exploring Natural dsRNA Delivery Systems
In pharmaceutical applications, liposome-encapsulated double-stranded RNAs (dsRNAs) or small interfering RNAs (siRNAs) can safely deliver RNA cargo, although they can still trigger an innate immune response in a sequence-dependent manner, unlike naked RNAs [71,72]. To enhance RNA delivery, capsid protein homologs, such as PEG10, derived from long terminal repeat retrotransposons, have been engineered. PEG10 can bind its own mRNA, facilitating vesicular secretion, while modified PNMA2 particles, based on their capsid structure, have been engineered for efficient RNA encapsulation and delivery in mammalian cells [72,73]. Additionally, a GNA:dsRBD fusion protein, which combines the lectin Galanthus nivalis agglutinin with a dsRNA-binding domain, has been shown to improve dsRNA uptake and RNA interference (RNAi) efficacy in lepidopteran midgut cells, significantly increasing insect mortality compared to naked dsRNA [11]. Furthermore, Arabidopsis Dicer-like 3 (DCL3), known for producing 24-nucleotide small RNAs, has been found to facilitate systemic RNA silencing through its RNA-binding domain (RBD), independent of its RNA processing activity, suggesting that engineered RBD domains could be useful for dsRNA delivery [74]. These advancements highlight innovative strategies for enhancing RNA delivery and RNAi efficacy across different organisms.
4.3. Exogenous dsRNAs: Activating Host Immune Responses for Enhanced Defense
Exogenous dsRNAs elicit antiviral defense by activating RNAi, the primary mechanism in plants against viruses [75]. Viral replication-associated dsRNAs are degraded by DCL proteins in the RNAi pathway [75]. Additionally, dsRNAs mimic viral replicative intermediates, triggering DCL-mediated cleavage and pattern-triggered immunity (PTI). In Arabidopsis, in vitro-generated and virus-derived dsRNAs induce PTI by acting as pathogen-associated molecular patterns. This signaling, involving SERK1 and a specific dsRNA receptor but not DCLs, restricts viral spread through callose deposition at plasmodesmata [47]. However, viral movement proteins can suppress this host response [75]. Although dsRNAs are sequence-specific, their potential toxicity to non-target organisms should be evaluated. Additionally, the biosafety of nanomaterials used as delivery vehicles must be independently assessed to ensure environmental safety [76].
5. Advances in Delivery Methods for Artificial dsRNAs in Plant Systems
5.1. Direct dsRNA Application in Plants
5.1.1. Spraying (Spray-Induced Gene Silencing—SIGS)
Spraying plants with exogenous dsRNA is one of the most promising direct application methods for RNA interference (RNAi). Exogenous dsRNA can either be taken up directly by pests or translocated from plant cells to pathogens. Locally sprayed dsRNAs have been shown to spread systemically in plants, inhibiting pathogens in untreated areas as well [54]. Enhancements such as encapsulation in hydroxide nanolayers (BioClay) increase stability against degradation and extend protection duration to over 20 days, making it more effective and environmentally friendly [77,78].
5.1.2. Trunk Injection and Root Absorption
Direct injection of dsRNA into tree trunks or application to plant roots allows systemic delivery through the plant’s vascular system (xylem and phloem). These methods facilitate RNA uptake and enable targeted gene silencing, offering an alternative to spraying for certain crops [79].
5.1.3. Cut Surface and Surfactant-Assisted Penetration Enhancement
Wound surfaces or cut plant tissues enhance dsRNA uptake, and surfactants like Silwet L-77 improve penetration efficiency for targeted applications [78]. Optimal dsRNA length and concentration depend on the pathogen, with viruses often requiring lengths over 200–300 nucleotides [58]. Tailored dsRNAs can target specific pathogens or related species [56]. Exogenous dsRNA applications reduce viral infection severity by altering pathogenicity [62]. Targeting viral coat protein genes delays disease onset, reduces virus titers, and provides protection for 20–70 days post-inoculation [54].
5.2. Symplastic and Apoplastic Delivery
The efficiency of RNAi depends on the RNA application method. Chemically synthesized 22-nt sRNAs triggered RNAi in Nb-GFP via high-pressure spraying but failed when delivered by petiole absorption, where sRNAs remained confined to the xylem and apoplast [80]. Similarly, a 499 nt GFP hpRNA applied through petiole absorption or trunk injection in Vitis vinifera and Malus domestica stayed restricted to these regions without initiating RNAi [80]. High-pressure spraying effectively delivers RNA for intracellular targets like plant mRNAs or viral RNAs, while petiole uptake or trunk injection retains intact dsRNAs, avoiding DCL processing, allowing pest/pathogen Dicer proteins to enhance RNAi efficacy [81,82]. This method offers a non-GMO alternative to transplastomic plants.
5.3. Optimizing RNA Delivery to Plants with Adjuvants
Efficient dsRNA application requires overcoming structural barriers such as stomata, cell walls, and the selectively permeable plasma membrane for internalization into plant cells. Post-endocytosis, dsRNA may be trapped in cytoplasmic vesicles, necessitating release into the cytoplasm for RNAi activation. Delivery to sufficient cells is crucial for inducing resistance, followed by movement through plasmodesmata and systemic translocation via the vascular system for maximal effect [14].
6. Harnessing Carrier Nanomolecules for Targeted RNA Delivery
Numerous carrier nanomolecules have been developed to overcome these barriers and facilitate dsRNA internalization. These carriers improve the stability and effectiveness of RNAi triggers by enabling cellular entry and protecting RNA from degradation.
Various nanomaterials have been explored for RNA delivery in plants, each offering unique advantages. Single-walled carbon nanotubes (SWNTs), with diameters around 1 nm, are smaller than plant cell wall pores (10–20 nm), allowing them to penetrate plant cells smoothly. The efficacy of SWNTs was demonstrated through delivery of siRNAs by binding complementary siRNA strands to nanotubes and infiltrating them into Nicotiana benthamiana leaves [83]. This facilitated the formation of active duplexes, leading to significant GFP knockdown, with noncovalent binding protecting the siRNAs from plant ribonucleases and enhancing silencing efficiency. Carbon dots, which are low cost and highly stable, have also shown success in drug delivery, including for plant applications. Positively charged carbon dots have been used to deliver 22 nt siRNAs, promoting efficient cellular uptake and sustained mRNA silencing in Nicotiana benthamiana and tomato plants [84,85]. Gold nanoclusters, conjugated with polyethyleneimine (PEI), bind siRNAs through electrostatic interactions, improving siRNA stability and promoting cellular internalization. PEI also facilitates endosomal escape via osmotic swelling from protonation, and cell-penetrating peptides further assist this process [86,87,88]. DNA-based nanostructures, including oligonucleotide tiles and 3D scaffolds, can be designed to attach siRNAs via complementary hybridization, with conjugation to SWNTs further boosting delivery efficacy [89]. Additionally, layered double hydroxide (LDH) clay nanosheets have emerged as promising carriers for dsRNA, effectively targeting viruses such as CMV and bean common mosaic virus. This formulation not only protects dsRNA from degradation but also facilitates sustained cellular uptake, offering prolonged stability under harsh conditions like UV radiation and rainfall [5].
Recent field experiments have demonstrated effective dsRNA delivery systems. PEI polymers conjugated with lipids formed nanoparticles carrying dsRNA targeting RNA Pol and CP of grapevine leafroll-associated virus-3 (GLRaV-3) [90]. Sprayed onto infected grapevines, these nanoparticles protected dsRNA from degradation, ensured systemic distribution via the phloem, reduced viral load, and improved grape quality after five biweekly applications [90]. Nanomaterials like SWNTs, carbon dots, gold nanoclusters, and LDH nanosheets enhance dsRNA stability, promote cellular uptake, and protect RNA under field conditions, enabling precise and robust RNAi-based solutions for plant viral diseases [14].
6.1. Spray-Induced Gene Silencing (SIGS)
Topical application of virus-specific dsRNAs has been shown to protect plants against various plant viruses [54,77,91]. This approach was first demonstrated against pepper mild mottle virus (PMMoV), alfalfa mosaic virus (AMV), and tobacco etch virus (TEV), where dsRNAs targeting viral replicase genes were mechanically applied, attenuating infections in host plants [34]. Subsequent studies have confirmed that external dsRNA application can confer viral resistance in diverse host plants against numerous viruses [58,77,91,92].
RNA-mediated virus resistance was first demonstrated [34] using in vitro transcribed dsRNA fragments targeting PMMoV replicase, TEV HcPro, and AMV RNA3 in Nicotiana benthamiana. Resistance required a certain dsRNA length. Applications of SIGS against different viruses along with their hosts and their targeted genes are mentioned in Table 1.

Table 1.
Application of SIGS against different viruses.
6.1.1. dsRNA Formulations
The stability and efficacy of dsRNA formulations remain a significant challenge, as studies have shown that the protective antiviral effects of dsRNA often last only a few days. This short duration necessitates frequent applications, particularly for long-duration crops grown in open fields. As a result, improving the stabilization and delivery methods of dsRNA molecules has become a priority for researchers. Strategies to overcome these challenges have included virus-like particle (VLP)-based delivery, BioClay nanosheets, direct trunk injection, high-pressure spraying, and biocompatible material formulations [54,98,99].
Virus-like particles (VLPs) offer an innovative approach by encapsulating dsRNA within virus particles or virion-like structures. Companies such as Apse RNA Containers (ARCs) have utilized bacteriophage MS2 capsid proteins to produce self-assembling VLPs. These VLPs are generated in E. coli systems, where target RNA molecules are packaged during bacterial growth, ensuring stability and efficient delivery [54,56].
BioClay is a layered double hydroxide (LDH) nanostructure that binds dsRNA, protecting it from degradation and enabling gradual release. BioClay not only resists environmental factors like watering but also provides prolonged antiviral effects. Successful applications of BioClay include the inhibition of cucumber mosaic virus and bean common mosaic virus in Nicotiana benthamiana and cowpea (Vigna unguiculata), respectively [5].
Direct trunk injection has emerged as a delivery method particularly suitable for woody plants. Commercially available systems like Arborjet inject dsRNA formulations directly into tree trunks for systemic delivery. However, the efficacy of this method against viruses in woody plants still requires further evaluation [56,80].
High-pressure spraying is another effective strategy, allowing systemic delivery of dsRNA through plant tissues. This method induces both local and systemic silencing and facilitates secondary siRNA production, particularly when using 22 nt dsRNA molecules [58,100].
Biocompatible and environmentally friendly materials are being explored. These materials aim to enhance stability and provide a gradual release of dsRNA molecules, making them suitable for field applications [98,99].
6.1.2. Challenges in dsRNA-Based Applications
The application of dsRNA-based crop protection faces challenges like short-lived antiviral effects requiring frequent reapplications, especially in open fields [58]. The high costs of in vitro synthesis, complex in vivo methods, and RNA degradation due to environmental factors like UV light and rainfall further complicate delivery [5,6,101]. Stabilization strategies such as BioClay and virus-like particles show potential but need refinement [56]. Regulatory hurdles, off-target concerns, and public perception akin to GMOs hinder adoption, highlighting the need for standardized formulations and delivery methods for consistent efficacy [55,58,98].
6.1.3. Nanomaterial-Based RNAi Delivery
Nanotechnology has significantly advanced agricultural practices by introducing eco-friendly solutions for crop protection, insect and pathogen control, yield improvement, and overall crop quality enhancement. Nanoparticles, due to their stability, specificity, and unique properties, have emerged as effective tools in delivering dsRNA/RNAi for gene silencing against pests and pathogens [102].
6.1.4. Nanoparticles as Nanopesticides
Nanoparticles like silver, gold, copper, zinc, and titanium are synthesized as nanopesticides to target plant-promoting disease resistance. Silica nanoparticles are widely used for manufacturing nanofertilizers and pesticides [102,103]. The insoluble active ingredient is fused with an inorganic nanoparticle coating or conjugated with polymers. Examples include fluorescent RNAi-NPs with a cationic polymer shell and polyphenylene dendrimer core [103,104,105].
6.1.5. Nanocarriers for dsRNA Delivery
Nanocarriers encapsulate dsRNA to prevent enzymatic degradation and ensure specific delivery to target genes viruses. This enables post-transcriptional gene silencing for effective control [106]. Metal nanoparticles such as zinc, copper, gold, and silver disrupt the pathogen’s cell wall, induce ROS accumulation, intercalate into DNA, and deactivate proteins by binding to thiol groups [107]. Liposome-mediated gold nanoparticles, guanidine-based polymers, and detergent-formulated nanocarriers ensure nucleolytic stability, rapid cellular uptake, and RNAi penetration [108]. Nanocarriers have shown success in preventing viruses like bean yellow mosaic virus and cucumber mosaic virus [102,109,110,111].
6.1.6. Types of Nanomaterials and Their Conjugates Used
Nanomaterials enhance the stability of dsRNA formulations through electrostatic interactions between positively charged nanomaterials and the phosphate groups of dsRNA. This interaction forms a nanoparticle/dsRNA complex with a net positive charge, enabling easy attachment to negatively charged cell membranes. Encapsulation by nanomaterials shields dsRNA from nuclease and chemical degradation during endocytosis [112].
6.1.7. Layered Double Hydroxide (LDH) Nanosheets
LDH nanosheets, also known as BioClay or nanoclay, are aluminosilicates (e.g., bentonite, hectorite) used as nanocarriers for RNAi delivery. These nanosheets protect dsRNA from UV radiation, enhance stability and adhesion to plant surfaces, promote cellular uptake, and effectively shield plants from diseases [113,114,115].
6.1.8. Boosting SIGS Effectiveness: Role of Nanoparticles in Stabilizing dsRNA
The short-term environmental stability of dsRNA is a major challenge for spray-induced gene silencing (SIGS), with research focusing on chemical modifications, delivery methods, and formulations to address this. Nanoparticles such as layered double hydroxides (LDHs) increased viral protection in tobacco from 5–7 to 20 days [114,116]. BioClay dsRNA prevented aphid-mediated bean common mosaic virus (BCMV) transmission in tobacco and cowpea, while carbon dots and chitosan improved dsRNA absorption and shelf life [5]. Artificial nanovesicles (AVs) protect dsRNA from UV radiation and nucleases, ensuring gradual release and enhanced stability, even after washing [114,117]. Though costly and complex, optimizing dsRNA formulations can improve SIGS effectiveness and scalability, making it a promising tool for crop protection [64].
6.2. Host-Induced Gene Silencing (HIGS)
Host-induced gene silencing (HIGS) is an RNA-based alternative to chemical pesticides and fungicides, involving transgenic expression of dsRNAs to silence target genes of pests or pathogens. HIGS has been developed to protect crops from viruses [118] and insects [119,120]. A review [121] reported effective HIGS applications, with average mortality or resistance rates of 90% for viruses and 50% for insects. Despite these advances, a major limitation is the reliance on stable plant transformation, which depends on the transformability and genetic stability of crop species [122]. Most crops lack established transformation protocols, and optimizing HIGS for new species can take years [5]. However, ongoing efforts to refine transformation methods offer promise for broader application of HIGS in crop protection [123].
Advances in hpRNA for Viral Disease Resistance
Dicer-like proteins (DCL3, DCL4, DCL2) process hpRNAs into small RNAs (sRNAs) for long-distance and cell-autonomous silencing [124,125,126], targeting viral coat proteins and replicase genes [127,128]. Transgenic Nicotiana benthamiana expressing hpRNA constructs showed resistance to citrus tristeza virus (CTV) and plum pox virus (PPV) [129,130], with field trials demonstrating efficacy against tomato yellow leaf curl virus (TYLCV) [125]. However, challenges persist, as transgenic citrus plants targeting CTV showed no resistance, likely due to host-specific factors or enhanced viral virulence [129]. Deep sequencing revealed off-target effects and transcriptome changes, emphasizing the need for optimized constructs and crop-specific promoters [124,126,131]. Applications of hpRNA against different viruses are mentioned in Table 2.

Table 2.
Applications of hpRNA against different viruses.
7. Direct dsRNA Application Techniques
RNAi is widely used in crop protection, primarily via transgenic plants, but GMO-related environmental and health concerns have limited its adoption in many countries. Non-transformative methods like spray-induced gene silencing (SIGS) provide eco-friendly, transgenic-free alternatives [91,133]. Effective gene silencing depends on delivery methods such as foliar spray, trunk injection, irrigation, seed coating, baits, or soil applications. Focus has shifted to exogenous dsRNA, siRNA, and hpRNA applications as sustainable solutions, paving the way for broader acceptance of RNAi technologies [134]. Different techniques for applying dsRNA molecules along with their applications against different viruses are mentioned in Table 3.

Table 3.
Different techniques for applying dsRNA molecules in plants.
7.1. Topical Applications of dsRNA
Non-transgenic strategies use exogenous dsRNA to induce RNAi-based resistance, similar to transgenic methods [95]. For instance, dsRNA from the PRSV-Tirupati isolate targeting CP and HC-Pro genes conferred complete resistance and up to 94% protection against PRSV in papaya cv. Pusa Nanha [139]. Escherichia coli-expressed intron-containing hpRNA (CP279) also provided over 2 months of PRSV resistance in papaya [96]. Naked dsRNA degrades quickly, but nanocarriers like clay nanosheets, chitosan, and carbon dots enhance stability and systemic movement, with clay nanosheets extending protection to 20 days [5]. Delivery methods include spraying, root soaking, and nanocarrier adhesion, offering reduced off-target effects, eco-friendliness, and social acceptability, making it a promising alternative for plant disease resistance [140,141].
7.1.1. Production of dsRNA for Topical Application
The synthesis of dsRNA can be achieved using in vitro or in vivo systems, employing expression vectors with essential elements like T7 or T3 DNA-dependent RNA polymerase (DdRp) promoters and multiple cloning sites (MCS) for gene incorporation. Promoters may be unidirectional (e.g., pGemT and pGemT-Easy vectors; Promega, Madison, WI, USA) or bidirectional (e.g., L4440 vector with two convergent DdRp promoters; Addgene, Watertown, MA, USA) [97].
7.1.2. In Vitro dsRNA Production
The ssRNA transcription method converts sense and antisense complementary DNA (cDNA) from two plasmids into ssRNA using T7 DdRp, followed by annealing to form dsRNA [34]. The single plasmid method synthesizes dsRNA directly using Pseudomonas syringae dsRNA bacteriophage phi 6, which is suitable for small-scale production [101]. Different methods, their features, and kits for in vitro dsRNA synthesis are given in Table 4.

Table 4.
Different methods, features, and kits for in vitro dsRNA synthesis.
7.1.3. In Vivo dsRNA Production
The in vivo method generates dsRNA within living cells by expressing inverted repeat sequences, commonly using E. coli strain HT115 (DE3), deficient in RNAse III and harboring an IPTG-inducible T7 DdRp for efficient dsRNA transcription [51,145,146,147]. While in vitro methods suit small-scale applications, in vivo systems are preferred for large-scale dsRNA production. Novel systems like bacteriophage-based ϕ6-expression use RdRp from ϕ6 to synthesize dsRNA from ssRNA templates, effectively managing viruses like tobacco mosaic virus (TMV) [6,101]. A modified E. coli system (pET28-BL21 DE3 RNase III) achieved higher yields (4.23 μg/mL) than the traditional L4440-HT115 (DE3) system (1.30 μg/mL), tripling expression efficiency [148]. And bacteriophage Phi6 RdRp enables enzymatic dsRNA production [149]. Yeast (Saccharomyces cerevisiae) [150], bacteria (Corynebacterium glutamicum) [151], and Bacillus thuringiensis [152,153] also offer scalable dsRNA production, which is crucial for cost-effective applications.
7.2. Vesicle-Mediated Cross-Kingdom RNA Trafficking
7.2.1. Intercellular Communication Based on Extracellular Vesicles (EVs)
Extracellular vesicles (EVs) have gained significant attention in biological research for their role in intercellular communication, transporting waste, toxins, nutrients, and RNA for silencing [154,155]. In animal systems, EVs from mammalian cells, including adipocytes [156], cancer cells [157,158], and immune cells [159,160], transmit miRNAs and functional RNAs to regulate gene expression in target cells. These findings demonstrate the critical role of EVs in sRNA transport across animal, plant, and microbial cells, though the precise mechanisms remain unclear [161].
7.2.2. RNA Components in Extracellular Vesicles (EVs)
Extracellular vesicles (EVs) transport various RNA types, including sRNAs, mRNAs, and non-coding RNAs, impacting intercellular communication [162,163]. Small RNAs, such as miRNAs and siRNAs, regulate gene expression via RNAi, participating in gene silencing and post-transcriptional regulation [164]. mRNAs carry genetic information for protein translation in recipient cells, influencing gene expression [165]. EVs also contain non-coding RNAs, like circRNAs and lncRNAs, which regulate gene expression and cellular functions through diverse mechanisms [154,166].
7.2.3. Extracellular Vesicle (EV)-Mediated RNA Transport
Extracellular vesicles (EVs) are key RNA carriers in the cross-kingdom transport of small RNAs (sRNAs) between plants and pathogens, enabling targeted gene silencing [76]. The selective loading of sRNAs into EVs is mediated by specific RNA-binding proteins and signaling molecules, ensuring that only specific sRNAs are encapsulated for transport to target organisms. This selective packaging is vital for precise cross-kingdom RNA interference (RNAi) [167].
Artificial nanovesicles, designed to mimic natural extracellular vesicles (EVs), provide a customizable and efficient RNA delivery system, enhancing RNA stability and precision to strengthen plant defences against pathogens [62]. EVs protect encapsulated RNAs from extracellular RNases and enhance delivery efficiency through specific target cell recognition via surface receptors [168]. RNA degradation from rainfall, UV light, and extracellular nucleases limits spray-induced gene silencing (SIGS) effectiveness [65,169]. Nanovesicles, naturally involved in cross-kingdom RNA transport, stabilize dsRNA and allow surface modifications for targeted delivery to specific tissues or pathogens [170,171,172]. Sprayed dsRNA can be processed into siRNA or transported intact, although factors like dsRNA structure, plant physiology, and environmental conditions affect efficacy [4,173]. Nanovesicle-based stabilization mitigates these challenges by reducing dsRNA degradation, improving absorption, and enabling slow or pathogen-triggered release [5,174], making nanovesicles a promising solution for RNAi-based plant protection strategies.
EVs contain various RNAs, including miRNAs, sRNAs (18–24 nucleotides), and tyRNAs (10–17 nucleotides), indicating a sophisticated RNA transport mechanism [162]. However, the processes governing the encapsulation of unconventional non-coding RNAs, their uptake by target cells, and their role in cellular communication remain largely unexplored. Customizing RNA and vesicle composition based on plant traits and genetic factors could enhance RNAi efficacy [175]. Research on EV-mediated RNA transport is critical for improving RNA delivery efficiency and stability. Plant EVs can encapsulate endogenous molecules and exogenous therapeutic substances [90,176]. Rich in bioactive lipids, proteins, RNA, and other pharmacologically active molecules, EVs have unique morphologies and compositions that support their application in plant protection [177].
8. Enhancing dsRNA Stability and Effectiveness
The commercialization of dsRNA-based biopesticides faces challenges, particularly environmental instability, as dsRNA degrades rapidly due to nucleases, rain, UV rays, and microbial [64,116,136,178]. Nanoparticle delivery systems like clay nanosheets and layered double hydroxides (LDHs) enhance dsRNA stability and provide prolonged pathogen defense, as demonstrated using Cy3-labeled RNA for cucumber mosaic virus (CMV), pepper mild mottle virus (PMMoV), and bean common mosaic virus [5,97]. Cationic oligosaccharides (ODAGal4) with phosphorothioate linkages and star polycations (SPc) improve dsRNA stability and pest targeting [179,180]. Insects have been models for delivering dsRNA targeting endonucleases to prevent degradation [181]. DNA nanostructures protect siRNA from nuclease activity, and carbon dots functionalized with polyethyleneimine (PEI) and golden nanoclusters facilitate siRNA delivery via foliar infiltration [85,182]. Single-walled carbon nanotubes (SWNTs) enhance dsRNA uptake efficiency [183], while cell-penetrating peptides (CPP) maintain stability [184]. Nanomaterials like CLPs and ALPS effectively spread dsRNA in maize to mitigate viral infections [185]. UV laser-induced artificial wounds improve dsRNA mobility in tomato plant vascular systems during foliar delivery [186].
9. Applications of Exogenous dsRNA in Viral Disease Resistance
Exogenous dsRNA has been effectively utilized to control various plant pathogens, including viruses [6,51,187]. Studies have shown that activating RNA interference (RNAi) through the application of dsRNA, siRNA, or hpRNA can protect plants against diseases [4]. Applications of exogenous dsRNA against different viruses are mentioned in Table 5.

Table 5.
Exogenous application of dsRNA against different viruses.
10. Risk Assessment and Regulatory Consideration
Regulatory frameworks differ across regions. In the USA, dsRNA products are classified as biochemical pesticides, requiring EPA approval under FIFRA and FFDCA [199,200]. Australia regulates them as agricultural chemical products through APVMA, while the OGTR considers SIGS non-GMO under specific conditions [201]. In the European Union, regulation follows Directive 2001/18/EC and Regulation (EC) 1829/2003 [European Parliament 2003]. HIGS-based products for food or feed fall under Regulation (EC) 1829/2003, while SIGS-based products without GMOs adhere to Regulation (EC) 1107/2009 [202]. Approval in the EU involves a two-step process: EFSA evaluates the active substance, followed by zonal assessment by Member States (MSs) [203]. Specific data requirements for dsRNA-based products are currently lacking, with evaluations based on chemical PPP standards (Regulations (EC) 284/2013 and 546/2011) [204,205]. The OECD Working Paper provides guidance on environmental risk assessments and off-target effects, but formal dsRNA-specific regulations are still pending [206]. Future adaptations may refine guidelines as understanding of dsRNA evolves.
10.1. GMO-free RNAi in Plants
RNAi technology holds immense potential in combating pests and pathogens in plants [207,208]. Traditionally, RNAi applications relied on recombinant viruses (virus-induced gene silencing), Agrobacterium tumefaciens-mediated transgenes, and transgenic plants producing dsRNA molecules for targeted host-induced gene silencing [209,210]. Notably, SmartStax Pro maize, engineered to express dsRNA against corn rootworm, was approved in 2017 by the EPA, FDA, and USDA, marking a significant milestone. However, RNAi-based transgenic crops face criticism due to anti-GMO sentiments, high commercialization costs (~USD 140 million), and limited public acceptance [211]. To address these challenges, innovative GMO-free RNAi approaches focus on the direct exogenous application of RNA molecules (dsRNA or sRNA) to plants, bypassing transgenes and recombinant viruses. These strategies aim to harness RNAi benefits while mitigating regulatory and public concerns [207,212,213,214,215,216,217]. GMO-free RNAi methods are exclusively explored in [210,211].
10.2. Safety Concerns of dsRNA Application in Plants
In plants, dsRNA can stimulate pattern-triggered immunity (PTI) independent of RNAi [218], and RNAi can trigger epigenetic changes such as RNA-directed DNA methylation (RdDM) [219]. For example, dsRNAs targeting Arabidopsis genes increased cytosine methylation. Long-term risk assessments are essential, as RNAi products may show delayed efficacy or non-lethal phenotypes [199,220]. Regulatory frameworks, as highlighted in [221], must address environmental fate, non-target effects, and biosafety, positioning dsRNA as a secure, eco-friendly, and targeted alternative for crop protection [222].
11. Challenges for Using RNAi Technology
The RNA interference (RNAi) mechanism relies on sequence homology, but siRNA can sometimes lack selectivity, leading to off-target effects, which pose challenges for plant disease management [112,223,224]. Highly conserved target genes across species increase the likelihood of off-target effects. Additionally, exogenous dsRNAs may cause epigenetic off-target effects in crops, potentially persisting transgenerationally [58]. Computational design programs and validation through bioassays are essential for assessing these effects comprehensively. Pathogens and pests can develop resistance to RNAi-based products, similar to traditional biopesticides, through mechanisms like genetic variation and single nucleotide polymorphisms (SNPs) [225]. RNAi strategies often downregulate target genes, potentially creating long-lasting resistance by limiting a pathogen’s ability to adapt. However, sequence mismatches between dsRNAs and target genes due to SNPs can reduce RNAi efficacy [226]. While synonymous SNPs incur minimal fitness penalties for pathogens, deviations in dsRNA–target compatibility diminish RNAi impact or induce resistance [113]. Hence, RNAi resistance must be carefully considered during application [227,228].
12. Conclusions
Exogenous dsRNA-induced RNAi offers a transformative and non-GMO approach to achieving viral resistance in plants by leveraging siRNA pathways. This technology provides precise, eco-friendly alternatives to traditional pest management practices. Efficient delivery systems, such as spray-induced gene silencing (SIGS), nanocarrier-based methods, and direct applications, have enhanced dsRNA stability and uptake, enabling practical agricultural use [5,54]. Despite its potential, challenges remain, including high production costs, environmental degradation of dsRNA, and the need for clear regulatory frameworks. Scalable production methods like microbial fermentation and nanotechnology-based stabilization reduce these hurdles, with innovations such as layered double hydroxide nanosheets and carbon dots significantly improving dsRNA durability and efficacy [56,64]. Future research should focus on optimizing delivery, ensuring safety, and addressing public concerns. Integration with other biotechnologies may expand its applications beyond viral resistance to broader plant health management. With sustained innovation, exogenous dsRNA-based technologies can revolutionize sustainable agriculture and bolster global food security [75].
Author Contributions
Conceptualization, Writing, Drafting, Literature Search and Review: E.V. and A.R. (Both authors contributed equally); Critical Analysis and Synthesis: P.L.B., B.S. and N.K.R.; Data Visualization and Presentation: S.K., Y.M.B., A.M. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Demilie, W.B. Plant disease detection and classification techniques: A comparative study of the performances. J. Big Data 2024, 11, 5. [Google Scholar] [CrossRef]
- Kanapiya, A.; Amanbayeva, U.; Tulegenova, Z.; Abash, A.; Zhangazin, S.; Dyussembayev, K.; Mukiyanova, G. Recent advances and challenges in plant viral diagnostics. Front. Plant Sci. 2024, 15, 1451790. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Merkel, L.; Amari, K. Exogenous Application of dsRNA in Plant Protection: Efficiency, Safety Concerns and Risk Assessment. Int. J. Mol. Sci. 2024, 25, 6530. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Li, P.; Jain, R.G. Clay nanosheets for topical delivery of RNAi for sustained protection against plant viruses. Nat. Plants 2017, 3, 16207. [Google Scholar] [CrossRef] [PubMed]
- Niehl, A.; Soininen, M.; Poranen, M.M.; Heinlein, M. Synthetic biology approach for plant protection using ds RNA. Plant Biotechnol. J. 2018, 16, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Llave, C.; Díaz-Ruíz, J.R. RNA interference as a new biotechnological tool for the control of virus diseases in plants. Virus Res. 2004, 102, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front Plant Sci 2020, 11, 51. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Konakalla, N.C.; Nitin, M.; Kaldis, A.; Masarapu, H.; Carpentier, S.; Voloudakis, A. dsRNA Molecules from the Tobacco Mosaic Virus p126 Gene Counteract TMV-Induced Proteome Changes at an Early Stage of Infection. Front. Plant Sci. 2021, 12, 663707. [Google Scholar] [CrossRef]
- Martinez, Z.; De Schutter, K.; Van Damme, E.J.M.; Vogel, E.; Wynant, N.; Vanden Broeck, J.; Christiaens, O.; Smagghe, G. Accelerated delivery of dsRNA in lepidopteran midgut cells by a Galanthus nivalis lectin (GNA)-dsRNAbinding domain fusion protein. Pestic. Biochem. Physiol. 2021, 175, 104853. [Google Scholar] [CrossRef]
- Konakalla, N.C.; Kaldis, A.; Berbati, M.; Masarapu, H.; Voloudakis, A.E. Exogenous application of double-stranded RNA molecules from TMV p126 and CP genes confers resistance against TMV in tobacco. Planta 2016, 244, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.W. Transgene silencing, RNA interference, and the antiviral defense mechanism directed by small interfering RNAs. Phytopathology 2023, 113, 616–625. [Google Scholar] [CrossRef]
- Voloudakis, A.E.; Kaldis, A.; Patil, B.L. RNA-based vaccination of plants for control of viruses. Annu. Rev. Virol. 2022, 9, 521–548. [Google Scholar] [CrossRef] [PubMed]
- Gal-On, A.; Shiboleth, Y.M. Cross-protection. In Natural Resistance Mechanisms of Plants to Viruses; Loebenstein, G., Ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 261–288. [Google Scholar]
- Salaman, R.N.; Smith, K.M.; MacClement, W.D.; Bawden, F.C.; Bernal, J.D. A discussion on new aspects of virus disease. Proc. R. Soc. B 1938, 125, 291–310. [Google Scholar]
- Ziebell, H.; Carr, J.P. Cross-protection: A century of mystery. Adv. Virus Res. 2010, 76, 211–264. [Google Scholar] [PubMed]
- Pechinger, K.; Chooi, K.M.; MacDiarmid, R.M.; Harper, S.J.; Ziebell, H. A new era for mild strain crossprotection. Viruses 2019, 11, 670. [Google Scholar] [CrossRef]
- Ziebell, H.; MacDiarmid, R. Prospects for engineering and improvement of cross-protective virusstrains. Curr. Opin. Virol. 2017, 26, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Folimonova, S.Y. Developing an understanding of cross-protection by Citrus tristeza virus. Front. Microbiol. 2013, 4, 76. [Google Scholar] [CrossRef] [PubMed]
- Muller, G.W.; Rezende, J.A.M. Preimmunization: Applications and perspectives in virus disease control. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Kluwer: Dordrecht, The Netherlands, 2006; Volume I, pp. 361–395. [Google Scholar]
- Jones, R.A.C.; Koenig, R.; Lesemann, D.E. Pepino mosaic virus, a new potexvirus from pepino (Solanum muricatum). Ann. Appl. Biol. 1980, 94, 61–68. [Google Scholar] [CrossRef]
- Sherwood, J.L.; Fulton, R.W. The specific involvement of coat protein in tobacco mosaic virus cross protection. Virology 1982, 119, 150–158. [Google Scholar] [CrossRef]
- Bergua, M.; Zwart, M.P.; El-Mohtar, C.; Shilts, T.; Elena, S.F.; Folimonova, S.Y. A viral protein mediate ssuperinfection exclusion at the whole-organism level but is not required for exclusion at the cellularlevel. J. Virol. 2014, 88, 11327–11338. [Google Scholar] [CrossRef]
- Sun, Y.-D.; Folimonova, S.Y. The p33 protein of Citrus tristeza virus affects viral pathogenicity by modulating a host immune response. New Phytol. 2019, 221, 2039–2053. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.A.; Singh, S.P.; Wang, M.-B.; Stoutjesdijk, P.A.; Green, A.G.; Waterhouse, P.M. Total silencing by intron-spliced hairpin RNAs. Nature 2000, 407, 319–320. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, P.M.; Helliwell, C.A. Exploring plant genomes by RNA-induced gene silencing. Nat. Rev. Genet. 2003, 4, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Béclin, C.; Boutet, S.; Waterhouse, P.; Vaucheret, H. A branched pathway for transgene-induced RNA silencing in plants. Curr. Biol. 2002, 12, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Dietzgen, R.G.; Mitter, N. Transgenic gene silencing strategies for virus control. Australas. Plant Pathol. 2006, 35, 605–618. [Google Scholar]
- Waterhouse, P.M.; Graham, M.W.; Wang, M.-B. Virus resistance and gene silencing in p lants can be induced by simultaneous expression of sense and antisense RNA. Proc. Natl. Acad. Sci. USA 1998, 95, 13959–13964. [Google Scholar] [CrossRef]
- Bucher, E.; Lohuis, D.; van Poppel, P.M.J.A.; Geerts-Dimitriadou, C.; Goldbach, R.; Prins, M. Multiple virus resistance at a high frequency using a single transgene construct. J. Gen. Virol. 2006, 87, 3697–3701. [Google Scholar] [CrossRef]
- Birch, R.G.; Shen, B.; Sawyer, B.J.B.; Huttner, E.; Tucker, W.Q.J.; Betzner, A.S. Evaluation and application of a luciferase fusion system for rapid in vivo analysis of RNAi targets and constructs in plants. Plant Biotechnol. J. 2010, 8, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.L.; Bagewadi, B.; Yadav, J.S.; Fauquet, C.M. Mapping and identification of cassava mosaic geminivirus DNA-A and DNA-B genome sequences for efficient siRNA expression and RNAi based virus resistance by transient agro-infiltration studies. Virus Res. 2016, 213, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Tenllado, F.; Dıaz-Ruız, J.R. Double-stranded RNA-mediated interference with plant virus infection. J. Virol. 2001, 75, 12288–12297. [Google Scholar] [CrossRef] [PubMed]
- Kalantidis, K.; Psaradakis, S.; Tabler, M.; Tsagris, M. The occurrence of CMV-specific short RNAs in transgenic tobacco expressing virus-derived double-stranded RNA is indicative of resistance to the virus. Mol. Plant-Microbe Interact. 2002, 15, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.B.; Waterhouse, P.M. Application of gene silencing in plants. Curr. Opin. Plant Biol. 2002, 5, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Peach, C.; Velten, J. Transgene expression variability (position effect) of CAT and GUS reporter genes driven by linked divergent T-DNA promoters. Plant Mol. Biol. 1991, 17, 49–60. [Google Scholar] [CrossRef]
- Vargas, M.; Martínez-García, B.; Díaz-Ruíz, J.R.; Tenllado, F. Transient expression of homologous hairpin RNA interferes with PVY transmission by aphids. Virol. J. 2008, 5, 42. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Du, P.; Wang, X.; Yu, Y.-Q.; Qiu, Y.-H.; Li, W.; Gal-On, A.; Zhou, C.; Li, Y.; Ding, S.-W. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14613–14618. [Google Scholar] [CrossRef]
- Zhao, M.; Cai, C.; Zhai, J.; Lin, F.; Li, L. Coordination of microRNAs, phasiRNAs, and NB-LRR genes in response to a plant pathogen: Insights from analyses of a set of soybean Rps gene near-isogenic lines. Plant Genome 2015, 8, plant genome2014.09.0044. [Google Scholar] [CrossRef]
- Reis, R.S.; Hart-Smith, G.; Eamens, A.L.; Wilkins, M.R.; Waterhouse, P.M. Gene regulation by translational inhibition is determined by Dicer partnering proteins. Nat. Plants 2015, 1, 14027. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mei, J.; Ren, G. Plant microRNAs: Biogenesis, homeostasis, and degradation. Front. Plant Sci. 2019, 10, 360. [Google Scholar] [CrossRef]
- Akmal, M.; Baig, M.S.; Khan, J.A. Suppression of cotton leaf curl disease symptoms in Gossypium hirsutum through over expression of host-encoded miRNAs. J. Biotechnol. 2017, 263, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Shweta; Akhter, Y.; Khan, J.A. Genome wide identification of cotton (Gossypium hirsutum)-encoded microRNA targets against Cotton leaf curl Burewala virus. Gene 2018, 638, 60–65. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Kong, X.; Hamera, S.; Wu, Y.; Chen, X.; Fang, R.; Yan, Y.; Humaira, S. A signaling cascade from miR444 to RDR1 in rice antiviral RNA silencing pathway. Plant Physiol. 2016, 170, 2365–2377. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Ryu, C.M. Plant perceptions of extracellular DNA and RNA. Mol. Plant 2016, 9, 956–958. [Google Scholar] [CrossRef]
- Niehl, A.; Wyrsch, I.; Boller, T.; Heinlein, M. Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. New Phytol. 2016, 211, 1008–1019. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Extracellular self-DNA as a damage-associated molecular pattern (DAMP) that triggers self-specific immunity induction in plants. Brain Behav. Immun. 2018, 72, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wang, X.; Duan, J.; Ma, J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science 2019, 365, 919–922. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Sanchez, A.R.; Romo-Quinones, C.; Rosas-Quijano, R.; Reyes, A.G.; Barraza, A.; Magallon-Barajas, F.; Angulo, C.; Mejía-Ruíz, C.H. Production of specific dsRNA against white spot syndrome virus in the yeast Yarrowia lipolytica. Aquac. Res. 2017, 49, 480–491. [Google Scholar] [CrossRef]
- Gan, D.; Zhang, J.; Jiang, H.; Jiang, T.; Zhu, S.; Cheng, B. Bacterially expressed dsRNA protects maize against SCMV infection. Plant Cell Rep. 2010, 29, 1261–1268. [Google Scholar] [CrossRef]
- Rodrigues, T.; Sridharan, K.; Manley, B.; Cunningham, D.; Narva, K. Development of dsRNA as a Sustainable Bioinsecticide: From Laboratory to Field. In Crop Protection Products for Sustainable Agriculture; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2021; Volume 1390, pp. 65–82. [Google Scholar] [CrossRef]
- Yin, G.H.; Sun, Z.N.; Song, Y.Z.; An, H.L.; Zhu, C.X.; Wen, F.J. Bacterially expressed doublestranded RNAs against hot-spot sequences of tobacco mosaic virus or potato virus Y genome have diferent ability to protect tobacco from viral infection. Appl. Biochem. Biotechnol. 2010, 162, 1901–1914. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Voloudakis, A.E.; Holeva, M.C.; Sarin, L.P.; Bamford, D.H.; Vargas, M.; Poranen, M.M. Efficient double-stranded RNA production methods for utilization in plant virus control. In Plant Virology Protocols, Methods in Molecular Biology; Uyeda, I., Masuta, C., Eds.; Humana Press: New York, NY, USA, 2015; Chapter 19. [Google Scholar]
- Zotti, M.; Dos Santos, E.A.; Cagliari, D.; Christiaens, O.; Taning, C.N.T.; Smagghe, G. RNA interference technology in crop protection against arthropod pests, pathogens and nematodes. Pest Manage. Sci. 2018, 74, 1239–1250. [Google Scholar] [CrossRef]
- Andrade, E.C.; Hunter, W.B. RNA interference—Natural gene-based technology for highly specific pest control (HiSPeC). In RNA Interference; Abdurakhmonov, I.Y., Ed.; InTechOpen: London, UK, 2016. [Google Scholar]
- Dalakouras, A.; Papadopoulou, K.K. Epigenetic modifcations: An unexplored facet of exogenous RNA application in plants. Plants 2020, 9, 673. [Google Scholar] [CrossRef]
- Dalakouras, A.; Koidou, V.; Papadopoulou, K. DsRNA-based pesticides: Considerations for efciency and risk assessment. Chemosphere 2024, 352, 141530. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Hamby, R.; Sanchez, J.N.; Cai, Q.; Yan, Q.; Jin, H. RNAs—A new frontier in crop protection. Curr. Opin. Biotechnol. 2021, 70, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Yang, W.J.; Tian, Y.; Fan, J.Y.; Ye, C.; Shang, F.; Ding, B.Y.; Zhang, J.; An, X.; Yang, L. Topical dsRNA delivery induces gene silencing and mortality in the pea aphid. Pest Manag. Sci. 2019, 75, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Niño-Sánchez, J.; Hamby, R.; Capriotti, L.; Chen, A.; Mezzetti, B.; Jin, H. Artifcial nanovesicles for dsRNA delivery in spray induced gene silencing for crop protection. Plant Biotechnol. J. 2023, 10, 550. [Google Scholar]
- Luo, X.; Nanda, S.; Zhang, Y.; Zhou, X.; Yang, C.; Pan, H. Risk assessment of RNAibased biopesticides. New Crops 2024, 1, 100019. [Google Scholar] [CrossRef]
- Rank, A.P.; Koch, A. Lab-to-feld transition of RNA spray applications–how far are we? Front. Plant Sci. 2021, 12, 755203. [Google Scholar] [CrossRef]
- Secic, E.; Kogel, K.H. Requirements for fungal uptake of dsRNA and gene silencing in RNAi-based crop protection strategies. Curr. Opin. Biotechnol. 2021, 70, 136–142. [Google Scholar] [CrossRef]
- Lai, D.Y. Approach to using mechanism-based structure activity relationship (SAR) analysis to assess human health hazard potential of nanomaterials. Food Chem. Toxicol. 2015, 85, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Efect of silver nano- particles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef]
- Cheon, S.H.; Kim, Z.H.; Choi, H.Y.; Kang, S.H.; Nam, H.J.; Kim, J.Y.; Kim, D.I. Efective delivery of siRNA to transgenic rice cells for enhanced transfection using PEIbased polyplexes. Biotechnol. Bioprocess Eng. 2017, 22, 577–585. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Shen, G.; Liu, H.; Fu, H.; Cui, D. Fluorescent carbon dots as an efcient siRNA nanocarrier for its interference therapy in gastric cancer cells. J. Nanobiotechnology 2014, 12, 58. [Google Scholar] [CrossRef]
- Havrdova, M.; Hola, K.; Skopalik, J.; Tomankova, K.; Petr, M.; Cepe, K.; Polakova, K.; Tucek, J.; Bourlinos, A.B.; Zboril, R. Toxicity of carbon dots—Efect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle. Carbon 2016, 99, 238–248. [Google Scholar] [CrossRef]
- Judge, A.D.; Sood, V.; Shaw, J.R.; Fang, D.; McClintock, K.; MacLachlan, I. Sequencedependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat. Biotechnol. 2005, 23, 457–462. [Google Scholar] [CrossRef]
- Madigan, V.; Zhang, Y.; Raghavan, R.; Wilkinson, M.E.; Faure, G.; Puccio, E.; Segel, M.; Lash, B.; Macrae, R.K.; Zhang, F. Human paraneoplastic antigen Ma2 (PNMA2) forms icosahedral capsids that can be engineered for mRNA delivery. Proc. Natl. Acad. Sci. USA 2024, 121, e2307812120. [Google Scholar] [CrossRef] [PubMed]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, B.S.; Wu, H.W.; Liu, C.L.; Guo, H.S.; Zhao, J.H. The RNA-binding domain of DCL3 is required for long-distance RNAi signaling. aBiotech 2024, 5, 17–28. [Google Scholar] [CrossRef]
- Huang, C.; Sede, A.R.; Elvira-Gonzalez, L.; Yan, Y.; Rodriguez, M.E.; Mutterer, J.; Boutant, E.; Shan, L.; Heinlein, M. dsRNA-induced immunity targets plasmodesmata and is suppressed by viral movement proteins. Plant Cell 2023, 35, 3845–3869. [Google Scholar] [CrossRef]
- Zhao, J.H.; Liu, Q.Y.; Xie, Z.M.; Guo, H.S. Exploring the challenges of RNAi-based strategies for crop protection. Adv. Biotechnol. 2024, 2, 23. [Google Scholar] [CrossRef]
- Mitter, N.; Worrall, E.A.; Robinson, K.E.; Xu, Z.P.; Carroll, B.J. Induction of virus resistance by exogenous application of double-stranded RNA. Curr. Opin. Virol. 2017, 26, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gu, K.; Duan, X.; Xiao, X.; Hou, Y.; Duan, Y.; Wang, J.; Yu, N.; Zhou, M. Secondary amplification of siRNA machinery limits the application of spray-induced gene silencing. Mol. Plant Pathol. 2018, 19, 2543–2560. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.B.; Hunter, W.B.; Park, A.L.; Gundersen-Rindal, D.E. Double-stranded RNA Oral Delivery Methods to Induce RNA Interference in Phloem and Plant-sap-feeding Hemipteran Insects. J. Vis. Exp. 2018, 4, e57390. [Google Scholar] [CrossRef]
- Dalakouras, A.; Jarausch, W.; Buchholz, G.; Bassler, A.; Braun, M.; Manthey, T.; Krczal, G.; Wassenegger, M. Delivery of hairpin RNAs and small RNAs into woody and herbaceous plants by trunk injection and petiole absorption. Front. Plant Sci. 2018, 9, 1253. [Google Scholar] [CrossRef] [PubMed]
- Bally, J.; Fishilevich, E.; Bowling, A.J.; Pence, H.E.; Narva, K.E.; Waterhouse, P.M. Improved insect-proofing: Expressing double-stranded RNA in chloroplasts. Pest Manag. Sci. 2018, 74, 1751–1758. [Google Scholar] [CrossRef]
- Bally, J.; McIntyre, G.J.; Doran, R.L.; Lee, K.; Perez, A.; Jung, H.; Naim, F.; Larrinua, I.M.; Narva, K.E.; Waterhouse, P.M. In-plant protection against Helicoverpa armigera by production of long hpRNA in chloroplasts. Front. Plant Sci. 2016, 7, 1453. [Google Scholar] [CrossRef]
- Demirer, G.S.; Zhang, H.; Goh, N.S.; Pinals, R.L.; Chang, R.; Landry, M.P. Carbon nanocarriers deliver siRNA to intact plant cells for efficient gene knockdown. Sci. Adv. 2020, 6, eaqz0495. [Google Scholar] [CrossRef]
- Hendrix, B.; Hoffer, P.; Sanders, R.; Schwartz, S.; Zheng, W. Systemic GFP silencing is associated with high transgene expression in Nicotiana benthamiana. PLoS ONE 2021, 16, e0245422. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Hendrix, B.; Hofer, P.; Sanders, R.A.; Zheng, W. Carbon dots for efcient small interfering RNA delivery and gene silencing in plants. Plant Physiol. 2020, 184, 647–657. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Tzeng, S.Y.; Green, J.J. Bioengineered nanoparticles for siRNA delivery. WIREs Nanomed. Nanobiotechnol. 2013, 5, 449–468. [Google Scholar] [CrossRef]
- Numata, K.; Ohtani, M.; Yoshizumi, T.; Demura, T.; Kodama, Y. Local gene silencing in plants via synthetic dsRNA and carrier peptide. Plant Biotechnol. J. 2014, 12, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xu, J.; Chen, J.; Wang, Z.; Wang, X.; Zhong, J. Protein nanoparticles for pickering emulsions: A comprehensive review on their shapes, preparation methods, and modifcation methods. Trends Food Sci. Technol. 2021, 113, 26–41. [Google Scholar] [CrossRef]
- Zhang, H.; Demirer, G.S.; Zhang, H.; Ye, T.; Goh, N.S.; Aditham, A.J.; Cunningham, F.J.; Fan, C.; Landry, M.P. DNA nanostructures coordinate gene silencing in mature plants. Proc. Natl. Acad. Sci. USA 2019, 116, 7543–7548. [Google Scholar] [CrossRef]
- Avital, A.; Muzika, N.S.; Persky, Z.; Bar, G.; Michaeli, Y.; Fridman, Y.; Karny, A.; Shklover, J.; Shainsky, J.; Savaldi-Goldstein, S.; et al. Foliar delivery of siRNA particles for treating viral infections in agricultural grapevines. Adv. Funct. Mater. 2021, 31, 2101003. [Google Scholar] [CrossRef] [PubMed]
- Das, P.R.; Sherif, S.M. Application of Exogenous dsRNAsinduced RNAi in Agriculture: Challenges and Triumphs. Front. Plant Sci. 2020, 11, 946. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Aleynova, O.A.; Kalachev, A.V.; Suprun, A.R.; Ogneva, Z.V.; Kiselev, K.V. Induction of transgene suppression in plants via external application of synthetic dsRNA. Int. J. Mol. Sci. 2019, 20, 1585. [Google Scholar] [CrossRef] [PubMed]
- Šafářová, D.; Brázda, P.; Navrátil, M. Effect of artificial dsRNA on infection of pea plants by pea seed-borne mosaic virus. Czech J. Genet. Plant Breed. 2014, 50, 105–108. [Google Scholar] [CrossRef]
- Lau, S.E.; Mazumdar, P.; Hee, T.W.; Song, A.L.A.; Othman, R.Y.; Harikrishna, J.A. Crude extracts of bacteriallyexpressed dsRNA protect orchid plants against Cymbidium mosaic virus during transplantation from in vitro culture. J. Hortic. Sci. Biotechnol. 2014, 89, 569–576. [Google Scholar] [CrossRef]
- Kaldis, A.; Berbati, M.; Melita, O.; Reppa, C.; Holeva, M.; Otten, P.; Voloudakis, A. Exogenously applied dsRNA molecules deriving from the Zucchini yellow mosaic virus (ZYMV) genome move systemically and protect cucurbits against ZYMV. Mol. Plant Pathol. 2018, 19, 883–895. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Yang, G.; Chen, Y.; Yan, P.; Tuo, D.; Li, X.; Zhou, P. Resistance of non-transgenic papaya plants to papaya ringspot virus (PRSV) mediated by intron-containing hairpin dsRNAs expressed in bacteria. Acta Virol. 2014, 58, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Worrall, E.A.; Hamid, A.; Mody, K.T.; Mitter, N.; Pappu, H.R. Nanotechnology for plant disease management. Agronomy 2018, 8, 285. [Google Scholar] [CrossRef]
- Pérez-de-Luque, A. Interaction of nanomaterials with plants: What do we need for real applications in agriculture? Front. Environ. Sci. 2017, 5, 12. [Google Scholar] [CrossRef]
- Vurro, M.; Miguel-Rojas, C.; Pérez-de-Luque, A. Safe nanotechnologies for increasing the effectiveness of environmentally friendly natural agrochemicals. Pest Manag. Sci. 2019, 75, 2403–2412. [Google Scholar] [CrossRef] [PubMed]
- Dalakouras, A.; Wassenegger, M.; McMillan, J.N.; Cardoza, V.; Maegele, I.; Dadami, E.; Runne, M.; Krczal, G.; Wassenegger, M. Induction of silencing in plants by high-pressure spraying of in vitro synthesized small RNAs. Front. Plant Sci. 2016, 7, 1327. [Google Scholar] [CrossRef]
- Aalto, A.P.; Sarin, L.P.; van Dijk, A.A.; Saarma, M.; Poranen, M.M.; Arumäe, U.; Bamford, D.H. Large-scale production of dsRNA and siRNA pools for RNA interference utilizing bacteriophage phi6 RNA-dependent RNA polymerase. RNA 2007, 13, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Kumari, A.; Mahanta, M.; Biswas, K.K.; Dudkiewicz, A.; Thakuria, D.; Abdelrhim, A.S.; Singh, S.B.; Muthukrishnan, G.; Sabarinathan, K.G.; et al. Advances in nanotechnology as a potential alternative for plant viral disease management. Front. Microbiol. 2022, 13, 935193. [Google Scholar] [CrossRef] [PubMed]
- Gebremichael, D.E.; Haile, Z.M.; Negrini, F.; Sabbadini, S.; Capriotti, L.; Mezzetti, B.; Baraldi, E. RNA interference strategies for future management of plant pathogenic fungi: Prospects and challenges. Plants 2021, 10, 650. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; Zhang, Y.; Zhao, X.; Zhang, W.; Guo, X.; He, L.; Liu, K.; Yang, H.; Hong, H.; Peng, J.; et al. Fluorescent nanoparticleRNAi-mediated silencing of sterol carrier protein-2 gene expression suppresses the growth, development, and reproduction of Helicoverpa armigera. Nanomaterials 2023, 13, 245. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nehra, M.; Dilbaghi, N.; Marrazza, G.; Tuteja, S.K.; Kim, K.-H. Nanovehicles for plant modifications towards pest- and disease-resistance traits. Trends Plant Sci. 2020, 25, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Hamid, A.; Saleem, S. Role of nanoparticles in management of plant pathogens and scope in plant transgenics for imparting disease resistance. Plant Prot. Sci. 2022, 58, 173–184. [Google Scholar] [CrossRef]
- Yan, S.; Ren, B.; Zeng, B.; Shen, J. Improving RNAi efficiency for pest control in crop species. BioTechniques 2020, 68, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zheng, Y.; Chao, Z.; Chen, H.; Zhang, Y.; Yin, M.; Shen, J.; Yan, S. Visualization of the process of a nanocarrier-mediated gene delivery: Stabilization, endocytosis and endosomal escape of genes for intracellular spreading. J. Nanobiotechnology 2022, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ji, S.; Bi, S.; Tang, Y.; Zhang, G.; Yan, S.; Wan, F.; Lü, Z.; Liu, W. A promising approach to an environmentally friendly pest management solution: Nanocarrier-delivered dsrna towards controlling the destructive invasive pest tuta absoluta. Environ. Sci. Nano 2023, 10, 1003. [Google Scholar] [CrossRef]
- Yan, S.; Yin, M.-Z.; Shen, J. Nanoparticle-based nontransformative RNA insecticides for sustainable pest control: Mechanisms, current status and challenges. Entomol. Gen. 2023, 43, 21–30. [Google Scholar] [CrossRef]
- Mamta, B.; Rajam, M.V. RNAi technology: A new platform for crop pest control. Physiol. Mol. Biol. Plants 2017, 23, 487–501. [Google Scholar] [CrossRef]
- He, L.; Huang, Y.; Tang, X. RNAi-based pest control: Production, application and the fate of dsrna. Front. Bioeng. Biotechnol. 2022, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Soto, A.; Chacón-Cerdas, R. RNAi Crop Protection Advances. Int. J. Mol. Sci. 2021, 22, 12148. [Google Scholar] [CrossRef]
- Menezes, P.S.; Yan, Y.; Yang, Y.; Mitter, N.; Mahony, T.J.; Mody, K.T. RNAi-based biocontrol of pests to improve the productivity and welfare of livestock production. Appl. Biosci. 2022, 1, 229–243. [Google Scholar] [CrossRef]
- Qiao, L.; Lan, C.; Capriotti, L.; Ah-Fong, A.; Nino Sanchez, J.; Hamby, R.; Heller, J.; Zhao, H.; Glass, N.L.; Judelson, H.S.; et al. Spray-induced gene silencing for disease control is dependent on the efficiency of pathogen RNA uptake. Plant Biotechnol. J. 2021, 19, 1756–1768. [Google Scholar] [CrossRef]
- Hoang, B.T.L.; Fletcher, S.J.; Brosnan, C.A.; Ghodke, A.B.; Manzie, N.; Mitter, N. RNAi as a foliar spray: Efficiency and challenges to field applications, Int. J. Mol. Sci. 2022, 23, 6639. [Google Scholar] [CrossRef]
- Abel, P.; Nelson, R.; De, B.; Hoffmann, N.; Rogers, S.; Fraley, R.T.; Beachy, R.N. Delay of disease development in transgenic plants that express the tobacco mosaic virus coat protein gene. Science 1986, 232, 738–743. [Google Scholar] [CrossRef] [PubMed]
- Baum, J.A.; Bogaert, T.; Clinton, W.; Heck, G.R.; Feldmann, P.; Ilagan, O.; Johnson, S.; Plaetinck, G.; Munyikwa, T.; Pleau, M. Control of coleopteran insect pests through RNA interference. Nat. Biotechnol. 2007, 25, 1322–1326. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.-B.; Cai, W.-J.; Wang, J.-W.; Hong, G.-J.; Tao, X.-Y.; Wang, L.-J.; Huang, Y.-P.; Chen, X.-Y. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol. 2007, 25, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Wassenegger, M. Host-induced gene silencing—Mechanisms and applications. New Phytol. 2021, 231, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Weiberg, A.; Wang, M.; Lin, F.-M.; Zhao, H.; Zhang, Z.; Kaloshian, I.; Huang, H.-D.; Jin, H. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science 2013, 342, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Beernink, B.M.; Amanat, N.; Li, V.H.; Manchur, C.L.; Whyard, S.; Belmonte, M.F. SIGS vs. HIGS: Opportunities and challenges of RNAi pest and pathogen control strategies. Can. J. Plant Pathol. 2024, 46, 675–689. [Google Scholar] [CrossRef]
- Fuentes, A.; Carlos, N.; Ruiz, Y.; Callard, D.; Sánchez, Y.; Ochagavía, M.E.; Seguin, J.; Malpica-López, N.; Hohn, T.; Lecca, M.R. Field trial and molecular characterization of RNAi-transgenic tomato plants that exhibit resistance to tomato yellow leaf curl geminivirus. Mol. Plant-Microbe Interact. 2016, 29, 197–209. [Google Scholar] [CrossRef]
- Fuentes, A.; Ramos, P.L.; Fiallo, E.; Callard, D.; Sánchez, Y.; Peral, R.; Rodríguez, R.; Pujol, M. Intron-hairpin RNA derived from replication associated protein C1 gene confers immunity to tomato yellow leaf curl virus infection in transgenic tomato plants. Transgenic Res. 2006, 15, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Pooggin, M.M. RNAi-mediated resistance to viruses: A critical assessment of methodologies. Curr. Opin. Virol. 2017, 26, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Simón-Mateo, C.; García, J.A. Antiviral strategies in plants based on RNA silencing. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2011, 1809, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.L.; Jones, L.; Baulcombe, D.C.; Maule, A.J. Size constraints for targeting posttranscriptional gene silencing and for RNA-directed methylation in Nicotiana benthamiana using a potato virus X vector. Plant J. 2001, 25, 417–425. [Google Scholar] [CrossRef]
- Batuman, O.; Mawassi, M.; Bar-Joseph, M. Transgenes consisting of a dsRNA of an RNAi suppressor plus the 3′ UTR provide resistance to Citrus tristeza virus sequences in Nicotiana benthamiana but not in citrus. Virus Genes 2006, 33, 319–327. [Google Scholar]
- Pandolfini, T.; Molesini, B.; Avesani, L.; Spena, A.; Polverari, A. Expression of selfcomplementary hairpin RNA under the control of the rolC promoter confers systemic disease resistance to plum pox virus without preventing local infection. BMC Biotechnol. 2003, 3, 7. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Sabbadini, S.; Miozzi, L.; Mezzetti, B.; Noris, E. Host-induced gene silencing and spray-induced gene silencing for crop protection against viruses. In RNAi for Plant Improvement and Protection; CABI: Wallingford, UK, 2021; pp. 72–85. [Google Scholar]
- Khalid, A.; Zhang, Q.; Yasir, M.; Li, F. Small RNA based genetic engineering for plant viral resistance: Application in crop protection. Front. Microbiol. 2017, 8, 43. [Google Scholar] [CrossRef]
- Vetukuri, R.R.; Dubey, M.; Kalyandurg, P.B.; Carlsson, A.S.; Whisson, S.C.; Ortiz, R. Spray-induced gene silencing: An innovative strategy for plant trait improvement and disease control. Crop Breed. Appl. Biotechnol. 2021, 21, e387921S11. [Google Scholar] [CrossRef]
- Cedillo-Jimenez, C.A.; Guevara-Gonzalez, R.G.; Cruz-Hernandez, A. Exogenous dsRNA sequence based on miR1917 downregulates its target gene related to ethylene signaling in tomato seedlings and fruit. Sci. Hortic. 2024, 331, 113090. [Google Scholar] [CrossRef]
- Bragg, Z.; Rieske, L.K. Feasibility of systemically applied dsRNAs for pest-specifc RNAi-induced gene silencing in white oak. Front. Plant Sci. 2022, 13, 830226. [Google Scholar] [CrossRef]
- Mann, S.K.; Kashyap, P.L.; Sanghera, G.S.; Singh, G.; Singh, S. RNA interference: An eco-friendly tool for plant disease management. Transgenic Plant J. 2008, 2, 110–126. [Google Scholar]
- Puyam, A.; Sharma, S.; Kashyap, P.L. RNA interference-a novel approach for plant disease management. J. Appl. Nat. Sci. 2017, 9, 1612–1618. [Google Scholar] [CrossRef]
- Hariharan, G.; Sivasubramaniam, N.; Prasannath, K. RNA interference as a promising strategy for plant disease management. In Food Security and Plant Disease Management; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Vadlamudi, T.; Patil, B.L.; Kaldis, A.; Gopal, D.V.R.S.; Mishra, R.; Berbati, M.; Voloudakis, A. dsRNA-mediated protection against two isolates of Papaya ringspot virus through topical application of dsRNA in papaya. J. Virol. Methods 2020, 275, 113750. [Google Scholar] [CrossRef] [PubMed]
- Dubrovina, A.S.; Kiselev, K.V. Exogenous RNAs for gene regulation and plant resistance. Int. J. Mol. Sci. 2019, 20, 2282. [Google Scholar] [CrossRef] [PubMed]
- Jyotika, R.K.; Harish, S.; Karthikeyan, G.; Kumar, K.K.; Murugan, M.; Jayakanthan, M.; Chen, T.C. Molecular approaches for the management of papaya ringspot virus infecting papaya: A comprehensive review. Mol. Biol. Rep. 2024, 51, 981. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.; Martinez de Alba, A.E.; Flores, R.; Gago, S. Doublestranded RNA interferes in a sequence-specific manner with the infection of representative members of the two viroid families. Virology 2008, 371, 44–53. [Google Scholar] [CrossRef]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Kogel, K.H. An RNAi-based control of Fusarium graminearum infections through spraying of long dsRNAs involves a plant passage and is controlled by the fungal silencing machinery. PLoS Path. 2016, 12, e1005901. [Google Scholar] [CrossRef]
- Makeyev, E.V.; Bamford, D.H. Replicase activity of purified recombinant protein P2 of doublestranded RNA bacteriophage φ6. EMBO J. 2000, 19, 124–133. [Google Scholar] [CrossRef]
- Ahn, S.J.; Donahue, K.; Koh, Y.; Martin, R.R.; Choi, M.Y. Microbial based double-stranded RNA production to develop cost-efective RNA interference application for insect pest management. Int. J. Insect Sci. 2019, 11, 1179543319840323. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Hua, F.; Bian, Z. Coronavirus disease (COVID-19): Emerging and future challenges for dental and oral medicine. J. Dent. Res. 2020, 99, 481–487. [Google Scholar] [CrossRef]
- Takif, H.E.; Chen, S.M.; Court, D.L. Genetic analysis of the rnc operon of Escherichia coli. J. Bacteriol. 1989, 171, 2581–2590. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhou, H.; Wei, Y.; Yan, S.; Shen, J. A novel plasmid–Escherichia coli system produces large batch dsRNAs for insect gene silencing. Pest Manag. Sci. 2020, 76, 2505–2512. [Google Scholar] [CrossRef] [PubMed]
- Levanova, A.A.; Poranen, M.M. Utilization of bacteriophage phi6 for the production of high-quality double-stranded RNA molecules. Viruses 2024, 16, 166. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.A.; Tabuloc, C.A.; Cervantes, K.R.; Chiu, J.C. Ingestion of genetically modifed yeast symbiont reduces ftness of an insect pest via RNA interference. Sci. Rep. 2016, 6, 22587. [Google Scholar] [CrossRef]
- Hashiro, S.; Mitsuhashi, M.; Chikami, Y.; Kawaguchi, H.; Niimi, T.; Yasueda, H. Construction of Corynebacterium glutamicum cells as containers encapsulating dsRNA overexpressed for agricultural pest control. Appl. Microbiol. Biotechnol. 2019, 103, 8485–8496. [Google Scholar] [CrossRef]
- Jiang, L.; Ding, L.; He, B.; Shen, J.; Xu, Z.; Yin, M. Systemic gene silencing in plants triggered by fluorescent nanoparticle-delivered doublestranded RNA. Nanoscale 2014, 6, 9965–9969. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Um, T.Y.; Jang, G.; Choi, Y.D.; Shin, C. Targeted gene suppression through double-stranded RNA application using easyto-use methods in Arabidopsis thaliana. Appl. Biol. Chem. 2022, 65, 4. [Google Scholar] [CrossRef]
- Huang, M.; Peng, X.; Yang, L.; Yang, S.; Li, X.; Tang, S.; Li, B.; Jin, H.; Wu, B.; Liu, J.; et al. Non-Coding RNA Derived from Extracellular Vesicles in Cancer Immune Escape: Biological Functions and Potential Clinical Applications. Cancer Lett. 2021, 501, 234–246. [Google Scholar] [CrossRef]
- Stotz, H.U.; Brotherton, D.; Inal, J. Communication Is Key: Extracellular Vesicles as Mediators of Infection and Defence during Host–Microbe Interactions in Animals and Plants. FEMS Microbiol. Rev. 2022, 46, fuab044. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, R.; Wang, G.; Brandao, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA Sequence Codes for Small Extracellular Vesicle Release and Cellular Retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Yoshioka, Y.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.; Kato, T.; Kiyono, T.; Takeshita, F.; Kajiyama, H.; Kikkawa, F.; et al. Malignant Extracellular Vesicles Carrying MMP1 mRNA Facilitate Peritoneal Dissemination in Ovarian Cancer. Nat. Commun. 2017, 8, 14470. [Google Scholar] [CrossRef]
- Zomer, A.; Maynard, C.; Verweij, F.J.; Kamermans, A.; Schäfer, R.; Beerling, E.; Schiffelers, R.M.; de Wit, E.; Berenguer, J.; Ellenbroek, S.I.J.; et al. In Vivo Imaging Reveals Extracellular Vesicle-Mediated Phenocopying of Metastatic Behavior. Cell 2015, 161, 1046–1057. [Google Scholar] [CrossRef]
- Dosil, S.G.; Lopez-Cobo, S.; Rodriguez-Galan, A.; Fernandez-Delgado, I.; Ramirez-Huesca, M.; Milan-Rois, P.; Castellanos, M.; Somoza, A.; Gómez, M.J.; Reyburn, H.T.; et al. Natural Killer (NK) Cell-Derived Extracellular-Vesicle Shuttled microRNAs Control T Cell Responses. eLife 2022, 11, e76319. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.Á.; Bernad, A.; Sánchez-Madrid, F. Unidirectional Transfer of microRNA-Loaded Exosomes from T Cells to Antigen-Presenting Cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Liu, G.; Kang, G.; Wang, S.; Huang, Y.; Cai, Q. Extracellular Vesicles: Emerging Players in Plant Defense against Pathogens. Front. Plant Sci. 2021, 12, 757925. [Google Scholar] [CrossRef]
- Baldrich, P.; Rutter, B.D.; Karimi, H.Z.; Podicheti, R.; Meyers, B.C.; Innes, R.W. Plant Extracellular Vesicles Contain Diverse Small RNA Species and Are Enriched in 10- to 17-Nucleotide “Tiny” RNAs. Plant Cell 2019, 31, 315–324. [Google Scholar] [CrossRef]
- Munhoz Da Rocha, I.F.; Amatuzzi, R.F.; Lucena, A.C.R.; Faoro, H.; Alves, L.R. Cross-Kingdom Extracellular Vesicles EV-RNA Communication as a Mechanism for Host–Pathogen Interaction. Front. Cell. Infect. Microbiol. 2020, 10, 593160. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Tian, Y.; Yang, Z.; Shi, J.; Kwak, K.J.; Tong, Y.; Estania, A.P.; Cao, J.; Hsu, W.-H.; Liu, Y.; et al. Intradermally Delivered mRNA-Encapsulating Extracellular Vesicles for Collagen-Replacement Therapy. Nat. Biomed. Eng. 2023, 7, 887–900. [Google Scholar] [CrossRef]
- Collado, A.; Gan, L.; Tengbom, J.; Kontidou, E.; Pernow, J.; Zhou, Z. Extracellular Vesicles and Their Non-Coding RNA Cargos: Emerging Players in Cardiovascular Disease. J. Physiol. 2023, 601, 4989–5009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Xu, J.; Fan, S.; Zhu, N.; Meng, Q.; Dai, S.; Yuan, X. Cross-Kingdom RNA Transport Based on Extracellular Vesicles Provides Innovative Tools for Plant Protection. Plants 2024, 13, 2712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhao, Y.-L.; Zhao, J.-H.; Wang, S.; Jin, Y.; Chen, Z.-Q.; Fang, Y.-Y.; Hua, C.-L.; Ding, S.-W.; Guo, H.-S. Cotton Plants Export microRNAs to Inhibit Virulence Gene Expression in a Fungal Pathogen. Nat. Plants 2016, 2, 16153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Apoplastic Proteases: Powerful Weapons against Pathogen Infection in Plants. Plant Commun. 2020, 1, 100085. [Google Scholar] [CrossRef]
- Cai, Q.; Qiao, L.; Wang, M.; He, B.; Lin, F.-M.; Palmquist, J.; Huang, S.-D.; Jin, H. Plants send small RNAs in extracellular vesicles to the fungal pathogen to silence virulence genes. Science 2018, 360, 1126–1129. [Google Scholar] [CrossRef]
- Suprun, A.R.; Kiselev, K.V.; Dubrovina, A.S. Exogenously Induced Silencing of Four MYB Transcription Repressor Genes and Activation of Anthocyanin Accumulation in Solanum lycopersicum. Int. J. Mol. Sci. 2023, 24, 9344. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H. Chitosan/dsRNA Polyplex Nanoparticles Advance Environmental RNA Interference Efficiency through Activating Clathrin-Dependent Endocytosis. Int. J. Biol. Macromol. 2023, 253, 127021. [Google Scholar] [CrossRef]
- Singewar, K.; Fladung, M. Double-Stranded RNA (dsRNA) Technology to Control Forest Insect Pests and Fungal Pathogens: Challenges and Opportunities. Funct. Integr. Genom. 2023, 23, 185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Goh, N.S.; Wang, J.W.; Pinals, R.L.; González-Grandío, E.; Demirer, G.S.; Butrus, S.; Fakra, S.C.; Del Rio Flores, A.; Zhai, R. Nanoparticle Cellular Internalization Is Not Required for RNA Delivery to Mature Plant Leaves. Nat. Nanotechnol. 2022, 17, 197–205. [Google Scholar] [CrossRef] [PubMed]
- De La Canal, L.; Pinedo, M. Extracellular vesicles: A missing component in plant cell wall remodeling. J. Exp. Bot. 2018, 69, 4655–4658. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, C.; Gan, Y.; Shao, L.; Chen, H.; Zhu, H. Exosome-Mediated siRNA Delivery to Suppress Postoperative Breast Cancer Metastasis. J. Control. Release 2020, 318, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Dad, H.A.; Gu, T.-W.; Zhu, A.-Q.; Huang, L.-Q.; Peng, L.-H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Bachman, P.; Fischer, J.; Song, Z.; Urbanczyk-Wochniak, E.; Watson, G. Environmental fate and dissipation of applied dsRNA in soil, aquatic systems, and plants. Front. Plant Sci. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed]
- Irie, A.; Sato, K.; Hara, R.I.; Wada, T.; Shibasaki, F. An artifcial cationic oligosaccharide combined with phosphorothioate linkages strongly improves siRNA stability. Sci. Rep. 2020, 10, 14845. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Booker, M.; Silver, S.J.; Friedman, A.; Hong, P.; Perrimon, N. Evidence of of-target efects associated with long dsRNAs in Drosophila melanogaster cell-based assays. Nat. Methods 2006, 3, 833–838. [Google Scholar] [CrossRef]
- Kaur, L.; Sohal, H.S.; Kaur, M.; Malhi, D.S.; Garg, S. A mini-review on nano technology in the tumour targeting strategies: Drug delivery to cancer cells. Anti-Cancer Agents Med. Chem. 2020, 20, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Xu, D.; Goh, N.S.; Demirer, G.S.; Cestellos-Blanco, S.; Chen, Y.; Landry, M.P.; Yang, P. Gold-nanocluster-mediated delivery of siRNA to intact plant cells for efficient gene knockdown. Nano Lett. 2021, 21, 5859–5866. [Google Scholar] [CrossRef] [PubMed]
- Demirer, G.S.; Silva, T.N.; Jackson, C.T.; Thomas, J.B.; Ehrhardt, D.W.; Rhee, S.Y.; Mortimer, J.C.; Landry, M.P. Nanotechnology to advance CRISPR–Cas genetic engineering of plants. Nat. Nanotechnol. 2021, 16, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Thagun, C.; Horii, Y.; Mori, M.; Fujita, S.; Ohtani, M.; Tsuchiya, K.; Kodama, Y.; Odahara, M.; Numata, K. Non-transgenic gene modulation via spray delivery of nucleic acid/peptide complexes into plant nuclei and chloroplasts. ACS Nano 2022, 16, 3506–3521. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Wang, M.; Cheng, L.; Si, M.; Feng, Z.; Feng, Z. Synergistic antibacterial mechanism of silver-copper bimetallic nanoparticles. Front. Bioeng. Biotechnol. 2024, 11, 1337543. [Google Scholar] [CrossRef] [PubMed]
- Thakre, N.; Carver, M.; Paredes-Montero, J.R.; Mondal, M.; Hu, J.; Saberi, E. UV-LASER adjuvant–surfactant-facilitated delivery of mobile dsRNA to tomato plant vasculature and evidence of biological activity by gene knockdown in the potato psyllid. Pest Manag. Sci. 2024, 80, 2141–2153. [Google Scholar] [CrossRef]
- Yin, G.; Sun, Z.; Liu, N.; Zhang, L.; Song, Y.; Zhu, C.; Wen, F. Production of double-stranded RNA for interference with TMV infection utilizing a bacterial prokaryotic expression system. Appl. Microbiol. Biotechnol. 2009, 84, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Melita, O.; Kaldis, A.; Berbati, M.; Reppa, C.; Holeva, M.; Lapidot, M.; Gelbart, D.; Otten, P.; Voloudakis, A. Topical application of double-stranded RNA molecules deriving from tomato yellow leaf curl virus reduces cognate virus infection in tomato. Biol. Plant 2021, 65, 100–110. [Google Scholar] [CrossRef]
- Borah, M.; Nath, P.D.; Chaudhury, S.P.; Biswas, K.K.; Patil, B.L.; Voloudakis, A. Topical application of dsRNAs targeting Citrus tristeza virus (CTV) reduces its titer in the CTV infected sweet orange (Citrus sinensis). Eur. J. Plant Pathol. 2024, 168, 273–278. [Google Scholar] [CrossRef]
- Necira, K.; Contreras, L.; Kamargiakis, S.; Kamoun, M.S.; Canto, T.; Tenllado, F. Comparative analysis of RNA interference and pattern-triggered immunity induced by dsRNA reveals different efficiencies in the antiviral response to Potato virus X. Mol. Plant Pathol. 2024, 25, e70008. [Google Scholar] [CrossRef] [PubMed]
- Frascati, F.; Rotunno, S.; Accotto, G.P.; Noris, E.; Vaira, A.M.; Miozzi, L. Exogenous application of dsRNA for Protection against tomato leaf curl new Delhi virus. Viruses 2014, 16, 436. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Malathi, V.G.; Renukadevi, P.; Kumar, S.M.; Raveendran, M.; Sudha, M.; Manivannan, N.; Karthikeyan, G. Exogenous delivery of dsRNA for management of mungbean yellow mosaic virus on blackgram. Planta 2023, 258, 94. [Google Scholar] [CrossRef] [PubMed]
- Patil, B.L.; Raghu, R.; Dangwal, M.; Byregowda, M.; Voloudakis, A. Exogenous dsRNA-mediated feld protection against Pigeonpea sterility mosaic emaravirus. J. Plant Biochem. Biotechnol. 2021, 30, 400–405. [Google Scholar] [CrossRef]
- Konakalla, N.C.; Bag, S.; Deraniyagala, A.S.; Culbreath, A.K.; Pappu, H.R. Induction of plant resistance in tobacco (Nicotiana tabacum) against tomato spotted wilt orthotospovirus through foliar application of dsRNA. Viruses 2021, 13, 662. [Google Scholar] [CrossRef] [PubMed]
- Holeva, M.C.; Sklavounos, A.; Rajeswaran, R.; Pooggin, M.M.; Voloudakis, A.E. Topical Application of Double-Stranded RNA Targeting 2b and CP Genes of Cucumber mosaic virus Protects Plants against Local and Systemic Viral Infection. Plants 2021, 10, 963. [Google Scholar] [CrossRef]
- Rego-Machado, C.M.; Nakasu, E.Y.; Silva, J.M.; Lucinda, N.; Nagata, T.; Inoue-Nagata, A.K. siRNA biogenesis and advances in topically applied dsRNA for controlling virus infections in tomato plants. Sci. Rep. 2020, 10, 22277. [Google Scholar] [CrossRef]
- Tabein, S.; Jansen, M.; Noris, E.; Vaira, A.M.; Marian, D.; Behjatnia, S.A.A. The induction of an efective dsRNA-mediated resistance against tomato spotted wilt virus by exogenous application of double-stranded RNA largely depends on the selection of the viral RNA target region. Front. Plant Sci. 2020, 11, 533338. [Google Scholar] [CrossRef] [PubMed]
- Namgial, T.; Kaldis, A.; Chakraborty, S.; Voloudakis, A. Topical application of double-stranded RNA molecules containing sequences of Tomato leaf curl virus and Cucumber mosaic virus confers protection against the cognate viruses. Physiol. Mol. Plant Pathol. 2019, 108, 101432. [Google Scholar] [CrossRef]
- Dietz-Pfeilstetter, A.; Mendelsohn, M.; Gathmann, A.; Klinkenbuß, D. Considerations and regulatory approaches in the USA and in the EU for dsRNA-based externally applied pesticides for plant protection. Front. Plant Sci. 2021, 12, 682387. [Google Scholar] [CrossRef]
- Leahy, J.; Mendelsohn, M.; Kough, J.; Jones, R.; Berckes, N. Biopesticide oversight and registration at the US Environmental Protection Agency. In Biopesticides: State of the Art and Future Opportunities; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2014; pp. 3–18. [Google Scholar]
- De Schutter, K.; Taning, C.N.T.; Van Daele, L.; Van Damme, E.J.M.; Dubruel, P.; Smagghe, G. RNAi-based biocontrol products: Market status, regulatory aspects, and risk assessment. Front. Insect Sci. 2022, 1, 818037. [Google Scholar] [CrossRef] [PubMed]
- EU. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009. Off. J. Eur. Union 2009, 309, 1–50. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:309:0001:0050:en:PDF (accessed on 28 March 2024).
- Schenkel, W.; Gathmann, A. Regulatory aspects of RNAi in plant production. In RNAi for Plant Improvement and Protection; CABI: Wallingford, UK, 2021; pp. 154–158. [Google Scholar]
- EU. EC, 2011 Commission Regulation (EU) No 546/2011 of 10 June 2011 Implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as Regards Uniform Principles for Evaluation and Authorisation of Plant Protection Products. Off. J. Eur. Union 2011, L155/127, 127–175. [Google Scholar]
- The European Commission ECRegulation (EC) No 284/2013. Off. J. Eur. Union 2013, 93, 1–68. Available online: https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2013:093:0085:0152:EN:PDF (accessed on 12 June 2024).
- OECD. Considerations for the Environmental Risk Assessment of the Application of Sprayed or Externally Applied ds-RNA-Based Pesticides. Series on Pesticides, No. 104. 2020, 1–174. Available online: https://images.chemycal.com/Media/Files/env-jm-mono(2020)26_(1).pdf (accessed on 12 June 2024).
- Eamens, A.; Wang, M.B.; Smith, N.A.; Waterhouse, P.M. RNA silencing in plants: Yesterday, today, and tomorrow. Plant Physiol. 2008, 147, 456–468. [Google Scholar] [CrossRef]
- Martínez de Alba, A.E.; Elvira-Matelot, E.; Vaucheret, H. Gene silencing in plants: A diversity of pathways. Biochim. Biophys. Acta 2013, 1829, 1300–1308. [Google Scholar] [CrossRef]
- Baulcombe, D. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef]
- Baulcombe, D.C. VIGS, HIGS and FIGS: Small RNA silencing in the interactions of viruses or filamentous organisms with their plant hosts. Curr. Opin. Plant Biol. 2015, 26, 141–146. [Google Scholar] [CrossRef]
- Rosa, C.; Kuo, Y.W.; Wuriyanghan, H.; Falk, B.W. RNA interference mechanisms and applications in plant pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; He, B.; Kogel, K.H.; Jin, H. Cross-kingdom RNA trafficking and environmental RNAi—Nature’s blueprint for modern crop protection strategies. Curr. Opin. Microbiol. 2018, 46, 58–64. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi efficiency, systemic properties, and novel delivery methods for pest insect control: What we know so far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Kogel, K.H. New wind in the sails: Improving the agronomic value of crop plants through RNAi-mediated gene silencing. Plant Biotechnol. J. 2014, 12, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Prins, M.; Laimer, M.; Noris, E.; Schubert, J.; Wassenegger, M.; Tepfer, M. Strategies for antiviral resistance in transgenic plants. Mol. Plant Pathol. 2008, 9, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Guo, J.; Peng, H.; Liu, P.; Kang, Z.; Guo, J. Host-induced gene silencing: A powerful strategy to control diseases of wheat and barley. Int. J. Mol. Sci. 2019, 20, 206. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.J.; Smagghe, G. RNAi technology for insect management and protection of beneficial insects from diseases: Lessons, challenges and risk assessments. Neotrop. Entomol. 2015, 44, 197–213. [Google Scholar] [CrossRef]
- Amari, K.; Niehl, A. Nucleic acid-mediated PAMP-triggered immunity in plants. Curr. Opin. Virol. 2020, 42, 32–39. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M. Revisiting RNA-directed DNA methylation. RNA Biol, 2013; 10, 453–455. [Google Scholar]
- Romeis, J.; Widmer, F. Assessing the risks of topically applied dsRNA-based products to non-target arthropods. Front. Plant Sci. 2020, 11, 679. [Google Scholar] [CrossRef]
- Joga, M.R.; Mogilicherla, K.; Smagghe, G.; Roy, A. RNA Interference-Based Forest Protection Products (FPPs) Against Wood-Boring Coleopterans: Hope or Hype? Front. Plant Sci. 2021, 12, 733608. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.M.; Barragán Borrero, V.; van Leeuwen, D.M.; Lever, M.A.; Mateescu, B.; Sander, M. Environmental fate of RNA interference pesticides: Adsorption and degradation of double-stranded RNA molecules in agricultural soils. Environ. Sci. Technol. 2019, 53, 3027–3036. [Google Scholar] [CrossRef]
- Chen, J.; Peng, Y.; Zhang, H.; Wang, K.; Zhao, C.; Zhu, G.; Palli, S.R.; Han, Z. Of-target efects of RNAi correlate with the mismatch rate between dsRNA and non-target mRNA. RNA Biol. 2021, 18, 1747–1759. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, J.; Meister, G. siRNA specificity: RNAi mechanisms and strategies to reduce of-target efects. Front. Plant Sci. 2021, 11, 526455. [Google Scholar] [CrossRef]
- James, C. A global overview of biotech (gm) crops: Adoption, impact and future prospects. GM Crops 2010, 1, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.D.; Liu, Z.C.; Huang, S.L.; Chen, Z.Q.; Sun, Y.W.; Duan, P.F.; Ma, Y.Z.; Xia, L.Q. RNAi-mediated plant protection against aphids. Pest Manag. Sci. 2016, 72, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Alshaer, W.; Zureigat, H.; Al Karaki, A.; Al-Kadash, A.; Gharaibeh, L.; Hatmal, M.M.; Aljabali, A.A.; Awidi, A. siRNA: Mechanism of action, challenges, and therapeutic approaches. Eur. J. Pharmacol. 2021, 905, 174178. [Google Scholar] [CrossRef]
- Mat Jalaluddin, N.S.; Othman, R.Y.; Harikrishna, J.A. Global trends in research and commercialization of exogenous and endogenous RNAi technologies for crops. Crit. Rev. Biotechnol. 2019, 39, 67–78. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).