Clinical Performance of MAGLUMI Diagnostic Tests for the Automated Detection of Dengue Virus
Abstract
1. Introduction
2. Material and Methods
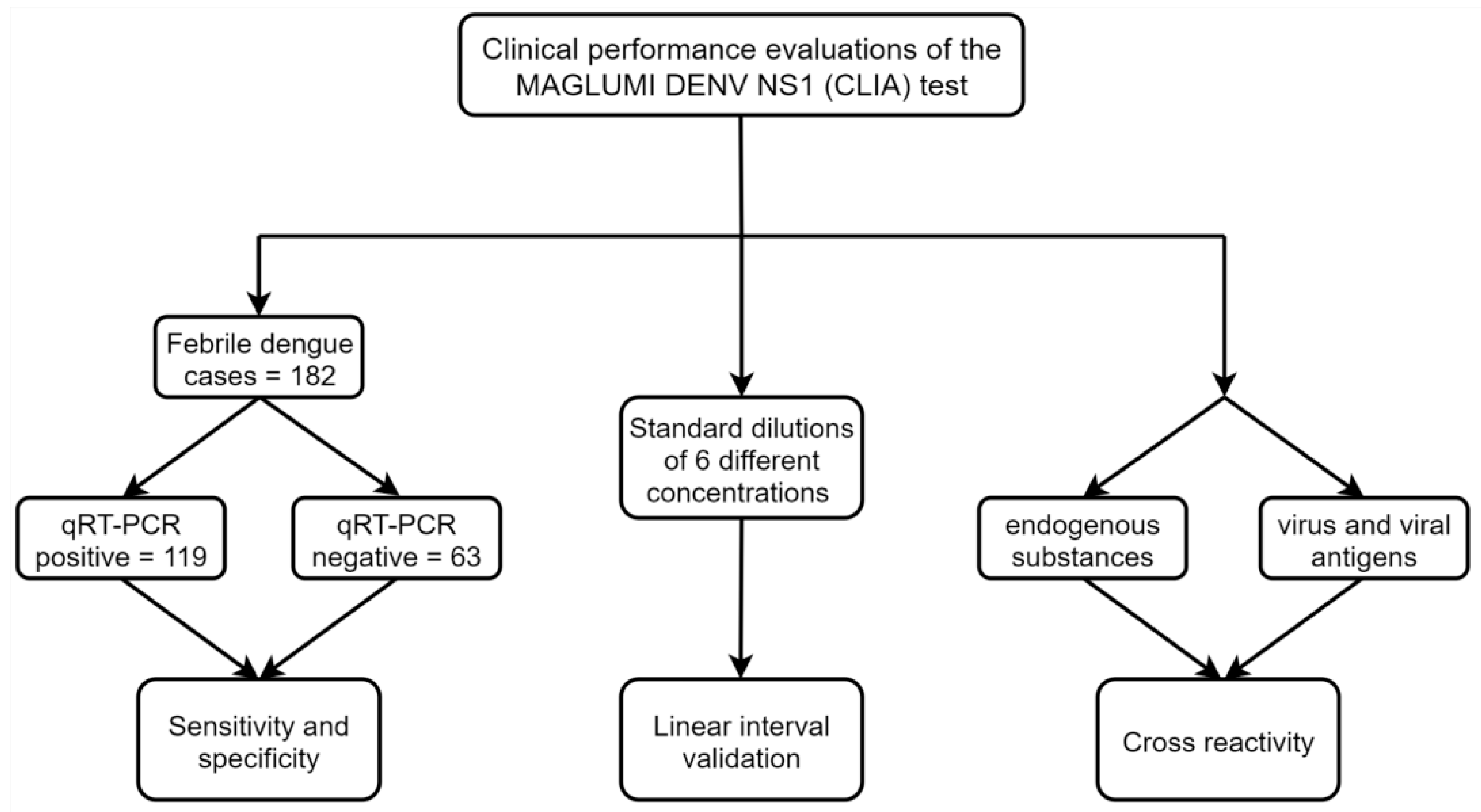
2.1. Clinical Samples
2.2. MAGLUMI Dengue Virus NS1 Antigen Test
| Coating Antibody | Labeling Antibody | |
| Immunogen | Dengue NS1 antigen | Dengue NS1 antigen |
| Source | mouse | mouse |
| Molecular weight (kDa) | 150 | 150 |
| SDS-PAGE bands (kDa) | 25, 55 | 25, 55 |
| Purity (SDS-page) | ≥95% | ≥95% |
| Concentration (mg/mL) | 2.449–3.178 | 2.114–2.869 |
2.3. Analytical Performance Verification
2.4. Endogenous Interference and Cross-Reactivity
2.5. Statistical Analysis
2.6. Ethics
3. Results
3.1. Diagnostic Specificity and Sensitivity
3.2. Analytical Specificity
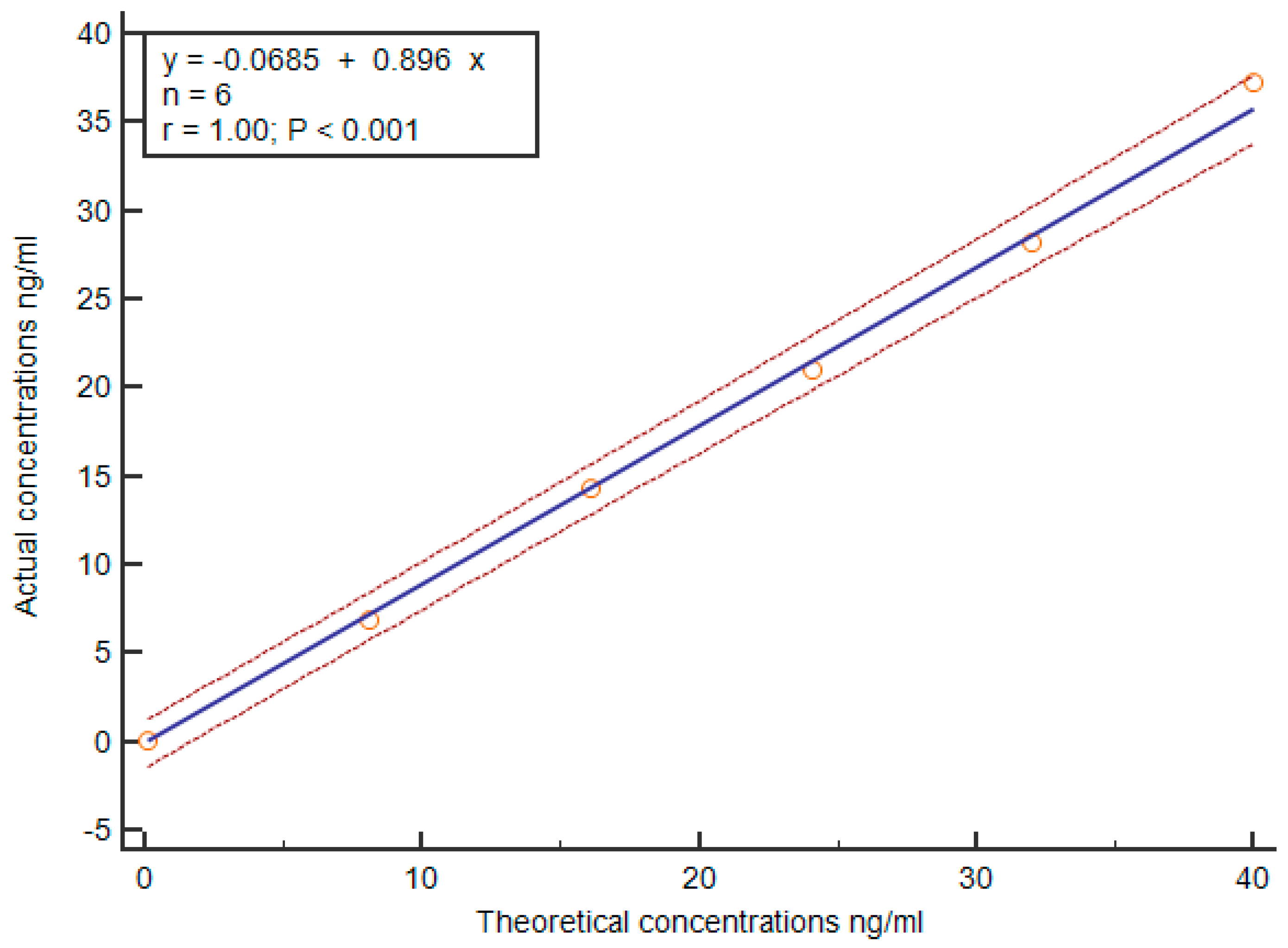
3.3. Analytic Sensitivity and Linearity Verification
3.4. Serum to Plasma Equivalency
3.5. Consistency Between Different Dengue Diagnostic Assays
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8 (Suppl. 12), S7–S16. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mairuhu, A.T.A.; Wagenaar, J.; Brandjes, D.P.M.; van Gorp, E.C.M. Dengue: An arthropod-borne disease of global importance. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 425–433. [Google Scholar] [CrossRef]
- Halstead, S.B. Etiologies of the experimental dengues of Siler and Simmons. Am. J. Trop. Med. Hyg. 1974, 23, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Kok, B.H.; Lim, H.T.; Lim, C.P.; Lai, N.S.; Leow, C.Y.; Leow, C.H. Dengue virus infection—A review of pathogenesis, vaccines, diagnosis and therapy. Virus Res. 2023, 324, 199018. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Halstead, S.B.; Mahalingam, S.; Marovich, M.A.; Ubol, S.; Mosser, D.M. Intrinsic antibody-dependent enhancement of microbial infection in mac rophages: Disease regulation by immune complexes. Lancet Infect. Dis. 2010, 10, 712–722. [Google Scholar] [CrossRef] [PubMed Central]
- Tatem, A.J.; Hay, S.I.; Rogers, D.J. Global traffic and disease vector dispersal. Proc. Natl. Acad. Sci. USA 2006, 103, 6242–6247. [Google Scholar] [CrossRef] [PubMed Central]
- Johansson, M.A.; Hombach, J.; Cummings, D.A.T. Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine 2011, 29, 5860–5868. [Google Scholar] [CrossRef] [PubMed Central]
- Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009.
- Tang, K.F.; Ooi, E.E. Diagnosis of dengue: An update. Expert. Rev. Anti Infect. Ther. 2012, 10, 895–907. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Calisher, C.H.; Gubler, D.J.; Chang, G.J.; Vorndam, A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 545–551. [Google Scholar] [CrossRef] [PubMed Central]
- Najioullah, F.; Viron, F.; Césaire, R. Evaluation of four commercial real-time RT-PCR kits for the detection of dengue viruses in clinical samples. Virol. J. 2014, 11, 164. [Google Scholar] [CrossRef] [PubMed Central]
- Young, P.R.; Hilditch, P.A.; Bletchly, C.; Halloran, W. An antigen capture enzyme-linked immunosorbent assay reveals high leve ls of the dengue virus protein NS1 in the sera of infected patients. J. Clin. Microbiol. 2000, 38, 1053–1057. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antunes, P.; Watterson, D.; Parmvi, M.; Burger, R.; Boisen, A.; Young, P.; Cooper, M.A.; Hansen, M.F.; Ranzoni, A.; Donolato, M. Quantification of NS1 dengue biomarker in serum via optomagnetic nanoc luster detection. Sci. Rep. 2015, 5, 16145. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Paranavitane, S.A.; Gomes, L.; Kamaladasa, A.; Adikari, T.N.; Wickramasinghe, N.; Jeewandara, C.; Shyamali, N.L.; Ogg, G.S.; Malavige, G.N. Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect. Dis. 2014, 14, 570. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, H.; Di, B.; Pan, Y.X.; Qiu, L.W.; Wang, Y.D.; Hao, W.; He, L.J.; Yuen, K.Y.; Che, X.Y. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: Implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 2006, 44, 2872–2878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Erra, E.O.; Korhonen, E.M.; Voutilainen, L.; Huhtamo, E.; Vapalahti, O.; Kantele, A. Dengue in travelers: Kinetics of viremia and NS1 antigenemia and their associations with clinical parameters. PLoS ONE 2013, 8, e65900. [Google Scholar] [CrossRef] [PubMed Central]
- Blacksell, S.D.; Jarman, R.G.; Bailey, M.S.; Tanganuchitcharnchai, A.; Jenjaroen, K.; Gibbons, R.V.; Paris, D.H.; Premaratna, R.; de Silva, H.J.; Lalloo, D.G.; et al. Evaluation of six commercial point-of-care tests for diagnosis of acut e dengue infections: The need for combining NS1 antigen and IgM/IgG an tibody detection to achieve acceptable levels of accuracy. Clin. Vaccine Immunol. 2011, 18, 2095–2101. [Google Scholar] [CrossRef] [PubMed Central]
- Chong, Z.L.; Sekaran, S.D.; Soe, H.J.; Peramalah, D.; Rampal, S.; Ng, C.-W. Diagnostic accuracy and utility of three dengue diagnostic tests for the diagnosis of acute dengue infection in Malaysia. BMC Infect. Dis. 2020, 20, 210. [Google Scholar] [CrossRef] [PubMed Central]
- Haider, M.; Yousaf, S.; Zaib, A.; Sarfraz, A.; Sarfraz, Z.; Cherrez-Ojeda, I. Diagnostic Accuracy of Various Immunochromatographic Tests for NS1 Antigen and IgM Antibodies Detection in Acute Dengue Virus Infection. Int. J. Environ. Res. Public Health 2022, 19, 8756. [Google Scholar] [CrossRef] [PubMed Central]
- Ndiaye, O.; Woolston, K.; Gaye, A.; Loucoubar, C.; Cocozza, M.; Fall, C.; Dia, F.; Adams, E.R.; Samb, M.; Camara, D.; et al. Laboratory Evaluation and Field Testing of Dengue NS1 and IgM/IgG Rapi d Diagnostic Tests in an Epidemic Context in Senegal. Viruses 2023, 15, 904. [Google Scholar] [CrossRef] [PubMed Central]
- Osorio, L.; Ramirez, M.; Bonelo, A.; Villar, L.A.; Parra, B. Comparison of the diagnostic accuracy of commercial NS1-based diagnost ic tests for early dengue infection. Virol. J. 2010, 7, 361. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.; Ryu, J.H.; Park, H.S.; Park, K.H.; Bae, H.; Yun, S.; Choi, A.-R.; Cho, S.-Y.; Park, C.; Lee, D.-G.; et al. Comparison of Six Commercial Diagnostic Tests for the Detection of Den gue Virus Non-Structural-1 Antigen and IgM/IgG Antibodies. Ann. Lab. Med. 2019, 39, 566–571. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sánchez-Vargas, L.A.; Sánchez-Marce, E.E.; Vivanco-Cid, H. Evaluation of the SD BIOLINE Dengue Duo rapid test in the course of acute and convalescent dengue infections in a Mexican endemic region. Diagn. Microbiol. Infect. Dis. 2014, 78, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Wu, Y.S.; Poh, C.L. Molecular Mechanisms of Antiviral Agents against Dengue Virus. Viruses 2023, 15, 705. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Norshidah, H.; Vignesh, R.; Lai, N.S. Updates on Dengue Vaccine and Antiviral: Where Are We Heading? Molecules 2021, 26, 6768. [Google Scholar] [CrossRef] [PubMed Central]
- Soo, K.-M.; Khalid, B.; Ching, S.-M.; Chee, H.-Y. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. PLoS ONE 2016, 11, e0154760. [Google Scholar] [CrossRef] [PubMed Central]
- NSirisena, P.D.N.; Mahilkar, S.; Sharma, C.; Jain, J.; Sunil, S. Concurrent dengue infections: Epidemiology & clinical implications. Indian J. Med. Res. 2021, 154, 669–679. [Google Scholar] [CrossRef] [PubMed Central]
- Xu, X.; Vaughan, K.; Weiskopf, D.; Grifoni, A.; Diamond, M.S.; Sette, A.; Peters, B. Identifying Candidate Targets of Immune Responses in Zika Virus Based on Homology to Epitopes in Other Flavivirus Species. PLoS Curr. 2016, 8. [Google Scholar] [CrossRef] [PubMed Central]
- George, J.; Valiant, W.G.; Mattapallil, M.J.; Walker, M.; Huang, Y.-J.S.; Vanlandingham, D.L.; Misamore, J.; Greenhouse, J.; Weiss, D.E.; Verthelyi, D.; et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Vire mia in Rhesus Macaques. Sci. Rep. 2017, 7, 10498. [Google Scholar] [CrossRef] [PubMed Central]
- Nascimento, E.J.M.; Huleatt, J.W.; Cordeiro, M.T.; Castanha, P.M.S.; George, J.K.; Grebe, E.; Welte, A.; Brown, M.; Burke, D.S.; Marques, E.T. Development of antibody biomarkers of long term and recent dengue viru s infections. J. Virol. Methods 2018, 257, 62–68. [Google Scholar] [CrossRef]
- Muller, D.A.; Depelsenaire, A.C.I.; Young, P.R. Clinical and Laboratory Diagnosis of Dengue Virus Infection. J. Infect. Dis. 2017, 215 (Suppl. S2), S89–S95. [Google Scholar] [CrossRef] [PubMed]
- Gan, V.C.; Tan, L.-K.; Lye, D.C.; Pok, K.-Y.; Mok, S.-Q.; Chua, R.C.-R.; Leo, Y.-S.; Ng, L.-C. Diagnosing dengue at the point-of-care: Utility of a rapid combined di agnostic kit in Singapore. PLoS ONE 2014, 9, e90037. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fry, S.R.; Meyer, M.; Semple, M.G.; Simmons, C.P.; Sekaran, S.D.; Huang, J.X.; McElnea, C.; Huang, C.-Y.; Valks, A.; Young, P.R.; et al. The diagnostic sensitivity of dengue rapid test assays is significantl y enhanced by using a combined antigen and antibody testing approach. PLoS Negl. Trop. Dis. 2011, 5, e1199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| MAGLUMI Denv NS1 Test | qPCR Reference Test | ||
|---|---|---|---|
| Positive | Negative | Total | |
| Positive | 117 | 1 | 118 |
| Negative | 2 | 62 | 64 |
| Total | 119 | 63 | 182 |
| Measure | Calculation | Estimate | 95% CI |
|---|---|---|---|
| Specificity | 62/63 | 98.41% | 91.54–99.72% |
| Sensitivity | 117/119 | 98.32% | 94.08–99.54% |
| FPR | 1/63 | 1.59% | 0.28–8.46% |
| FNR | 2/119 | 1.68% | 0.46–5.92% |
| PPV | 117/118 | 99.15% | 95.36–99.85% |
| NPV | 62/64 | 96.88% | 89.30–99.14% |
| Accuracy | 179/182 | 98.35% | 95.27–99.44% |
| Serotype | # Samples | Reactive | Non-Reactive | |
|---|---|---|---|---|
| Dengue qPCR positive samples | Unknown | 4 | 3 | 1 |
| Serotype I | 99 | 98 | 1 | |
| Serotype II | 14 | 14 | 0 | |
| Serotype III | 2 | 2 | 0 | |
| Serotype IV | 0 | 0 | 0 | |
| Total | 119 | 117 | 2 | |
| Sensitivity | 98.32% | |||
| 95% CI | 94.08–99.54% | |||
| Reactive | Non-Reactive | ||
|---|---|---|---|
| Cross reacting substances/agents | Autoimmune diseases | 0 | 6 |
| HAMA | 0 | 3 | |
| Rheumatoid factor positive | 0 | 6 | |
| TBE virus | 0 | 4 | |
| Yellow fever virus | 0 | 3 | |
| Japanese encephalitis virus | 0 | 4 | |
| West Nile virus | 0 | 3 | |
| Zika virus | 0 | 3 | |
| Zika NS1 recombinant antigen | 0 | 2 | |
| Chikungunya virus | 0 | 4 | |
| Measles virus | 0 | 4 | |
| Rubella virus | 0 | 4 | |
| Scarlet fever virus | 0 | 4 | |
| Leptospirosis | 0 | 3 | |
| HAV recombinant antigen | 0 | 3 | |
| HBsAg positive | 0 | 5 | |
| HCV recombinant antigen | 0 | 3 | |
| Interfering substances | Acetaminophen | 0 | 3 |
| Ibuprofen | 0 | 3 | |
| Aspirin | 0 | 3 | |
| Dexamethasone | 0 | 3 | |
| Hemolytic Low | 0 | 2 | |
| Hemolytic Medium | 0 | 2 | |
| Hemolytic High | 0 | 2 | |
| Lipemic Low | 0 | 2 | |
| Lipemic Medium | 0 | 1 | |
| Lipemic High | 0 | 1 | |
| Bilirubin | 0 | 4 | |
| Total | 0 | 90 | |
| analytical specificity | 100% | ||
| 95% CI | 97.87–100% |
| Lot ID | Lot 1 | Lot 2 | Lot 3 |
|---|---|---|---|
| LoB (AU/mL) | 0.471 | 0.480 | 0.482 |
| LoD (AU/mL) | 0.725 | 0.702 | 0.743 |
| LoQ (AU/mL) | 0.863 | 0.917 | 0.914 |
| Sample Type | Negative Samples | Positive Samples | |||
|---|---|---|---|---|---|
| Reactive | Non-Reactive | Reactive | Non-Reactive | ||
| Serum | / | 0 | 8 | 11 | 0 |
| Glass powder | 0 | 8 | 11 | 0 | |
| Maleic acid and α-olefin | 0 | 8 | 11 | 0 | |
| Plasma | Sodium citrate | 0 | 8 | 11 | 0 |
| K2EDTA | 0 | 8 | 11 | 0 | |
| Lithium heparin | 0 | 8 | 11 | 0 | |
| Sodium heparin | 0 | 8 | 11 | 0 | |
| Sample Size | EUROIMMUN Denv NS1 | EUROIMMUN Denv IgG | EUROIMMUN Denv IgM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 114 | + | − | 195 | + | − | 134 | + | − | |||
| MAGLUMI Denv NS1 | + | 6 | 1 | MAGLUMI Denv IgG | + | 77 | 2 | MAGLUMI Denv IgM | + | 21 | 1 |
| − | 1 | 106 | − | 2 | 116 | − | 0 | 112 | |||
| Sensitivity | 85.71% | 100.00% | 100.00% | ||||||||
| Specificity | 99.07% | 98.31% | 99.12% | ||||||||
| Accuracy | 98.25% | 98.97% | 99.25% | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, B.; Fang, Z.; Li, C.; Liu, K.; Wang, T.; Huang, K.; Yang, F.; Huang, Y.; Wu, C.; Li, Y.; et al. Clinical Performance of MAGLUMI Diagnostic Tests for the Automated Detection of Dengue Virus. Viruses 2025, 17, 106. https://doi.org/10.3390/v17010106
Peng B, Fang Z, Li C, Liu K, Wang T, Huang K, Yang F, Huang Y, Wu C, Li Y, et al. Clinical Performance of MAGLUMI Diagnostic Tests for the Automated Detection of Dengue Virus. Viruses. 2025; 17(1):106. https://doi.org/10.3390/v17010106
Chicago/Turabian StylePeng, Bo, Zhonggang Fang, Cong Li, Kun Liu, Ting Wang, Ke Huang, Fan Yang, Yalan Huang, Chunli Wu, Yue Li, and et al. 2025. "Clinical Performance of MAGLUMI Diagnostic Tests for the Automated Detection of Dengue Virus" Viruses 17, no. 1: 106. https://doi.org/10.3390/v17010106
APA StylePeng, B., Fang, Z., Li, C., Liu, K., Wang, T., Huang, K., Yang, F., Huang, Y., Wu, C., Li, Y., Huang, D., Zhang, Q., Tang, Y., Liu, X., Rao, W., & Shi, X. (2025). Clinical Performance of MAGLUMI Diagnostic Tests for the Automated Detection of Dengue Virus. Viruses, 17(1), 106. https://doi.org/10.3390/v17010106







