Detection and Phylogenetic Characterization of Influenza D in Swedish Cattle
Abstract
1. Introduction
2. Materials and Methods
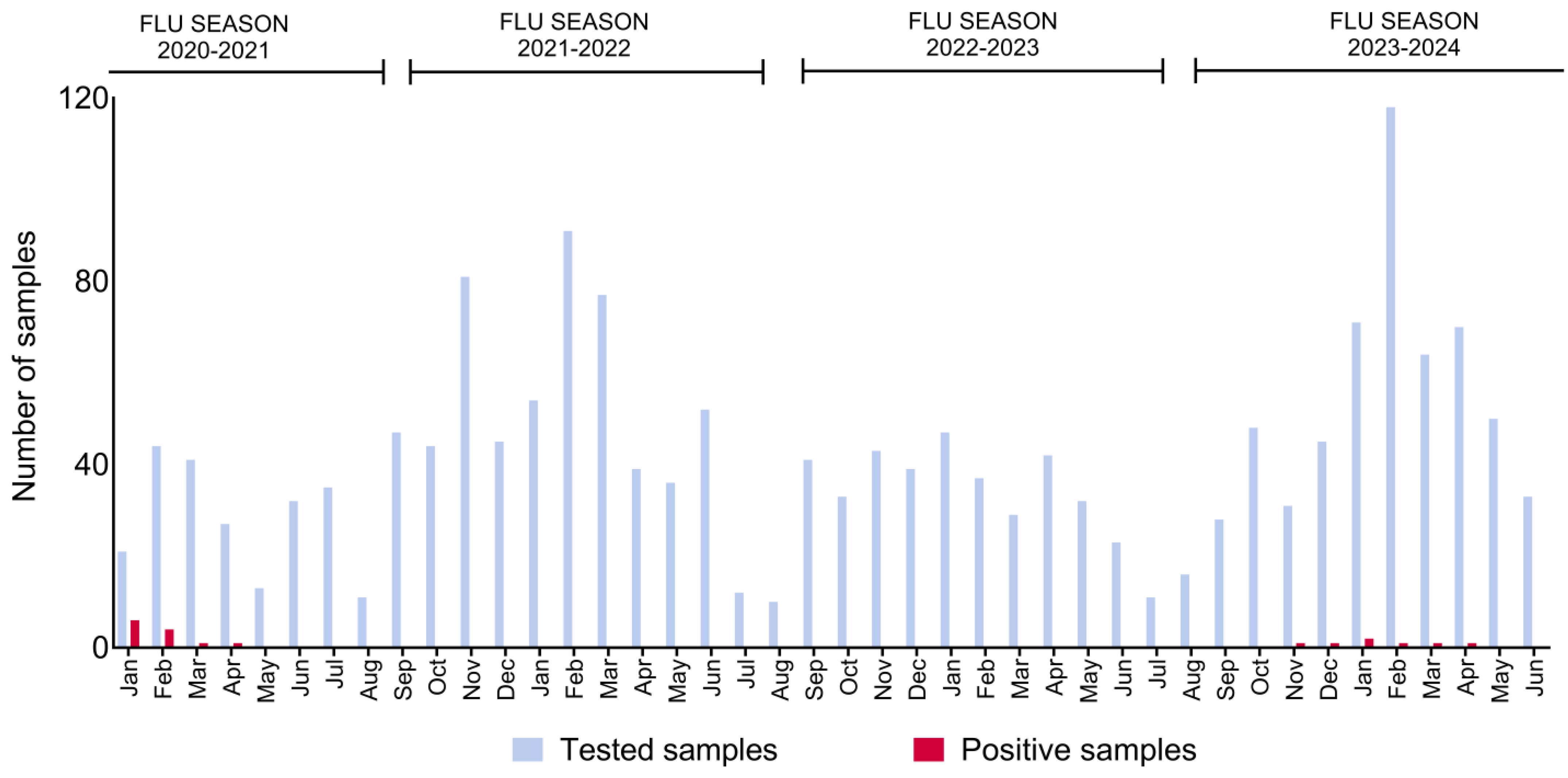
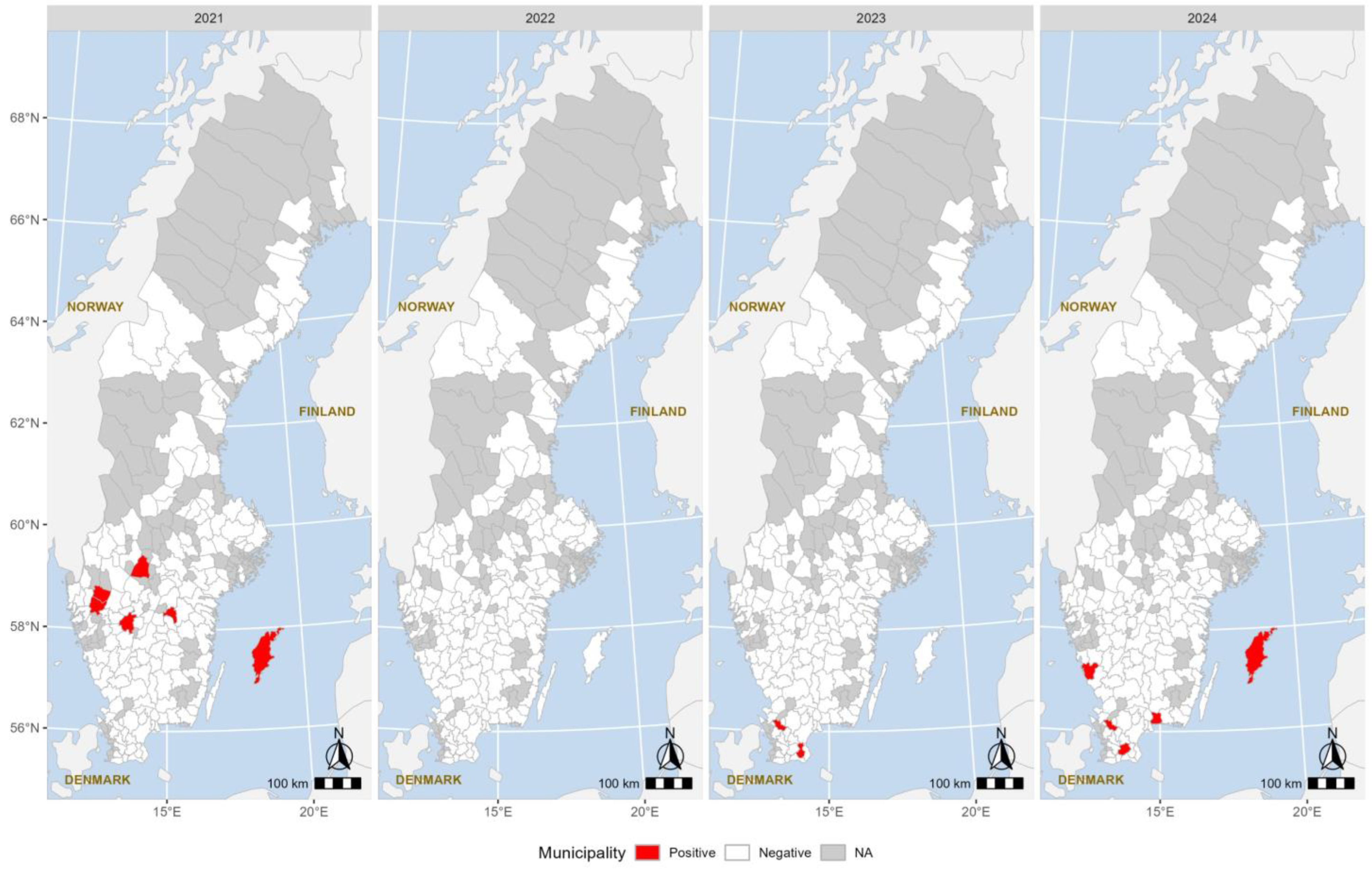
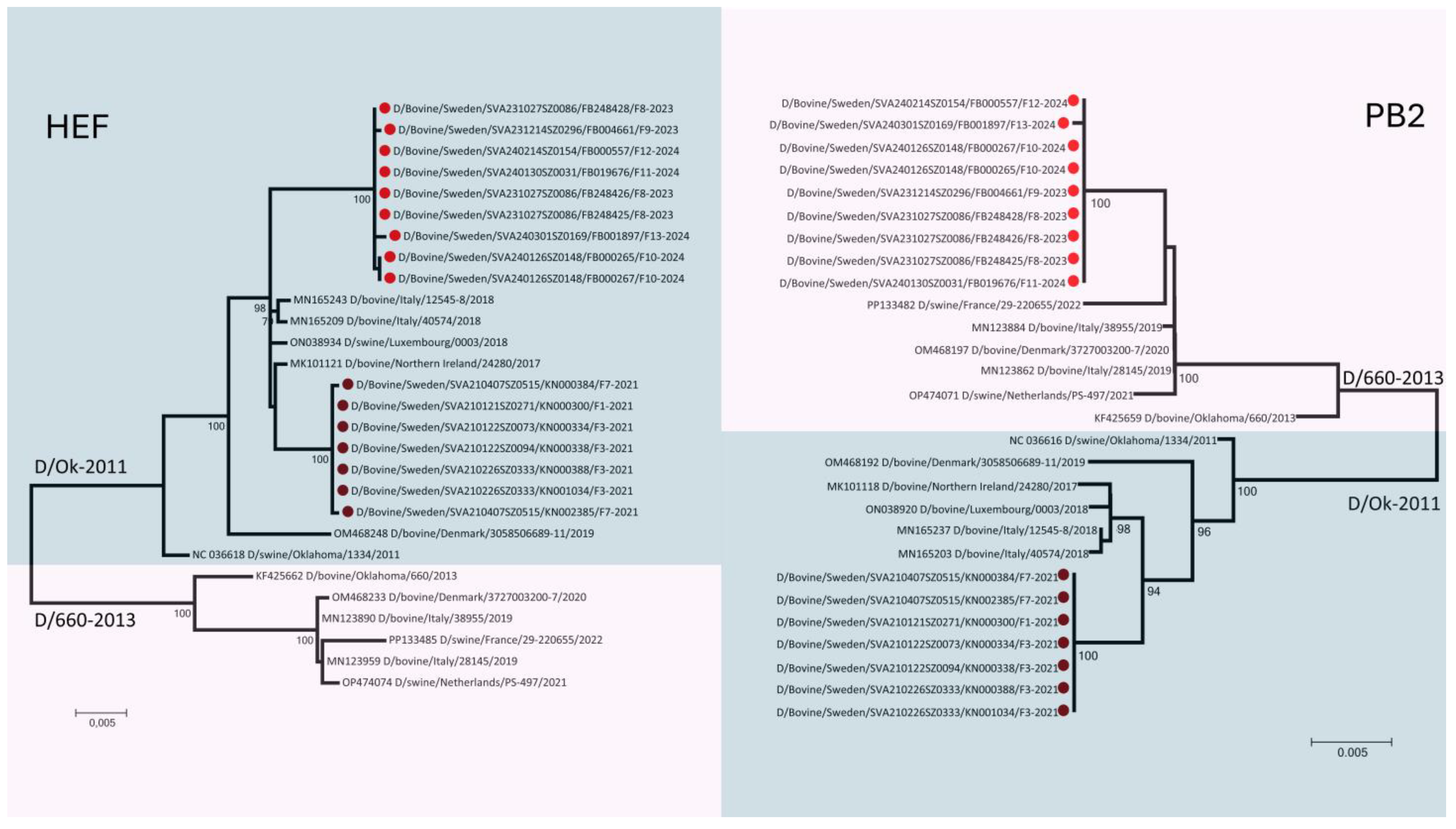
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hause, B.M.; Ducatez, M.; Collin, E.A.; Ran, Z.; Liu, R.; Sheng, Z.; Armien, A.; Kaplan, B.; Chakravarty, S.; Hoppe, A.D.; et al. Isolation of a novel swine influenza virus from Oklahoma in 2011 which is distantly related to human influenza C viruses. PLoS Pathog. 2013, 9, e1003176. [Google Scholar] [CrossRef]
- Mekata, H.; Yamamoto, M.; Hamabe, S.; Tanaka, H.; Omatsu, T.; Mizutani, T.; Hause, B.M.; Okabayashi, T. Molecular epidemiological survey and phylogenetic analysis of bovine influenza D virus in Japan. Transbound. Emerg. Dis. 2018, 65, e355–e360. [Google Scholar] [CrossRef]
- Wan, X.F.; Ferguson, L.; Oliva, J.; Rubrum, A.; Eckard, L.; Zhang, X.J.; Woolums, A.R.; Lion, A.; Meyer, G.; Murakami, S.; et al. Limited Cross-Protection Provided by Prior Infection Contributes to High Prevalence of Influenza D Viruses in Cattle. J. Virol. 2020, 94, e00240-20. [Google Scholar] [CrossRef] [PubMed]
- Mitra, N.; Cernicchiaro, N.; Torres, S.; Li, F.; Hause, B.M. Metagenomic characterization of the virome associated with bovine respiratory disease in feedlot cattle identified novel viruses and suggests an etiologic role for influenza D virus. J. Gen. Virol. 2016, 97, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.F.; Kondov, N.O.; Deng, X.; Van Eenennaam, A.; Neibergs, H.L.; Delwart, E. A metagenomics and case-control study to identify viruses associated with bovine respiratory disease. J. Virol. 2015, 89, 5340–5349. [Google Scholar] [CrossRef] [PubMed]
- Salem, E.; Hagglund, S.; Cassard, H.; Corre, T.; Naslund, K.; Foret, C.; Gauthier, D.; Pinard, A.; Delverdier, M.; Zohari, S.; et al. Pathogenesis, Host Innate Immune Response, and Aerosol Transmission of Influenza D Virus in Cattle. J. Virol. 2019, 93, e01853-18. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Marchi, S.; Manini, I.; Kistner, O.; Li, F.; Piu, P.; Manenti, A.; Biuso, F.; Sreenivasan, C.; Druce, J.; et al. Influenza D Virus: Serological Evidence in the Italian Population from 2005 to 2017. Viruses 2020, 12, 30. [Google Scholar] [CrossRef]
- Trombetta, C.M.; Montomoli, E.; Di Bartolo, I.; Ostanello, F.; Chiapponi, C.; Marchi, S. Detection of antibodies against influenza D virus in swine veterinarians in Italy in 2004. J. Med. Virol. 2022, 94, 2855–2859. [Google Scholar] [CrossRef]
- White, S.K.; Ma, W.; McDaniel, C.J.; Gray, G.C.; Lednicky, J.A. Serologic evidence of exposure to influenza D virus among persons with occupational contact with cattle. J. Clin. Virol. 2016, 81, 31–33. [Google Scholar] [CrossRef]
- Bailey, E.S.; Choi, J.Y.; Zemke, J.; Yondon, M.; Gray, G.C. Molecular surveillance of respiratory viruses with bioaerosol sampling in an airport. Trop. Dis. Travel. Med. Vaccines 2018, 4, 11. [Google Scholar] [CrossRef]
- Choi, J.Y.; Zemke, J.; Philo, S.E.; Bailey, E.S.; Yondon, M.; Gray, G.C. Aerosol Sampling in a Hospital Emergency Room Setting: A Complementary Surveillance Method for the Detection of Respiratory Viruses. Front. Public Health 2018, 6, 174. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; Mettier, J.; Sedano, L.; Delverdier, M.; Bourges-Abella, N.; Hause, B.; Loupias, J.; Pardo, I.; Bleuart, C.; Bordignon, P.J.; et al. Murine Model for the Study of Influenza D Virus. J. Virol. 2020, 94, e01662-19. [Google Scholar] [CrossRef]
- Sreenivasan, C.; Thomas, M.; Sheng, Z.Z.; Hause, B.M.; Collin, E.A.; Knudsen, D.E.B.; Pillatzki, A.; Nelson, E.; Wang, D.; Kaushik, R.S.; et al. Replication and Transmission of the Novel Bovine Influenza D Virus in a Guinea Pig Model. J. Virol. 2015, 89, 11990–12001. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, M.; Kelly, J.; Laloli, L.; Sturmer, I.; Portmann, J.; Stalder, H.; Dijkman, R. Determining the Replication Kinetics and Cellular Tropism of Influenza D Virus on Primary Well-Differentiated Human Airway Epithelial Cells. Viruses 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, M.; Chiapponi, C.; Moreno, A.; Zohari, S.; O’Donovan, T.; Quinless, E.; Sausy, A.; Oliva, J.; Salem, E.; Fusade-Boyer, M.; et al. Evolutionary and temporal dynamics of emerging influenza D virus in Europe (2009-22). Virus Evol. 2022, 8, veac081. [Google Scholar] [CrossRef]
- Collin, E.A.; Sheng, Z.Z.; Lang, Y.K.; Ma, W.J.; Hause, B.; Li, F. Cocirculation of Two Distinct Genetic and Antigenic Lineages of Proposed Influenza D Virus in Cattle. J. Virol. 2015, 89, 1036–1042. [Google Scholar] [CrossRef]
- Chiapponi, C.; Faccini, S.; Fusaro, A.; Moreno, A.; Prosperi, A.; Merenda, M.; Baioni, L.; Gabbi, V.; Rosignoli, C.; Alborali, G.L.; et al. Detection of a New Genetic Cluster of Influenza D Virus in Italian Cattle. Viruses 2019, 11, 1110. [Google Scholar] [CrossRef] [PubMed]
- Gorin, S.; Richard, G.; Herve, S.; Eveno, E.; Blanchard, Y.; Jardin, A.; Rose, N.; Simon, G. Characterization of Influenza D Virus Reassortant Strain in Swine from Mixed Pig and Beef Farm, France. Emerg. Infect. Dis. 2024, 30, 1672–1676. [Google Scholar] [CrossRef]
- Saegerman, C.; Gaudino, M.; Savard, C.; Broes, A.; Ariel, O.; Meyer, G.; Ducatez, M.F. Influenza D virus in respiratory disease in Canadian, province of Quebec, cattle: Relative importance and evidence of new reassortment between different clades. Transbound. Emerg. Dis. 2022, 69, 1227–1245. [Google Scholar] [CrossRef]
- Alvarez, I.; Hagglund, S.; Naslund, K.; Eriksson, A.; Ahlgren, E.; Ohlson, A.; Ducatez, M.F.; Meyer, G.; Valarcher, J.F.; Zohari, S. Detection of Influenza D-Specific Antibodies in Bulk Tank Milk from Swedish Dairy Farms. Viruses 2023, 15, 829. [Google Scholar] [CrossRef] [PubMed]
- Henritzi, D.; Hoffmann, B.; Wacheck, S.; Pesch, S.; Herrler, G.; Beer, M.; Harder, T.C. A newly developed tetraplex real-time RT-PCR for simultaneous screening of influenza virus types A, B, C and D. Influenza Other Resp. 2019, 13, 71–82. [Google Scholar] [CrossRef]
- Barrington, G.M.; Parish, S.M. Bovine neonatal immunology. Vet. Clin. N. Am. Food Anim. Pract. 2001, 17, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.S.; Li, F.; Wang, D. The first decade of research advances in influenza D virus. J. Gen. Virol. 2021, 102, jgv001529. [Google Scholar] [CrossRef]
- He, D.; Lui, R.; Wang, L.; Tse, C.K.; Yang, L.; Stone, L. Global Spatio-temporal Patterns of Influenza in the Post-pandemic Era. Sci. Rep. 2015, 5, 11013. [Google Scholar] [CrossRef]
- Horimoto, T.; Hiono, T.; Mekata, H.; Odagiri, T.; Lei, Z.; Kobayashi, T.; Norimine, J.; Inoshima, Y.; Hikono, H.; Murakami, K.; et al. Nationwide Distribution of Bovine Influenza D Virus Infection in Japan. PLoS ONE 2016, 11, e0163828. [Google Scholar] [CrossRef]
- Welliver, R.C., Sr. Temperature, humidity, and ultraviolet B radiation predict community respiratory syncytial virus activity. Pediatr. Infect. Dis. J. 2007, 26, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhu, H.; Horby, P.W.; Wang, Q.; Zhou, J.; Jiang, H.; Liu, L.; Zhang, T.; Zhang, Y.; Chen, X.; et al. Specificity, kinetics and longevity of antibody responses to avian influenza A(H7N9) virus infection in humans. J. Infect. 2020, 80, 310–319. [Google Scholar] [CrossRef]
- Hagglund, S.; Naslund, K.; Svensson, A.; Lefverman, C.; Enul, H.; Pascal, L.; Siltenius, J.; Holzhauer, M.; Delabouglise, A.; Osterberg, J.; et al. Longitudinal study of the immune response and memory following natural bovine respiratory syncytial virus infections in cattle of different age. PLoS ONE 2022, 17, e0274332. [Google Scholar] [CrossRef] [PubMed]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Lion, A.; Secula, A.; Rancon, C.; Boulesteix, O.; Pinard, A.; Deslis, A.; Hagglund, S.; Salem, E.; Cassard, H.; Naslund, K.; et al. Enhanced Pathogenesis Caused by Influenza D Virus and Mycoplasma bovis Coinfection in Calves: A Disease Severity Linked with Overexpression of IFN-gamma as a Key Player of the Enhanced Innate Immune Response in Lungs. Microbiol. Spectr. 2021, 9, e01690-21. [Google Scholar] [CrossRef]
- Ferguson, L.; Olivier, A.K.; Genova, S.; Epperson, W.B.; Smith, D.R.; Schneider, L.; Barton, K.; McCuan, K.; Webby, R.J.; Wan, X.F. Pathogenesis of Influenza D Virus in Cattle. J. Virol. 2016, 90, 5636–5642. [Google Scholar] [CrossRef] [PubMed]
- Noremark, M.; Frossling, J.; Lewerin, S.S. Application of routines that contribute to on-farm biosecurity as reported by Swedish livestock farmers. Transbound. Emerg. Dis. 2010, 57, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Goecke, N.B.; Liang, Y.; Otten, N.D.; Hjulsager, C.K.; Larsen, L.E. Characterization of Influenza D Virus in Danish Calves. Viruses 2022, 14, 423. [Google Scholar] [CrossRef]
- Wan, Y.M.; Kang, G.B.; Sreenivasan, C.; Daharsh, L.; Zhang, J.F.; Fan, W.J.; Wang, D.; Moriyama, H.; Li, F.; Li, Q.S. A DNA Vaccine Expressing Consensus Hemagglutinin-Esterase Fusion Protein Protected Guinea Pigs from Infection by Two Lineages of Influenza D Virus. J. Virol. 2018, 92, e00110-18. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.P.; Gordon, M.L. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 2022, 58, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, W.; Wang, Z.; Zhao, M.; Zhang, X.; Kong, Y.; Wang, X.; Feng, N.; Wang, T.; Yan, F.; et al. Amino acid sites related to the PB2 subunits of IDV affect polymerase activity. Virol. J. 2021, 18, 230. [Google Scholar] [CrossRef]
| Farm ID | Production Type | Sample Type | Age Group | Number of Tested Animals | Number of IDV-Positive Animals |
|---|---|---|---|---|---|
| 1 | F | NS | 3–6 w | 4 | 4 |
| 2 | S | NS | 1 y | 4 | 4 |
| 3 | F | NS | 12–14 w | 4 | 4 |
| NS | 3–5 w | 3 | 3 | ||
| NS | 8–28 w | 3 | 3 | ||
| NS | 4 w | 4 | 4 | ||
| NS | 8 w | 3 | 3 | ||
| NS | 4 w | 3 | 3 | ||
| 4 | F | NS | 8–12 w | 3 | 1 |
| 5 | n.d | NS | n.d | 1 | 1 |
| 6 | F | NS | 28 w | 1 | 1 |
| 7 | F | NS | n.d | 4 | 2 |
| 8 | F | NS | 4–14 w | 4 | 4 |
| 9 | F and S | Ns | 49 w | 1 | 1 |
| 10 | D | NS | 2–6 w | 4 | 4 |
| 11 | n.d | NS | n.d | 1 | 1 |
| 12 | F | NS | 4–14 w | 3 | 3 |
| 13 | n.d | Lung | 4 y | 1 | 1 |
| 14 | F | NS | 8–16 w | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, I.; Banihashem, F.; Persson, A.; Hurri, E.; Kim, H.; Ducatez, M.; Geijer, E.; Valarcher, J.-F.; Hägglund, S.; Zohari, S. Detection and Phylogenetic Characterization of Influenza D in Swedish Cattle. Viruses 2025, 17, 17. https://doi.org/10.3390/v17010017
Alvarez I, Banihashem F, Persson A, Hurri E, Kim H, Ducatez M, Geijer E, Valarcher J-F, Hägglund S, Zohari S. Detection and Phylogenetic Characterization of Influenza D in Swedish Cattle. Viruses. 2025; 17(1):17. https://doi.org/10.3390/v17010017
Chicago/Turabian StyleAlvarez, Ignacio, Fereshteh Banihashem, Annie Persson, Emma Hurri, Hyeyoung Kim, Mariette Ducatez, Erika Geijer, Jean-Francois Valarcher, Sara Hägglund, and Siamak Zohari. 2025. "Detection and Phylogenetic Characterization of Influenza D in Swedish Cattle" Viruses 17, no. 1: 17. https://doi.org/10.3390/v17010017
APA StyleAlvarez, I., Banihashem, F., Persson, A., Hurri, E., Kim, H., Ducatez, M., Geijer, E., Valarcher, J.-F., Hägglund, S., & Zohari, S. (2025). Detection and Phylogenetic Characterization of Influenza D in Swedish Cattle. Viruses, 17(1), 17. https://doi.org/10.3390/v17010017






