Recent Advances in Our Understanding of Human Inflammatory Dendritic Cells in Human Immunodeficiency Virus Infection
Abstract
1. Introduction
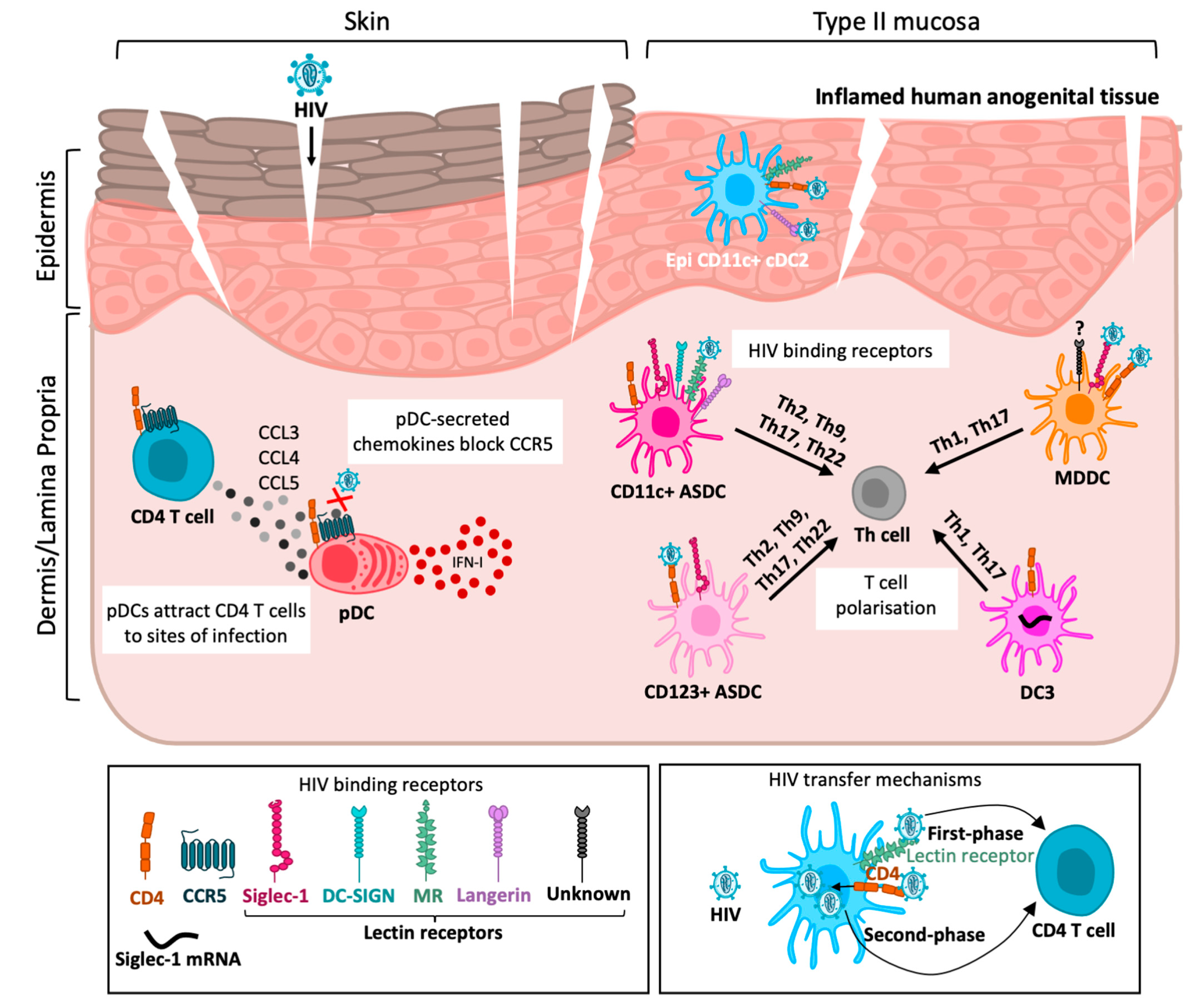
2. Human Anogenital Tissue
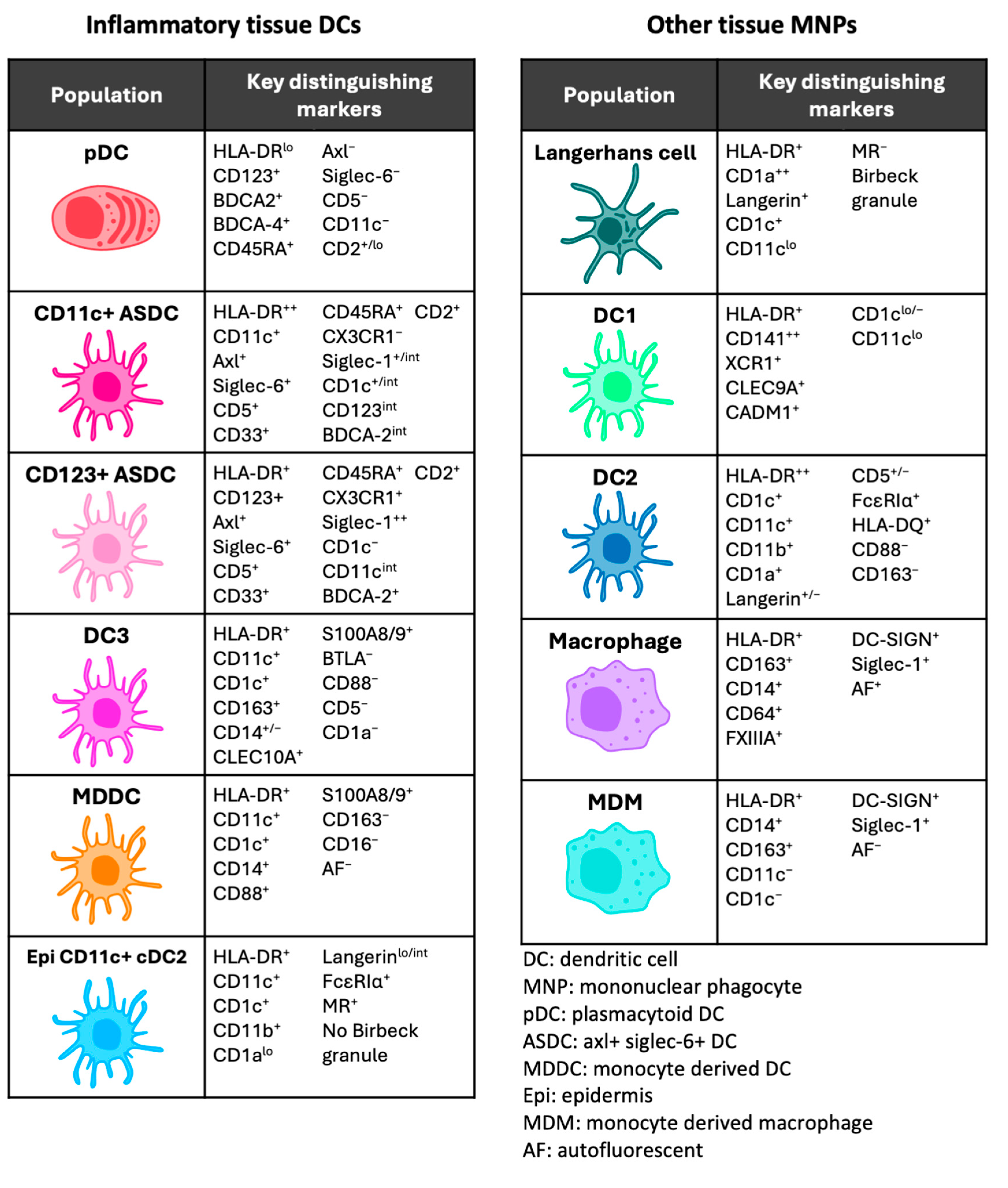
3. Dendritic Cells in Human Tissue
4. Dendritic Cell—T-Cell Transmission Mechanisms
5. Plasmacytoid Dendritic Cells
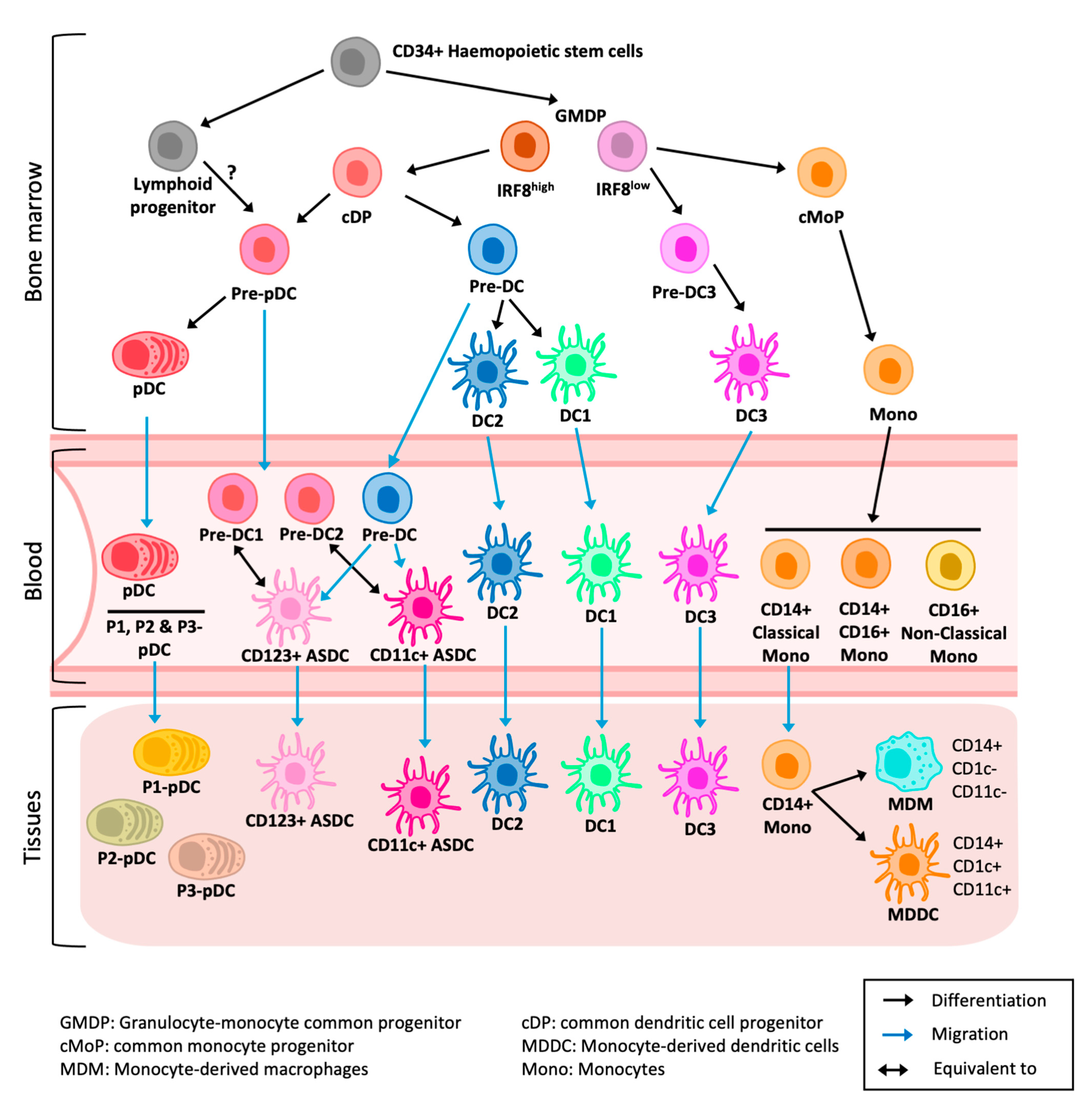
5.1. Origins and Discovery
5.2. Inflammation and Immunological Functions
5.3. HIV Interactions
6. ASDCs
6.1. Origins and Discovery
6.2. Inflammation and Immunological Functions
6.3. HIV Uptake, Infection and Transfer to T Cells by ASDCs
7. DC3
7.1. Origins and Discovery
7.2. Inflammation and Immunological Functions
7.3. HIV Interactions
8. Other Inflammatory Tissue Dendritic Cells
8.1. Monocyte-Derived Dendritic Cell
8.2. Epidermal CD11c+ Dendritic Cell
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baggaley, R.F.; Owen, B.N.; Silhol, R.; Elmes, J.; Anton, P.; McGowan, I.; van der Straten, A.; Shacklett, B.; Dang, Q.; Swann, E.M.; et al. Does per-act HIV-1 transmission risk through anal sex vary by gender? An updated systematic review and meta-analysis. Am. J. Reprod. Immunol. 2018, 80, e13039. [Google Scholar] [CrossRef] [PubMed]
- Stannah, J.; Silhol, R.; Elmes, J.; Owen, B.; Shacklett, B.L.; Anton, P.; McGowan, I.; van der Straten, A.; Dimitrov, D.; Baggaley, R.F.; et al. Increases in HIV Incidence Following Receptive Anal Intercourse Among Women: A Systematic Review and Meta-analysis. AIDS Behav. 2019, 24, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Cuadros, D.F.; Crowley, P.H.; Augustine, B.; Stewart, S.L.; García-Ramos, G. Effect of variable transmission rate on the dynamics of HIV in sub-Saharan Africa. BMC Infect. Dis. 2011, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Wawer, M.J.; Gray, R.H.; Sewankambo, N.K.; Serwadda, D.; Li, X.; Laeyendecker, O.; Kiwanuka, N.; Kigozi, G.; Kiddugavu, M.; Lutalo, T.; et al. Rates of HIV-1 Transmission per Coital Act, by Stage of HIV-1 Infection, in Rakai, Uganda. J. Infect. Dis. 2005, 191, 1403–1409. [Google Scholar] [CrossRef]
- Yi, T.J.; Shannon, B.; Prodger, J.; McKinnon, L.; Kaul, R. Genital immunology and HIV susceptibility in young women. Am. J. Reprod. Immunol. 2012, 69, 74–79. [Google Scholar] [CrossRef]
- Masson, L.; Passmore, J.-A.S.; Liebenberg, L.J.; Werner, L.; Baxter, C.; Arnold, K.B.; Williamson, C.; Little, F.; Mansoor, L.E.; Naranbhai, V.; et al. Genital Inflammation and the Risk of HIV Acquisition in Women. Clin. Infect. Dis. 2015, 61, 260–269. [Google Scholar] [CrossRef]
- Esra, R.T.; Olivier, A.J.; Passmore, J.-A.S.; Jaspan, H.B.; Harryparsad, R.; Gray, C.M. Does HIV Exploit the Inflammatory Milieu of the Male Genital Tract for Successful Infection? Front. Immunol. 2016, 7, 245. [Google Scholar] [CrossRef]
- Wall, K.M.; Kilembe, W.; Vwalika, B.; Haddad, L.B.; Hunter, E.; Lakhi, S.; Chavuma, R.; Khu, N.H.; Brill, I.; Vwalika, C.; et al. Risk of heterosexual HIV transmission attributable to sexually transmitted infections and non-specific genital inflammation in Zambian discordant couples, 1994–2012. Leuk. Res. 2017, 46, 1593–1606. [Google Scholar] [CrossRef]
- Passmore, J.-A.S.; Jaspan, H.B.; Masson, L. Genital inflammation, immune activation and risk of sexual HIV acquisition. Curr. Opin. HIV AIDS 2016, 11, 156–162. [Google Scholar] [CrossRef]
- Jewkes, R.K.; Dunkle, K.; Nduna, M.; Shai, N. Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: A cohort study. Lancet 2010, 376, 41–48. [Google Scholar] [CrossRef]
- Laga, M.; Manoka, A.; Kivuvu, M.; Malele, B.; Tuliza, M.; Nzila, N.; Goeman, J.; Behets, F.; Batter, V.; Alary, M.; et al. Non-ulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women. AIDS 1993, 7, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Mlisana, K.; Naicker, N.; Werner, L.; Roberts, L.; van Loggerenberg, F.; Baxter, C.; Passmore, J.-A.S.; Grobler, A.C.; Sturm, A.W.; Williamson, C.; et al. Symptomatic Vaginal Discharge Is a Poor Predictor of Sexually Transmitted Infections and Genital Tract Inflammation in High-Risk Women in South Africa. J. Infect. Dis. 2012, 206, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Masson, L.; Mlisana, K.; Little, F.; Werner, L.; Mkhize, N.N.; Ronacher, K.; Gamieldien, H.; Williamson, C.; Mckinnon, L.R.; Walzl, G.; et al. Defining genital tract cytokine signatures of sexually transmitted infections and bacterial vaginosis in women at high risk of HIV infection: A cross-sectional study. Sex. Transm. Infect. 2014, 90, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Atashili, J.; Poole, C.; Ndumbe, P.M.; A Adimora, A.; Smith, J.S. Bacterial vaginosis and HIV acquisition: A meta-analysis of published studies. AIDS 2008, 22, 1493–1501. [Google Scholar] [CrossRef]
- Liu, C.M.; Prodger, J.L.; Tobian, A.A.R.; Abraham, A.G.; Kigozi, G.; Hungate, B.A.; Aziz, M.; Nalugoda, F.; Sariya, S.; Serwadda, D.; et al. Penile Anaerobic Dysbiosis as a Risk Factor for HIV Infection. mBio 2017, 8, e00996-17. [Google Scholar] [CrossRef]
- Prodger, J.L.; Abraham, A.G.; Tobian, A.A.; Park, D.E.; Aziz, M.; Roach, K.; Gray, R.H.; Buchanan, L.; Kigozi, G.; Galiwango, R.M.; et al. Penile bacteria associated with HIV seroconversion, inflammation, and immune cells. J. Clin. Investig. 2021, 6. [Google Scholar] [CrossRef]
- McKinnon, L.R.; Liebenberg, L.J.; Yende-Zuma, N.; Archary, D.; Ngcapu, S.; Sivro, A.; Nagelkerke, N.; Lerma, J.G.G.; Kashuba, A.D.; Masson, L.; et al. Genital inflammation undermines the effectiveness of tenofovir gel in preventing HIV acquisition in women. Nat. Med. 2018, 24, 491–496. [Google Scholar] [CrossRef]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noël-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef]
- Dabee, S.; Barnabas, S.L.; Lennard, K.S.; Jaumdally, S.Z.; Gamieldien, H.; Balle, C.; Happel, A.-U.; Murugan, B.D.; Williamson, A.-L.; Mkhize, N.; et al. Defining characteristics of genital health in South African adolescent girls and young women at high risk for HIV infection. PLoS ONE 2019, 14, e0213975. [Google Scholar] [CrossRef]
- Caputo, V.; Libera, M.; Sisti, S.; Giuliani, B.; Diotti, R.A.; Criscuolo, E. The initial interplay between HIV and mucosal innate immunity. Front. Immunol. 2023, 14, 1104423. [Google Scholar] [CrossRef]
- Koumans, E.H.; Sternberg, M.; Bruce, C.B.; McQuillan, G.; Kendrick, J.; Sutton, M.; Markowitz, L.E. The Prevalence of Bacterial Vaginosis in the United States, 2001–2004; Associations with Symptoms, Sexual Behaviors, and Reproductive Health. Sex. Transm. Dis. 2007, 34, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Klebanoff, M.A.; Schwebke, J.R.; Zhang, J.; Nansel, T.R.; Yu, K.-F.; Andrews, W.W. Vulvovaginal Symptoms in Women with Bacterial Vaginosis. Obstet. Gynecol. 2004, 104, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Anahtar, M.N.; Byrne, E.H.; Doherty, K.E.; Bowman, B.A.; Yamamoto, H.S.; Soumillon, M.; Padavattan, N.; Ismail, N.; Moodley, A.; Sabatini, M.E.; et al. Cervicovaginal Bacteria Are a Major Modulator of Host Inflammatory Responses in the Female Genital Tract. Immunity 2015, 42, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Lennard, K.; Dabee, S.; Barnabas, S.L.; Havyarimana, E.; Blakney, A.; Jaumdally, S.Z.; Botha, G.; Mkhize, N.N.; Bekker, L.-G.; Lewis, D.A.; et al. Microbial Composition Predicts Genital Tract Inflammation and Persistent Bacterial Vaginosis in South African Adolescent Females. Infect. Immun. 2017, 86, e00410-17. [Google Scholar] [CrossRef] [PubMed]
- Gosmann, C.; Anahtar, M.N.; Handley, S.A.; Farcasanu, M.; Abu-Ali, G.; Bowman, B.A.; Padavattan, N.; Desai, C.; Droit, L.; Moodley, A.; et al. Lactobacillus-Deficient Cervicovaginal Bacterial Communities Are Associated with Increased HIV Acquisition in Young South African Women. Immunity 2017, 46, 29–37. [Google Scholar] [CrossRef]
- Hearps, A.; Tyssen, D.; Srbinovski, D.; Bayigga, L.; Diaz, D.J.D.; Aldunate, M.; Cone, R.; Gugasyan, R.; Anderson, D.; Tachedjian, G. Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunol. 2017, 10, 1480–1490. [Google Scholar] [CrossRef]
- Aldunate, M.; Tyssen, D.; Johnson, A.; Zakir, T.; Sonza, S.; Moench, T.; Cone, R.; Tachedjian, G. Vaginal concentrations of lactic acid potently inactivate HIV. J. Antimicrob. Chemother. 2013, 68, 2015–2025. [Google Scholar] [CrossRef]
- Tyssen, D.; Wang, Y.-Y.; Hayward, J.A.; Agius, P.A.; DeLong, K.; Aldunate, M.; Ravel, J.; Moench, T.R.; Cone, R.A.; Tachedjian, G. Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid. mSphere 2018, 3, e00055-18. [Google Scholar] [CrossRef]
- Nelson, S.G.; Liu, C.M. Penile microbiome: Decoding its impact on HIV risk. Curr. Opin. HIV AIDS 2024, 19, 241–245. [Google Scholar] [CrossRef]
- Fulcher, J.A.; Li, F.; Tobin, N.H.; Zabih, S.; Elliott, J.; Clark, J.L.; D’Aquila, R.; Mustanski, B.; Kipke, M.D.; Shoptaw, S.; et al. Gut dysbiosis and inflammatory blood markers precede HIV with limited changes after early seroconversion. EBioMedicine 2022, 84, 104286. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Cole, M.; Morris, A.; Martinson, J.; Mckay, H.; Mimiaga, M.; Margolick, J.; Fitch, A.; Methe, B.; et al. Signature changes in gut microbiome are associated with increased susceptibility to HIV-1 infection in MSM. Microbiome 2021, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.B.; Burgener, A.; Birse, K.; Romas, L.; Dunphy, L.J.; Shahabi, K.; Abou, M.; Westmacott, G.R.; McCorrister, S.; Kwatampora, J.; et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2016, 9, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Borgdorff, H.; Gautam, R.; Armstrong, S.D.; Xia, D.; Ndayisaba, G.F.; van Teijlingen, N.H.; Geijtenbeek, T.B.H.; Wastling, J.M.; van de Wijgert, J.H.H.M. Cervicovaginal microbiome dysbiosis is associated with proteome changes related to alterations of the cervicovaginal mucosal barrier. Mucosal Immunol. 2016, 9, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Zevin, A.S.; Xie, I.Y.; Birse, K.; Arnold, K.; Romas, L.; Westmacott, G.; Novak, R.M.; McCorrister, S.; McKinnon, L.R.; Cohen, C.R.; et al. Microbiome Composition and Function Drives Wound-Healing Impairment in the Female Genital Tract. PLoS Pathog. 2016, 12, e1005889. [Google Scholar] [CrossRef]
- Said, A.; Weindl, G. Regulation of Dendritic Cell Function in Inflammation. J. Immunol. Res. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Y.-Y.; Jiang, A.-P.; Jiang, J.-F.; Wang, J.-H. Stimulation of Cryptococcus neoformans isolated from skin lesion of AIDS patient matures dendritic cells and promotes HIV-1 trans-infection. Biochem. Biophys. Res. Commun. 2012, 423, 709–714. [Google Scholar] [CrossRef]
- Turville, S.G.; Santos, J.J.; Frank, I.; Cameron, P.U.; Wilkinson, J.; Miranda-Saksena, M.; Dable, J.; Stössel, H.; Romani, N.; Piatak, M.; et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood 2004, 103, 2170–2179. [Google Scholar] [CrossRef]
- Rhodes, J.W.; Botting, R.A.; Bertram, K.M.; Vine, E.E.; Rana, H.; Baharlou, H.; Vegh, P.; O’neil, T.R.; Ashhurst, A.S.; Fletcher, J.; et al. Human anogenital monocyte-derived dendritic cells and langerin+cDC2 are major HIV target cells. Nat. Commun. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Shang, L.; Duan, L.; Perkey, K.; Wietgrefe, S.; Zupancic, M.; Smith, A.; Southern, P.; Johnson, R.; Haase, A. Epithelium-innate immune cell axis in mucosal responses to SIV. Mucosal Immunol. 2016, 10, 508–519. [Google Scholar] [CrossRef]
- Haniffa, M.; Shin, A.; Bigley, V.; McGovern, N.; Teo, P.; See, P.; Wasan, P.S.; Wang, X.-N.; Malinarich, F.; Malleret, B.; et al. Human Tissues Contain CD141hi Cross-Presenting Dendritic Cells with Functional Homology to Mouse CD103+ Nonlymphoid Dendritic Cells. Immunity 2012, 37, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Silvin, A.; I Yu, C.; Lahaye, X.; Imperatore, F.; Brault, J.-B.; Cardinaud, S.; Becker, C.; Kwan, W.-H.; Conrad, C.; Maurin, M.; et al. Constitutive resistance to viral infection in human CD141 + dendritic cells. Sci. Immunol. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Bertram, K.M.; Botting, R.A.; Baharlou, H.; Rhodes, J.W.; Rana, H.; Graham, J.D.; Patrick, E.; Fletcher, J.; Plasto, T.M.; Truong, N.R.; et al. Identification of HIV transmitting CD11c+ human epidermal dendritic cells. Nat. Commun. 2019, 10, 2759. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef]
- Dutertre, C.-A.; Becht, E.; Irac, S.E.; Khalilnezhad, A.; Narang, V.; Khalilnezhad, S.; Ng, P.Y.; van den Hoogen, L.L.; Leong, J.Y.; Lee, B.; et al. Single-Cell Analysis of Human Mononuclear Phagocytes Reveals Subset-Defining Markers and Identifies Circulating Inflammatory Dendritic Cells. Immunity 2019, 51, 573–589.e8. [Google Scholar] [CrossRef]
- Bourdely, P.; Anselmi, G.; Vaivode, K.; Ramos, R.N.; Missolo-Koussou, Y.; Hidalgo, S.; Tosselo, J.; Nuñez, N.; Richer, W.; Vincent-Salomon, A.; et al. Transcriptional and Functional Analysis of CD1c+ Human Dendritic Cells Identifies a CD163+ Subset Priming CD8+CD103+ T Cells. Immunity 2020, 53, 335–352.e8. [Google Scholar] [CrossRef]
- Cytlak, U.; Resteu, A.; Pagan, S.; Green, K.; Milne, P.; Maisuria, S.; McDonald, D.; Hulme, G.; Filby, A.; Carpenter, B.; et al. Differential IRF8 Transcription Factor Requirement Defines Two Pathways of Dendritic Cell Development in Humans. Immunity 2020, 53, 353–370.e8. [Google Scholar] [CrossRef]
- Bourdely, P.; Petti, L.; Khou, S.; Meghraoui-Kheddar, A.; Elaldi, R.; Cazareth, J.; Mossadegh-Keller, N.; Boyer, J.; Sieweke, M.H.; Poissonnet, G.; et al. Autofluorescence identifies highly phagocytic tissue-resident macrophages in mouse and human skin and cutaneous squamous cell carcinoma. Front. Immunol. 2022, 13, 903069. [Google Scholar] [CrossRef]
- McGovern, N.; Schlitzer, A.; Gunawan, M.; Jardine, L.; Shin, A.; Poyner, E.; Green, K.; Dickinson, R.; Wang, X.-N.; Low, D.; et al. Human Dermal CD14 + Cells Are a Transient Population of Monocyte-Derived Macrophages. Immunity 2014, 41, 465–477. [Google Scholar] [CrossRef]
- McDonald, D.; Wu, L.; Bohks, S.M.; KewalRamani, V.N.; Unutmaz, D.; Hope, T.J. Recruitment of HIV and Its Receptors to Dendritic Cell-T Cell Junctions. Science 2003, 300, 1295–1297. [Google Scholar] [CrossRef]
- Cunningham, A.L.; Harman, A.; Nasr, N. Initial HIV mucosal infection and dendritic cells. EMBO Mol. Med. 2013, 5, 658–660. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.L.; Harman, A.; Nasr, N. Immunobiology of dendritic cells and the influence of HIV infection. Adv. Exp. Med. Biol. 2013, 762, 1–44. [Google Scholar]
- Turville, S.G.; Cameron, P.U.; Handley, A.; Lin, G.; Pöhlmann, S.; Doms, R.W.; Cunningham, A.L. Diversity of receptors binding HIV on dendritic cell subsets. Nat. Immunol. 2002, 3, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N.; Lai, J.; Botting, R.A.; Mercier, S.K.; Harman, A.N.; Kim, M.; Turville, S.; Center, R.J.; Domagala, T.; Gorry, P.R.; et al. Inhibition of Two Temporal Phases of HIV-1 Transfer from Primary Langerhans Cells to T Cells: The Role of Langerin. J. Immunol. 2014, 193, 2554–2564. [Google Scholar] [CrossRef] [PubMed]
- de Witte, L.; Nabatov, A.; Pion, M.; Fluitsma, D.; Jong, M.A.W.P.d.; de Gruijl, T.; Piguet, V.; van Kooyk, Y.; Geijtenbeek, T.B.H. Langerin is a natural barrier to HIV-1 transmission by Langerhans cells. Nat. Med. 2007, 13, 367–371. [Google Scholar] [CrossRef]
- Lai, J.; Bernhard, O.K.; Turville, S.G.; Harman, A.N.; Wilkinson, J.; Cunningham, A.L. Oligomerization of the Macrophage Mannose Receptor Enhances gp120-mediated Binding of HIV-1. J. Biol. Chem. 2009, 284, 11027–11038. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Kwon, D.S.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Middel, J.; Cornelissen, I.L.; Nottet, H.S.; KewalRamani, V.N.; Littman, D.R.; et al. DC-SIGN, a Dendritic Cell–Specific HIV-1-Binding Protein that Enhances trans-Infection of T Cells. Cell 2000, 100, 587–597. [Google Scholar] [CrossRef]
- Puryear, W.B.; Akiyama, H.; Geer, S.D.; Ramirez, N.P.; Yu, X.; Reinhard, B.M.; Gummuluru, S. Interferon-Inducible Mechanism of Dendritic Cell-Mediated HIV-1 Dissemination Is Dependent on Siglec-1/CD169. PLoS Pathog. 2013, 9, e1003291. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, B.; Chen, J.; Sun, X.; Yang, W.; Yang, T.; Yu, H.; Chen, P.; Chen, K.; Huang, X.; et al. Type III interferon inhibits bladder cancer progression by reprogramming macrophage-mediated phagocytosis and orchestrating effective immune responses. J. Immunother. Cancer 2024, 12, e007808. [Google Scholar] [CrossRef]
- Pino, M.; Erkizia, I.; Benet, S.; Erikson, E.; Fernández-Figueras, M.T.; Guerrero, D.; Dalmau, J.; Ouchi, D.; Rausell, A.; Ciuffi, A.; et al. HIV-1 immune activation induces Siglec-1 expression and enhances viral trans-infection in blood and tissue myeloid cells. Retrovirology 2015, 12, 1–15. [Google Scholar] [CrossRef]
- Izquierdo-Useros, N.; Lorizate, M.; Contreras, F.-X.; Rodriguez-Plata, M.T.; Glass, B.; Erkizia, I.; Prado, J.G.; Casas, J.; Fabriàs, G.; Kräusslich, H.-G.; et al. Sialyllactose in Viral Membrane Gangliosides Is a Novel Molecular Recognition Pattern for Mature Dendritic Cell Capture of HIV-1. PLoS Biol. 2012, 10, e1001315. [Google Scholar] [CrossRef]
- Yu, H.J.; Reuter, M.A.; McDonald, D. HIV Traffics through a Specialized, Surface-Accessible Intracellular Compartment during trans-Infection of T Cells by Mature Dendritic Cells. PLoS Pathog. 2008, 4, e1000134. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Useros, N.; Naranjo-Gómez, M.; Archer, J.; Hatch, S.C.; Erkizia, I.; Blanco, J.; Borràs, F.E.; Puertas, M.C.; Connor, J.H.; Fernández-Figueras, M.T.; et al. Capture and transfer of HIV-1 particles by mature dendritic cells converges with the exosome-dissemination pathway. Blood 2009, 113, 2732–2741. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, N.; Gea-Mallorquí, E.; Brouiller, F.; Jouve, M.; Silvin, A.; See, P.; Dutertre, C.-A.; Ginhoux, F.; Benaroch, P. Constitutive Siglec-1 expression confers susceptibility to HIV-1 infection of human dendritic cell precursors. Proc. Natl. Acad. Sci. USA 2019, 116, 21685–21693. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.A.; Gilbert, C.; Richard, M.; Beaulieu, A.D.; Tremblay, M.J. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood 2008, 112, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.A.; Azzi, A.; Lin, S.-X.; Allaire, G.; St-Gelais, K.P.; Tremblay, M.J.; Gilbert, C. Dendritic Cell Immunoreceptor Is a New Target for Anti-AIDS Drug Development: Identification of DCIR/HIV-1 Inhibitors. PLoS ONE 2013, 8, e67873. [Google Scholar] [CrossRef]
- Jin, W.; Li, C.; Du, T.; Hu, K.; Huang, X.; Hu, Q. DC-SIGN plays a stronger role than DCIR in mediating HIV-1 capture and transfer. Virology 2014, 458–459, 83–92. [Google Scholar] [CrossRef]
- Jolly, C.; Kashefi, K.; Hollinshead, M.; Sattentau, Q.J. HIV-1 Cell to Cell Transfer across an Env-induced, Actin-dependent Synapse. J. Exp. Med. 2004, 199, 283–293. [Google Scholar] [CrossRef]
- Sourisseau, M.; Sol-Foulon, N.; Porrot, F.; Blanchet, F.; Schwartz, O. Inefficient Human Immunodeficiency Virus Replication in Mobile Lymphocytes. J. Virol. 2007, 81, 1000–1012. [Google Scholar] [CrossRef]
- Aggarwal, A.; Iemma, T.L.; Shih, I.; Newsome, T.P.; McAllery, S.; Cunningham, A.L.; Turville, S.G. Mobilization of HIV Spread by Diaphanous 2 Dependent Filopodia in Infected Dendritic Cells. PLoS Pathog. 2012, 8, e1002762. [Google Scholar] [CrossRef]
- Lennert, K.; Remmele, W. Karyometrische Untersuchungen an Lymphknotenzellen des Menschen. Acta Haematol. 1958, 19, 99–113. [Google Scholar] [CrossRef]
- O’Doherty, U.; Peng, M.; Gezelter, S.; Swiggard, W.J.; Betjes, M.; Bhardwaj, N.; Steinman, R.M. Human blood contains two subsets of dendritic cells, one immunologically mature and the other immature. Immunology 1994, 82, 487–493. [Google Scholar]
- Rhodes, J.W.; Tong, O.; Harman, A.N.; Turville, S.G. Human Dendritic Cell Subsets, Ontogeny, and Impact on HIV Infection. Front. Immunol. 2019, 10, 1088. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ohteki, T.; Ginhoux, F.; Shortman, K.; Spits, H. Reclassifying plasmacytoid dendritic cells as innate lymphocytes. Nat. Rev. Immunol. 2022, 23, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B.; Idoyaga, J.; Dalod, M.; Barrat, F.; Naik, S.; Trinchieri, G.; Tussiwand, R.; Cella, M.; Colonna, M. Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature. Nat. Rev. Immunol. 2023, 23, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ohteki, T.; Ginhoux, F.; Shortman, K.; Spits, H. Reply to ‘Reclassification of plasmacytoid dendritic cells as innate lymphocytes is premature’. Nat. Rev. Immunol. 2023, 23, 338–339. [Google Scholar] [CrossRef]
- Cella, M.; Jarrossay, D.; Facchetti, F.; Alebardi, O.; Nakajima, H.; Lanzavecchia, A.; Colonna, M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999, 5, 919–923. [Google Scholar] [CrossRef]
- Siegal, F.P.; Kadowaki, N.; Shodell, M.; Fitzgerald-Bocarsly, P.A.; Shah, K.; Ho, S.; Antonenko, S.; Liu, Y.-J. The Nature of the Principal Type 1 Interferon-Producing Cells in Human Blood. Science 1999, 284, 1835–1837. [Google Scholar] [CrossRef]
- Takagi, H.; Fukaya, T.; Eizumi, K.; Sato, Y.; Sato, K.; Shibazaki, A.; Otsuka, H.; Hijikata, A.; Watanabe, T.; Ohara, O.; et al. Plasmacytoid Dendritic Cells Are Crucial for the Initiation of Inflammation and T Cell Immunity In Vivo. Immunity 2011, 35, 958–971. [Google Scholar] [CrossRef]
- Naik, S.H.; Sathe, P.; Park, H.-Y.; Metcalf, D.; I Proietto, A.; Dakic, A.; Carotta, S.; O’Keeffe, M.; Bahlo, M.; Papenfuss, A.; et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007, 8, 1217–1226. [Google Scholar] [CrossRef]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription Factor E2-2 Is an Essential and Specific Regulator of Plasmacytoid Dendritic Cell Development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef]
- Onai, N.; Kurabayashi, K.; Hosoi-Amaike, M.; Toyama-Sorimachi, N.; Matsushima, K.; Inaba, K.; Ohteki, T. A Clonogenic Progenitor with Prominent Plasmacytoid Dendritic Cell Developmental Potential. Immunity 2013, 38, 943–957. [Google Scholar] [CrossRef]
- Honda, K.; Taniguchi, T. IRFs: Master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 2006, 6, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Beignon, A.-S.; McKenna, K.; Skoberne, M.; Manches, O.; DaSilva, I.; Kavanagh, D.G.; Larsson, M.; Gorelick, R.J.; Lifson, J.D.; Bhardwaj, N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor- viral RNA interactions. J. Clin. Investig. 2005, 115, 3265–3275. [Google Scholar] [CrossRef] [PubMed]
- Fonteneau, J.-F.; Larsson, M.; Beignon, A.-S.; McKenna, K.; Dasilva, I.; Amara, A.; Liu, Y.-J.; Lifson, J.D.; Littman, D.R.; Bhardwaj, N. Human Immunodeficiency Virus Type 1 Activates Plasmacytoid Dendritic Cells and Concomitantly Induces the Bystander Maturation of Myeloid Dendritic Cells. J. Virol. 2004, 78, 5223–5232. [Google Scholar] [CrossRef] [PubMed]
- Tong, O.; Duette, G.; O’neil, T.R.; Royle, C.M.; Rana, H.; Johnson, B.; Popovic, N.; Dervish, S.; Brouwer, M.A.E.; Baharlou, H.; et al. Plasmacytoid dendritic cells have divergent effects on HIV infection of initial target cells and induce a pro-retention phenotype. PLoS Pathog. 2021, 17, e1009522. [Google Scholar] [CrossRef]
- van Dijk, F.A.W.; Tong, O.; O’neil, T.R.; Bertram, K.M.; Hu, K.; Baharlou, H.; Vine, E.E.; Jenns, K.; Gosselink, M.P.; Toh, J.W.; et al. Characterising plasmacytoid and myeloid AXL+ SIGLEC-6+ dendritic cell functions and their interactions with HIV. PLoS Pathog. 2024, 20, e1012351. [Google Scholar] [CrossRef]
- Gilliet, M.; Cao, W.; Liu, Y.-J. Plasmacytoid dendritic cells: Sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008, 8, 594–606. [Google Scholar] [CrossRef]
- See, P.; Dutertre, C.-A.; Chen, J.; Günther, P.; McGovern, N.; Irac, S.E.; Gunawan, M.; Beyer, M.; Händler, K.; Duan, K.; et al. Mapping the human DC lineage through the integration of high-dimensional techniques. Science 2017, 356. [Google Scholar] [CrossRef]
- Alculumbre, S.G.; Saint-André, V.; Di Domizio, J.; Vargas, P.; Sirven, P.; Bost, P.; Maurin, M.; Maiuri, P.; Wery, M.; Roman, M.S.; et al. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat. Immunol. 2017, 19, 63–75. [Google Scholar] [CrossRef]
- Onodi, F.; Bonnet-Madin, L.; Meertens, L.; Karpf, L.; Poirot, J.; Zhang, S.-Y.; Picard, C.; Puel, A.; Jouanguy, E.; Zhang, Q.; et al. SARS-CoV-2 induces human plasmacytoid predendritic cell diversification via UNC93B and IRAK4. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Cuevas, E.S.; Bendriss-Vermare, N.; Mouret, S.; De Fraipont, F.; Charles, J.; Valladeau-Guilemond, J.; Chaperot, L.; Aspord, C. Diversification of circulating and tumor-infiltrating plasmacytoid DCs towards the P3 (CD80+ PDL1-)-pDC subset negatively correlated with clinical outcomes in melanoma patients. Clin. Transl. Immunol. 2022, 11, e1382. [Google Scholar] [CrossRef]
- Sandler, N.G.; Bosinger, S.E.; Estes, J.D.; Zhu, R.T.R.; Tharp, G.K.; Boritz, E.; Levin, D.; Wijeyesinghe, S.; Makamdop, K.N.; del Prete, G.Q.; et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 2014, 511, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Bibollet-Ruche, F.; Sherrill-Mix, S.; Learn, G.H.; Plenderleith, L.; Smith, A.G.; Barbian, H.J.; Russell, R.M.; Gondim, M.V.P.; Bahari, C.Y.; et al. Resistance to type 1 interferons is a major determinant of HIV-1 transmission fitness. Proc. Natl. Acad. Sci. USA 2017, 114, E590–E599. [Google Scholar] [CrossRef] [PubMed]
- Foster, T.L.; Wilson, H.; Iyer, S.S.; Coss, K.; Doores, K.; Smith, S.; Kellam, P.; Finzi, A.; Borrow, P.; Hahn, B.H.; et al. Resistance of Transmitted Founder HIV-1 to IFITM-Mediated Restriction. Cell Host Microbe 2016, 20, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Lederle, A.; Su, B.; Holl, V.; Penichon, J.; Schmidt, S.; Decoville, T.; Laumond, G.; Moog, C. Neutralizing Antibodies Inhibit HIV-1 Infection of Plasmacytoid Dendritic Cells by an FcγRIIa Independent Mechanism and Do Not Diminish Cytokines Production. Sci. Rep. 2014, 4, srep05845. [Google Scholar] [CrossRef]
- Fong, L.; Mengozzi, M.; Abbey, N.W.; Herndier, B.G.; Engleman, E.G. Productive Infection of Plasmacytoid Dendritic Cells with Human Immunodeficiency Virus Type 1 Is Triggered by CD40 Ligation. J. Virol. 2002, 76, 11033–11041. [Google Scholar] [CrossRef]
- Scarlatti, G.; Tresoldi, E.; Björndal, Å.; Fredriksson, R.; Colognesi, C.; Deng, H.K.; Malnati, M.S.; Plebani, A.; Siccardi, A.G.; Littman, D.R.; et al. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat. Med. 1997, 3, 1259–1265. [Google Scholar] [CrossRef]
- Tsai, A.; Irrinki, A.; Kaur, J.; Cihlar, T.; Kukolj, G.; Sloan, D.D.; Murry, J.P. Toll-Like Receptor 7 Agonist GS-9620 Induces HIV Expression and HIV-Specific Immunity in Cells from HIV-Infected Individuals on Suppressive Antiretroviral Therapy. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Shi, H.M.; He, H.; Sun, C.; Fu, J.M.; Ghosh, D.; Deng, C.; Sheng, Y. Association of toll-like receptor polymorphisms with acquisition of HIV infection and clinical findings. Medicine 2020, 99, e23663. [Google Scholar] [CrossRef]
- Sutter, K.; Dickow, J.; Dittmer, U. Interferon α subtypes in HIV infection. Cytokine Growth Factor Rev. 2018, 40, 13–18. [Google Scholar] [CrossRef]
- Almeida, M.; Cordero, M.; Almeida, J.; Orfao, A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. Aids 2005, 19, 261–271. [Google Scholar]
- Hervas-Stubbs, S.; Perez-Gracia, J.L.; Rouzaut, A.; Sanmamed, M.F.; Le Bon, A.; Melero, I. Direct Effects of Type I Interferons on Cells of the Immune System. Clin. Cancer Res. 2011, 17, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-Hernández, M.; Leylek, R.; Wagar, L.E.; Engleman, E.G.; Keler, T.; Marinkovich, M.P.; Davis, M.M.; Nolan, G.P.; Idoyaga, J. High-Dimensional Phenotypic Mapping of Human Dendritic Cells Reveals Interindividual Variation and Tissue Specialization. Immunity 2017, 47, 1037–1050.e6. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, G.; Vegh, P.; Fletcher, J.; Poyner, E.F.M.; Stephenson, E.; Goh, I.; Botting, R.A.; Huang, N.; Olabi, B.; Dubois, A.; et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021, 371, eaba6500. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kim, M.; Yoon, D.-Y.; Choi, S.-J.; Kwon, Y.-N.; Kim, W.-S.; Park, S.-H.; Sung, J.-J.; Park, M.; Lee, J.S.; et al. AXL+SIGLEC6+ dendritic cells in cerebrospinal fluid and brain tissues of patients with autoimmune inflammatory demyelinating disease of CNS. Clin. Immunol. 2023, 253, 109686. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Gomes, T.; Hardman, C.S.; Braga, F.A.V.; Gutowska-Owsiak, D.; Salimi, M.; Gray, N.; Duncan, D.A.; Reynolds, G.; Johnson, D.; et al. Re-evaluation of human BDCA-2+ DC during acute sterile skin inflammation. J. Exp. Med. 2019, 217. [Google Scholar] [CrossRef]
- Jardine, L.; Wiscombe, S.; Reynolds, G.; McDonald, D.; Fuller, A.; Green, K.; Filby, A.; Forrest, I.; Ruchaud-Sparagano, M.-H.; Scott, J.; et al. Lipopolysaccharide inhalation recruits monocytes and dendritic cell subsets to the alveolar airspace. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Brouiller, F.; Nadalin, F.; Bonté, P.-E.; Ait-Mohamed, O.; Delaugerre, C.; Lelièvre, J.-D.; Ginhoux, F.; Ruffin, N.; Benaroch, P. Single-cell RNA-seq analysis reveals dual sensing of HIV-1 in blood Axl+ dendritic cells. iScience 2023, 26, 106019. [Google Scholar] [CrossRef]
- O’neil, T.R.; Hu, K.; Truong, N.R.; Arshad, S.; Shacklett, B.L.; Cunningham, A.L.; Nasr, N. The Role of Tissue Resident Memory CD4 T Cells in Herpes Simplex Viral and HIV Infection. Viruses 2021, 13, 359. [Google Scholar] [CrossRef]
- Yin, X.; Yu, H.; Jin, X.; Li, J.; Guo, H.; Shi, Q.; Yin, Z.; Xu, Y.; Wang, X.; Liu, R.; et al. Human Blood CD1c+ Dendritic Cells Encompass CD5high and CD5low Subsets That Differ Significantly in Phenotype, Gene Expression, and Functions. J. Immunol. 2017, 198, 1553–1564. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Zhou, Y.; Yang, Y.; Xie, B.; Cao, X. Siglec1 suppresses antiviral innate immune response by inducing TBK1 degradation via the ubiquitin ligase TRIM27. Cell Res. 2015, 25, 1121–1136. [Google Scholar] [CrossRef]
- Bernhard, O.K.; Lai, J.; Wilkinson, J.; Sheil, M.M.; Cunningham, A.L. Proteomic Analysis of DC-SIGN on Dendritic Cells Detects Tetramers Required for Ligand Binding but No Association with CD4. J. Biol. Chem. 2004, 279, 51828–51835. [Google Scholar] [CrossRef] [PubMed]
- Harman, A.N.; Wilkinson, J.; Bye, C.R.; Bosnjak, L.; Stern, J.L.; Nicholle, M.; Lai, J.; Cunningham, A.L. HIV Induces Maturation of Monocyte-Derived Dendritic Cells and Langerhans Cells. J. Immunol. 2006, 177, 7103–7113. [Google Scholar] [CrossRef] [PubMed]
- Segura, E. Human dendritic cell subsets: An updated view of their ontogeny and functional specialization. Eur. J. Immunol. 2022, 52, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Nakamizo, S.; Dutertre, C.-A.; Khalilnezhad, A.; Zhang, X.M.; Lim, S.; Lum, J.; Koh, G.; Foong, C.; Yong, P.J.A.; Tan, K.J.; et al. Single-cell analysis of human skin identifies CD14+ type 3 dendritic cells co-producing IL1B and IL23A in psoriasis. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef] [PubMed]
- Santegoets, S.J.; Duurland, C.L.; Jordanova, E.J.; van Ham, V.J.; Ehsan, I.; Loof, N.M.; Narang, V.; Dutertre, C.A.; Ginhoux, F.; van Egmond, S.L.; et al. CD163+ cytokine-producing cDC2 stimulate intratumoral type 1 T cell responses in HPV16-induced oropharyngeal cancer. J. Immunother. Cancer 2020, 8, e001053. [Google Scholar] [CrossRef] [PubMed]
- Subtil, B.; van der Hoorn, I.A.E.; Cuenca-Escalona, J.; Becker, A.M.D.; Alvarez-Begue, M.; Iyer, K.K.; Janssen, J.; van Oorschot, T.; Poel, D.; Gorris, M.A.J.; et al. cDC2 plasticity and acquisition of a DC3-like phenotype mediated by IL-6 and PGE2 in a patient-derived colorectal cancer organoids model. Eur. J. Immunol. 2024, 54, e2350891. [Google Scholar] [CrossRef]
- Qiu, G.; Zhong, S.; Xie, J.; Feng, H.; Sun, S.; Gao, C.; Xu, X.; Kang, B.; Xu, H.; Zhao, C.; et al. Expanded CD1c+CD163+ DC3 Population in Synovial Tissues Is Associated with Disease Progression of Osteoarthritis. J. Immunol. Res. 2022, 2022, 1–11. [Google Scholar] [CrossRef]
- Chen, W.; Jin, B.; Cheng, C.; Peng, H.; Zhang, X.; Tan, W.; Tang, R.; Lian, X.; Diao, H.; Luo, N.; et al. Single-cell profiling reveals kidney CD163+dendritic cell participation in human lupus nephritis. Ann. Rheum. Dis. 2024, 83, 608–623. [Google Scholar] [CrossRef]
- Parthasarathy, S.; de Lara, L.M.; Carrillo-Salinas, F.J.; Werner, A.; Borchers, A.; Iyer, V.; Vogell, A.; Fortier, J.M.; Wira, C.R.; Rodriguez-Garcia, M. Human genital dendritic cell heterogeneity confers differential rapid response to HIV-1 exposure. Front. Immunol. 2024, 15, 1472656. [Google Scholar] [CrossRef]
- Becker, A.M.; Decker, A.H.; Flórez-Grau, G.; Bakdash, G.; Röring, R.J.; Stelloo, S.; Vermeulen, M.; Piet, B.; Aarntzen, E.H.; Verdoes, M.; et al. Inhibition of CSF-1R and IL-6R prevents conversion of cDC2s into immune incompetent tumor-induced DC3s boosting DC-driven therapy potential. Cell Rep. Med. 2024, 5, 101386. [Google Scholar] [CrossRef]
- Bakdash, G.; Buschow, S.I.; Gorris, M.A.; Halilovic, A.; Hato, S.V.; Sköld, A.E.; Schreibelt, G.; Sittig, S.P.; Torensma, R.; Boer, T.D.-D.; et al. Expansion of a BDCA1+CD14+ Myeloid Cell Population in Melanoma Patients May Attenuate the Efficacy of Dendritic Cell Vaccines. Cancer Res. 2016, 76, 4332–4346. [Google Scholar] [CrossRef] [PubMed]
- Winheim, E.; Rinke, L.; Lutz, K.; Reischer, A.; Leutbecher, A.; Wolfram, L.; Rausch, L.; Kranich, J.; Wratil, P.R.; Huber, J.E.; et al. Impaired function and delayed regeneration of dendritic cells in COVID-19. PLoS Pathog. 2021, 17, e1009742. [Google Scholar] [CrossRef] [PubMed]
- Delia, D.; Cattoretti, G.; Polli, N.; Fontanella, E.; Aiello, A.; Giardini, R.; Rilke, F.; Della Porta, G. CD1c but neither CD1a nor CD1b molecules are expressed on normal, activated, and malignant human B cells: Identification of a new B-cell subset. Blood 1988, 72, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Fairhurst, R.M.; Wang, C.-X.; A Sieling, P.; Modlin, R.L.; Braun, J. CD1-restricted T cells and resistance to polysaccharide-encapsulated bacteria. Immunol. Today 1998, 19, 257–259. [Google Scholar] [CrossRef]
- Stewart, A.; Ng, J.C.-F.; Wallis, G.; Tsioligka, V.; Fraternali, F.; Dunn-Walters, D.K. Single-Cell Transcriptomic Analyses Define Distinct Peripheral B Cell Subsets and Discrete Development Pathways. Front. Immunol. 2021, 12, 602539. [Google Scholar] [CrossRef]
- Weller, S. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004, 104, 3647–3654. [Google Scholar] [CrossRef]
- Golinski, M.-L.; Demeules, M.; Derambure, C.; Riou, G.; Maho-Vaillant, M.; Boyer, O.; Joly, P.; Calbo, S. CD11c+ B Cells Are Mainly Memory Cells, Precursors of Antibody Secreting Cells in Healthy Donors. Front. Immunol. 2020, 11, 32. [Google Scholar] [CrossRef]
- Haniffa, M.; Ginhoux, F.; Wang, X.-N.; Bigley, V.; Abel, M.; Dimmick, I.; Bullock, S.; Grisotto, M.; Booth, T.; Taub, P.; et al. Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J. Exp. Med. 2009, 206, 371–385. [Google Scholar] [CrossRef]
- Harman, A.N.; Bye, C.R.; Nasr, N.; Sandgren, K.J.; Kim, M.; Mercier, S.K.; Botting, R.A.; Lewin, S.R.; Cunningham, A.L.; Cameron, P.U. Identification of Lineage Relationships and Novel Markers of Blood and Skin Human Dendritic Cells. J. Immunol. 2013, 190, 66–79. [Google Scholar] [CrossRef]
- Watchmaker, P.B.; Lahl, K.; Lee, M.; Baumjohann, D.; Morton, J.; Kim, S.J.; Zeng, R.; Dent, A.; Ansel, K.M.; Diamond, B.; et al. Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat. Immunol. 2013, 15, 98–108. [Google Scholar] [CrossRef]
- Tang-Huau, T.-L.; Gueguen, P.; Goudot, C.; Durand, M.; Bohec, M.; Baulande, S.; Pasquier, B.; Amigorena, S.; Segura, E. Human in vivo-generated monocyte-derived dendritic cells and macrophages cross-present antigens through a vacuolar pathway. Nat. Commun. 2018, 9, 2570. [Google Scholar] [CrossRef] [PubMed]
- Richter, L.; Landsverk, O.J.B.; Atlasy, N.; Bujko, A.; Yaqub, S.; Horneland, R.; Øyen, O.; Aandahl, E.M.; Lundin, K.E.A.; Stunnenberg, H.G.; et al. Transcriptional profiling reveals monocyte-related macrophages phenotypically resembling DC in human intestine. Mucosal Immunol. 2018, 11, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Michea, P.; Noël, F.; Zakine, E.; Czerwinska, U.; Sirven, P.; Abouzid, O.; Goudot, C.; Scholer-Dahirel, A.; Vincent-Salomon, A.; Reyal, F.; et al. Adjustment of dendritic cells to the breast-cancer microenvironment is subset specific. Nat. Immunol. 2018, 19, 885–897. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 1994, 179, 1109–1118. [Google Scholar] [CrossRef]
- Singh, T.P.; Zhang, H.H.; Borek, I.; Wolf, P.; Hedrick, M.N.; Singh, S.P.; Kelsall, B.L.; Clausen, B.E.; Farber, J.M. Monocyte-derived inflammatory Langerhans cells and dermal dendritic cells mediate psoriasis-like inflammation. Nat. Commun. 2016, 7, 13581. [Google Scholar] [CrossRef]
- Menezes, S.; Melandri, D.; Anselmi, G.; Perchet, T.; Loschko, J.; Dubrot, J.; Patel, R.; Gautier, E.L.; Hugues, S.; Longhi, M.P.; et al. The Heterogeneity of Ly6Chi Monocytes Controls Their Differentiation into iNOS+ Macrophages or Monocyte-Derived Dendritic Cells. Immunity 2016, 45, 1205–1218. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Jiang, J.; Cao, Z.; Zhai, F.; Sun, W.; Cheng, X. A subset of CD1c+ dendritic cells is increased in patients with tuberculosis and promotes Th17 cell polarization. Tuberculosis 2018, 113, 189–199. [Google Scholar] [CrossRef]
- Salvadé, V.; Manuel, O.; Golshayan, D.; Obregon, C. Monocyte-derived dendritic cells can be detected in urine of kidney transplant recipients with pathogenic asymptomatic bacteriuria. Front. Transplant. 2024, 3, 1366104. [Google Scholar] [CrossRef]
- Martin, J.C.; Chang, C.; Boschetti, G.; Ungaro, R.; Giri, M.; Grout, J.A.; Gettler, K.; Chuang, L.-S.; Nayar, S.; Greenstein, A.J.; et al. Single-Cell Analysis of Crohn’s Disease Lesions Identifies a Pathogenic Cellular Module Associated with Resistance to Anti-TNF Therapy. Cell 2019, 178, 1493–1508.e20. [Google Scholar] [CrossRef]
- Perez-Zsolt, D.; Cantero-Pérez, J.; Erkizia, I.; Benet, S.; Pino, M.; Serra-Peinado, C.; Hernández-Gallego, A.; Castellví, J.; Tapia, G.; Arnau-Saz, V.; et al. Dendritic Cells From the Cervical Mucosa Capture and Transfer HIV-1 via Siglec-1. Front. Immunol. 2019, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Duluc, D.; Gannevat, J.; Anguiano, E.; Zurawski, S.; Carley, M.; Boreham, M.; Stecher, J.; Dullaers, M.; Banchereau, J.; Oh, S. Functional diversity of human vaginal APC subsets in directing T-cell responses. Mucosal Immunol. 2012, 6, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Garcia, M.; Shen, Z.; Barr, F.; Boesch, A.; Ackerman, M.; Kappes, J.; Ochsenbauer, C.; Wira, C. Dendritic cells from the human female reproductive tract rapidly capture and respond to HIV. Mucosal Immunol. 2016, 10, 531–544. [Google Scholar] [CrossRef]
- Vine, E.E.; Rhodes, J.W.; Warner van Dijk, F.A.; Byrne, S.N.; Bertram, K.M.; Cunningham, A.L.; Harman, A.N. HIV trans-mitting mononuclear phagocytes; integrating the old and new. Mucosal Immunol. 2022. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kraft, S.; Hanau, D.; Bieber, T. Immunomorphological and Ultrastructural Characterization of Langerhans Cells and a Novel, Inflammatory Dendritic Epidermal Cell (IDEC) Population in Lesional Skin of Atopic Eczema. J. Investig. Dermatol. 1996, 106, 446–453. [Google Scholar] [CrossRef]
- Pena-Cruz, V.; Agosto, L.M.; Akiyama, H.; Olson, A.; Moreau, Y.; Larrieux, J.-R.; Henderson, A.; Gummuluru, S.; Sagar, M. HIV-1 replicates and persists in vaginal epithelial dendritic cells. J. Clin. Investig. 2018, 128, 3439–3444. [Google Scholar] [CrossRef]
- Bertram, K.M.; O’neil, T.R.; Vine, E.E.; Baharlou, H.; Cunningham, A.L.; Harman, A.N. Defining the landscape of human epidermal mononuclear phagocytes. Immunity 2023, 56, 459–460. [Google Scholar] [CrossRef]
- Novak, N.; Valenta, R.; Bohle, B.; Laffer, S.; Haberstok, J.; Kraft, S.; Bieber, T. FcεRI engagement of Langerhans cell–like dendritic cells and inflammatory dendritic epidermal cell–like dendritic cells induces chemotactic signals and different T-cell phenotypes in vitro. J. Allergy Clin. Immunol. 2004, 113, 949–957. [Google Scholar] [CrossRef]
- FACT SHEET 2024 Global HIV Statistics; UNAIDS: Geneva, Switzerland, 2024; Available online: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed on 31 december 2024).
- Van der Sluis, R.M.; Egedal, J.H.; Jakobsen, M.R. Plasmacytoid Dendritic Cells as Cell-Based Therapeutics: A Novel Immunotherapy to Treat Human Immunodeficiency Virus Infection? Front. Cell. Infect. Microbiol. 2020, 10, 249. [Google Scholar] [CrossRef]
| Citation | Source Tissue | Defining Markers | Separated into CD11c+ and CD123+ Subsets? | Immune Functions | HIV Interactions |
|---|---|---|---|---|---|
| Villani et al., 2017 [44] | Blood and tonsil | Axl+ Siglec-6+ CD5+ CD11c+/− CD123+/− | Yes | Both subsets are potent stimulators of CD4 and CD8 T-cell proliferation | Not investigated |
| See et al., 2019 [89] | Blood and spleen | Siglec-6+ CD123+ CX3CR1+ CD45RA+ CD33+ CD5+ CD2+ | No | Induced proliferation and polarisation of CD4 T cells | Not investigated |
| Alcántara-Hernández et al., 2017 [104] | Blood, tonsil and spleen | Axl+ CD123+/int CD11c+/− CD2+ | Yes | ASDCs have a higher T-cell-stimulating capacity compared to pDCs | Not investigated |
| Warner van Dijk et al., 2024 [87] | Blood and anogenital tissue | Axl+ Siglec-6+ CD5+ CD11c+/− CD123+/− CX3CR1+/− | Yes | CD11c+ ASDCs are more potent inducers of CD4 T-cell activation and proliferation compared to CD123+ ASDCs. Both ASDC subsets polarise T cells towards Th2, Th9, Th17, Th22 and Treg | CD11c+ ASDCs are more efficient at first-phase transfer to CD4 T cells CD123+ ASDCs are more efficient at second-phase transfer to CD4 T cells |
| Kang et al., 2023 [106] | Cerebrospinal fluid (demyelinating diseases) | Axl+ Siglec-6+ | No | Stimulate CD4 T cells and mature LAMP3+ DCs Bind B and T cells | Not investigated |
| Chen, 2020 [107] | Skin (blisters and wounds) | Axl+ Siglec-6+ BDCA-2+ CD123int | No | Identified as an early infiltrator in inflammation | Not investigated |
| Jardine, 2019 [108] | Bronchoalveolar lavage | Axl+ Siglec-6+ | Yes | Too few cell numbers for functional investigation | Not investigated |
| Ruffin, 2019 [64] | Blood | Axl+ CD123+ CD45RA+ Siglec-1+ | No | Not investigated | CD123+ ASDCs are productively infected with HIV and transfer the virus to CD4 T cells |
| Brouiller, 2023 [109] | Blood | Axl+ | Yes/No (investigated as both combined and separate) | Not investigated | Productive HIV onfection of ASDCs was mediated by Vpx which neutralises SAMHD1, a restrictive factor that limits productive infection |
| Citation | Source Tissue | Defining Markers | Immune Functions | HIV Interactions |
|---|---|---|---|---|
| Villani et al., 2017 [44] | Blood and tonsil | CD1c+ CD163+ | Stimulates naïve CD4+ and CD8+ T cells | High protein expression of HIV co-receptor CXCR4 RNA expression of HIV lectin receptor siglec-1 |
| Dutertre et al., 2019 [45] | Blood (lupus erythematosus) | CD1c+ CD163+ CD5− CD88− CD14+/− | Stimulates naïve CD4+ T cells. Induces Th1, Th2 and Th17 polarisation | RNA expression of HIV lectin receptor siglec-1 which is increased on inflammatory DC3s |
| Bourdely et al., 2020 [46] | Blood and primary breast tumours | CD1c+ CD163+ CD88− CD14+/− | Stimulates naïve CD4+ and CD8+ T cells Secretes pro-inflammatory cytokines IL-23 and TNFα | Not investigated |
| Cytlak et al., 2020 [47] | Blood, spleen, dermis | CD1c+ CD163+ CD14+ BTLA− | Secretes pro-inflammatory cytokines IL-8, TNFα and IL-1β | Not investigated |
| Nakamizo et al., 2021 [116] | Blood and body skin (psoriasis) | CD1c+ CD14+ CD88− | Co-expression of markers IL-6 and IL-23 indicates their potential role in Th17 cell differentiation | Not investigated |
| Jardine et al., 2019 [108] | Bronchoalveolar lavage | BTLA- CD5- CD163+ CD14+ S100A8/9+ | Induces Th1 and Th17 polarisation | Not investigated |
| Chen et al., 2024 [120] | Kidney (lupus nephritis) | CD163+ CD1c+ CD88- | Induces Th1 and Th17 polarisation. High DC3 numbers associated with poor renal prognosis | Not investigated |
| Subtil et al., 2024 [118] | Primary malignant colorectal tumor and liver metastasis | CD14+ CD1c+ CD163+ | Impaired T-cell-activating and -proliferating capacity compared to DC2s | Not investigated |
| Santegoets et al., 2020 [117] | Oropharyngeal squamous cell carcinoma tumor | CD1c+ CD163+ CD14− | Secretes cytokines IL-12 and IL-18 Primes Th1 polarisation | High protein expression of HIV co-receptor CXCR4 |
| Qiu et al., 2022 [119] | Synovium and synovial fluid (osteoarthritis) | CD1c+ CD163+ CD88− | Secretes pro inflammatory cytokines TNFα, IL-23 and IL12p70 Primes CD8+ T cells | Not investigated |
| Parthasarathy et al., 2024 [121] | Cervix and endometrium | CD1c+ CD14+ (combined with MDDC) | Upregulation of genes associated with the initiation of inflammation and antiviral roles | High protein expression of HIV entry receptors CD4, CCR5 and CXCR4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Warner van Dijk, F.A.; Bertram, K.M.; O’Neil, T.R.; Li, Y.; Buffa, D.J.; Harman, A.N.; Cunningham, A.L.; Nasr, N. Recent Advances in Our Understanding of Human Inflammatory Dendritic Cells in Human Immunodeficiency Virus Infection. Viruses 2025, 17, 105. https://doi.org/10.3390/v17010105
Warner van Dijk FA, Bertram KM, O’Neil TR, Li Y, Buffa DJ, Harman AN, Cunningham AL, Nasr N. Recent Advances in Our Understanding of Human Inflammatory Dendritic Cells in Human Immunodeficiency Virus Infection. Viruses. 2025; 17(1):105. https://doi.org/10.3390/v17010105
Chicago/Turabian StyleWarner van Dijk, Freja A., Kirstie M. Bertram, Thomas R. O’Neil, Yuchen Li, Daniel J. Buffa, Andrew N. Harman, Anthony L. Cunningham, and Najla Nasr. 2025. "Recent Advances in Our Understanding of Human Inflammatory Dendritic Cells in Human Immunodeficiency Virus Infection" Viruses 17, no. 1: 105. https://doi.org/10.3390/v17010105
APA StyleWarner van Dijk, F. A., Bertram, K. M., O’Neil, T. R., Li, Y., Buffa, D. J., Harman, A. N., Cunningham, A. L., & Nasr, N. (2025). Recent Advances in Our Understanding of Human Inflammatory Dendritic Cells in Human Immunodeficiency Virus Infection. Viruses, 17(1), 105. https://doi.org/10.3390/v17010105









