Development and Validation of an Enzyme-Linked Immunosorbent Assay-Based Protocol for Evaluation of Respiratory Syncytial Virus Vaccines
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Protein Construct Design, Expression, and Purification
2.3. Development and Optimization of the ELISA-Based Protocol
2.4. Validation of the ELISA-Based Protocol
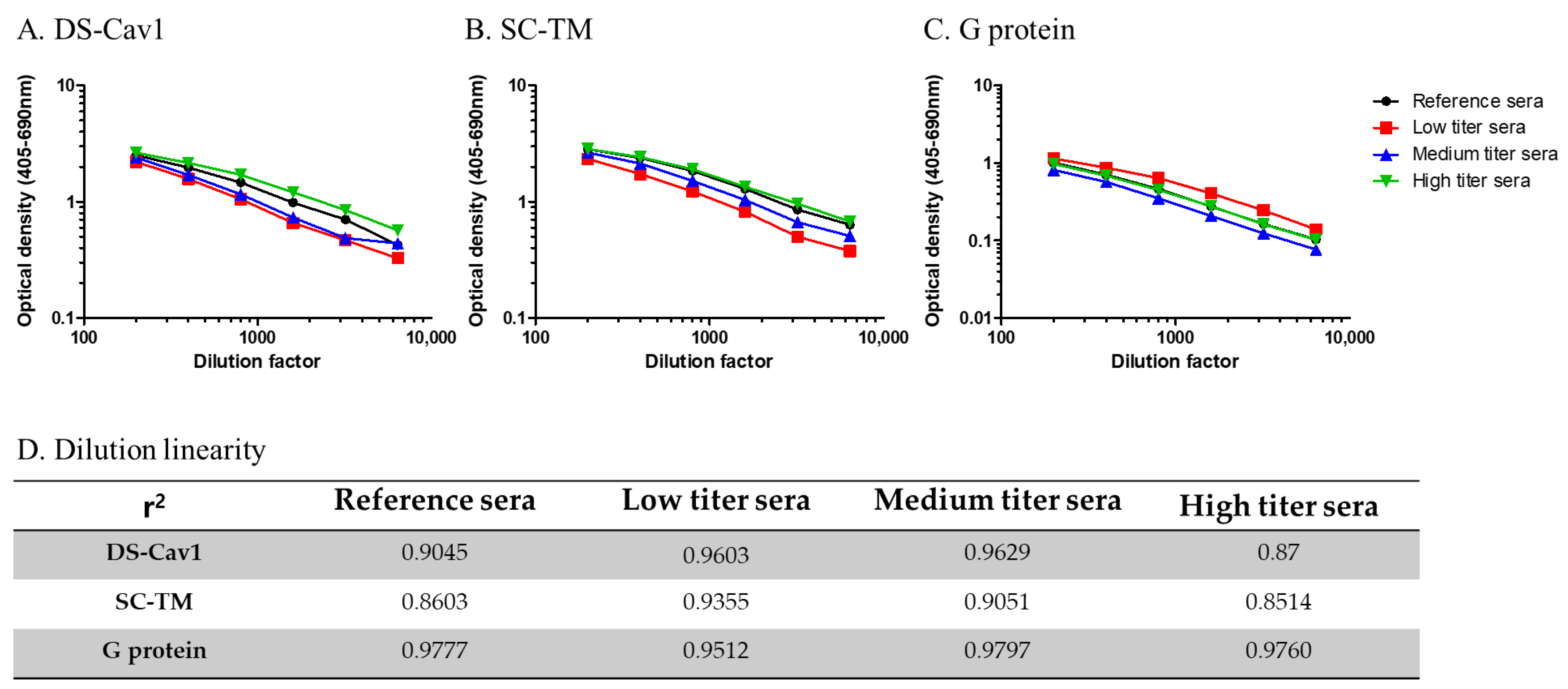
- Accuracy: Low, medium, and high titers of National Institutes of Health-provided reference serum were serially diluted 2-fold, from 1:200 to 1:6400. All experiments were performed in duplicate for each concentration. The average optical density values were plotted against the antiserum concentrations, and the r values were derived by correlating the calculated antibody titers against the three RSV antigens.
- Precision: One sample was used from each age group. Five samples were diluted 2-fold, from 1:200 to 1:25,600. The experiment was performed six times repetitively, and the coefficient of variation was calculated for each sample concentration.
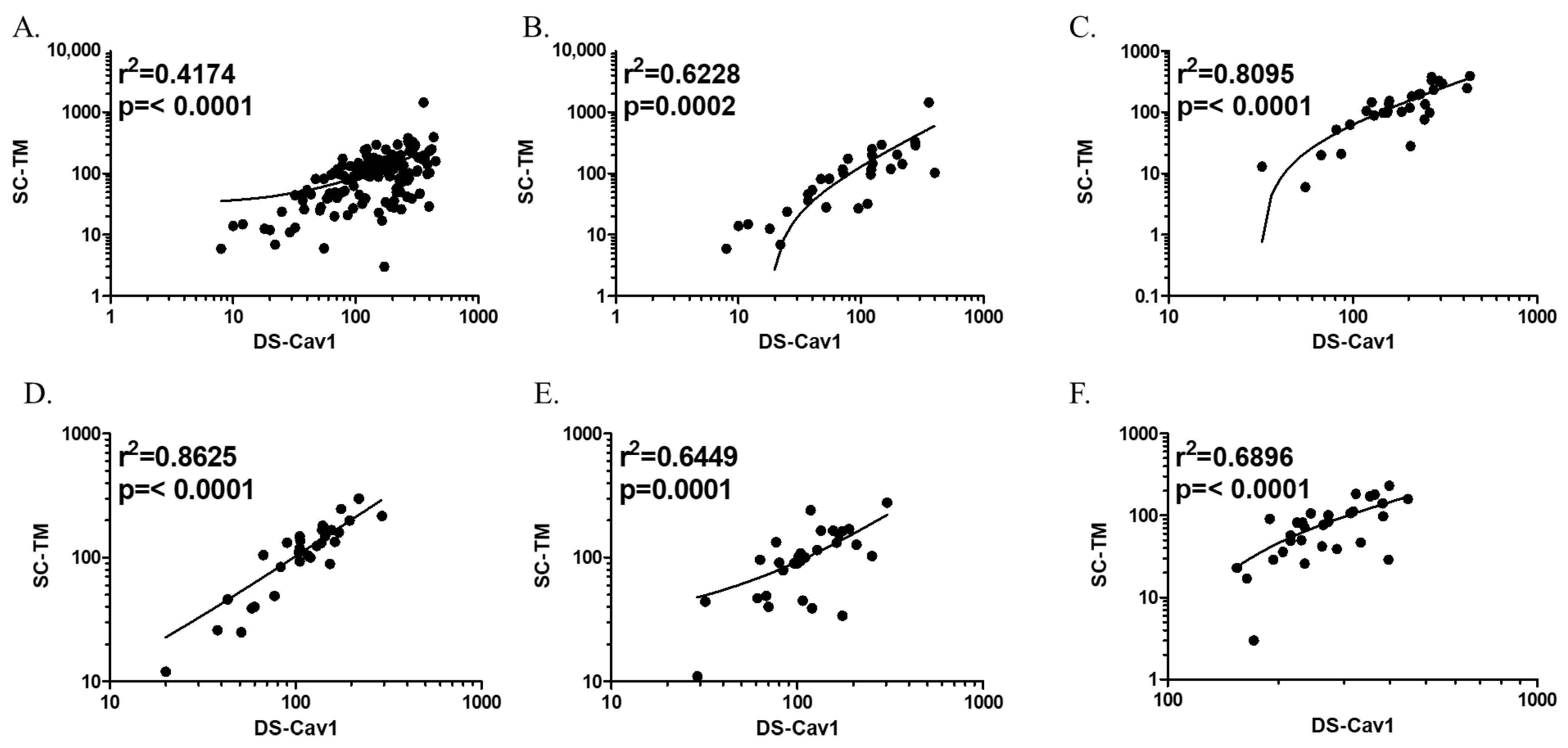
- Specificity: All 150 samples were adsorbed with each of the 3 homologous/heterologous RSV antigens (10 μg/mL of DS-Cav1, SC-TM, and G protein) for an hour with gentle shaking (300 rpm) at RT, following which the antibody titers were measured. Post-adsorption antibody titers were compared with those without adsorption (pre-adsorption). Cross-reactivity was determined based on whether the antibody titers were significantly lower after adsorption. Because RSV infection is mainly prevalent in young children, and because antibody specificity might be affected by the difference in the duration of time elapsed since the last RSV infection among the age groups studied, we investigated whether there was a difference in the antibody specificity based on age.
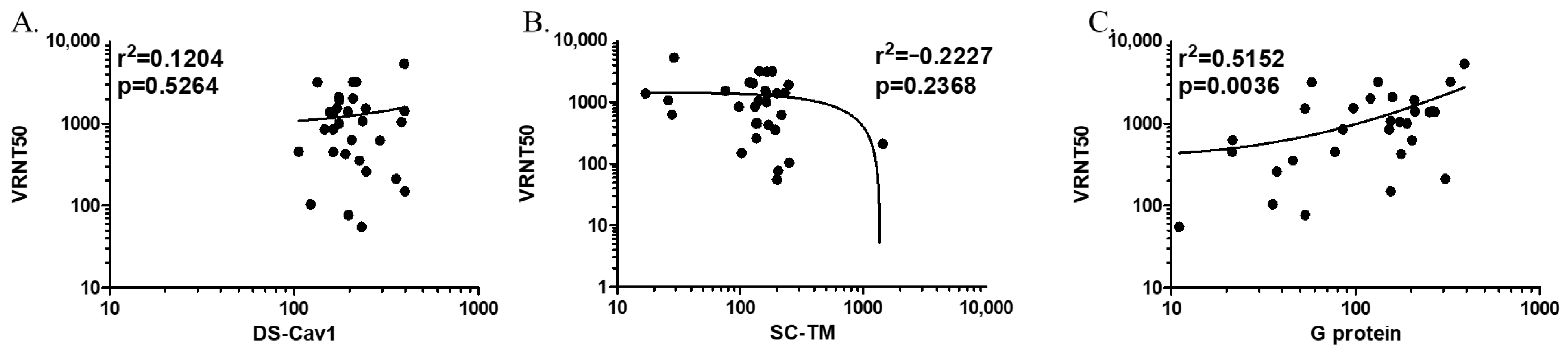
2.5. Correlation of Antibody Titers Determined by ELISA with Neutralizing Antibody Titers
2.6. Statistical Analyses
3. Results
3.1. Purification of Prefusion F Proteins and G Protein
3.2. Development and Optimization of ELISA-Based Protocol
3.3. Validation of the ELISA-Based Protocol
3.4. Correlation with Neutralizing Antibody Titers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in adults. Clin. Microbiol. Rev. 2000, 13, 371–384. [Google Scholar] [CrossRef]
- Lee, N.; Lui, G.C.Y.; Wong, K.T.; Li, T.C.M.; Tse, E.C.M.; Chan, J.Y.C.; Yu, J.; Wong, S.S.M.; Choi, K.W.; Wong, R.Y.K.; et al. High Morbidity and Mortality in Adults Hospitalized for Respiratory Syncytial Virus Infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.G.; Noh, J.Y.; Choi, W.S.; Park, J.J.; Bin Suh, Y.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci. Rep. 2020, 10, 12106. [Google Scholar] [CrossRef]
- WHO. RSV Vaccine Research and Development Technology Roadmap. 2017. Available online: https://www.who.int/publications/i/item/WHO-IVB-17.12 (accessed on 1 January 2017).
- PATH. RSV Vaccine and mAb Snapshot. 2023. Available online: https://www.path.org/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 17 June 2023).
- Mejias, A.; Rodríguez-Fernández, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.A.; Settembre, E.C.; Shaw, C.A.; Dey, A.K.; Rappuoli, R.; Mandl, C.W.; Dormitzer, P.R.; Carfi, A. Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers. Proc. Natl. Acad. Sci. USA 2011, 108, 9619–9624. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.E.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-Based Design of a Fusion Glycoprotein Vaccine for Respiratory Syncytial Virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef]
- Krarup, A.; Truan, D.; Furmanova-Hollenstein, P.; Bogaert, L.; Bouchier, P.; Bisschop, I.J.M.; Widjojoatmodjo, M.N.; Zahn, R.; Schuitemaker, H.; McLellan, J.S.; et al. A highly stable prefusion RSV F vaccine derived from structural analysis of the fusion mechanism. Nat. Commun. 2015, 6, 8143. [Google Scholar] [CrossRef]
- Flynn, J.A.; Durr, E.; Swoyer, R.; Cejas, P.J.; Horton, M.S.; Galli, J.D.; Cosmi, S.A.; Espeseth, A.S.; Bett, A.J.; Zhang, L. Stability Characterization of a Vaccine Antigen Based on the Respiratory Syncytial Virus Fusion Glycoprotein. PLoS ONE 2016, 11, e0164789. [Google Scholar] [CrossRef]
- Fuentes, S.; Coyle, E.M.; Golding, H.; Khurana, S. Nonglycosylated G-Protein Vaccine Protects against Homologous and Heterologous Respiratory Syncytial Virus (RSV) Challenge, while Glycosylated G Enhances RSV Lung Pathology and Cytokine Levels. J. Virol. 2015, 89, 8193–8205. [Google Scholar] [CrossRef]
- Fuentes, S.; Klenow, L.; Golding, H.; Khurana, S. Preclinical evaluation of bacterially produced RSV-G protein vaccine: Strong protection against RSV challenge in cotton rat model. Sci. Rep. 2017, 7, srep42428. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.N.; Power, U.F.; Robert, A.; Haeuw, J.-F.; Helffer, K.; Perez, A.; Asin, M.-A.; Corvaia, N.; Libon, C. The Respiratory Syncytial Virus G Protein Conserved Domain Induces a Persistent and Protective Antibody Response in Rodents. PLoS ONE 2012, 7, e34331. [Google Scholar] [CrossRef] [PubMed]
- Bin Seo, Y.; Cheong, H.J.; Song, J.Y.; Noh, J.Y.; Kim, I.S.; Song, D.J.; Kim, W.J. Epidemiologic differences of four major respiratory viruses between children, adolescents, and adults in Korea. J. Infect. Chemother. 2014, 20, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Jounai, N.; Yoshioka, M.; Tozuka, M.; Inoue, K.; Oka, T.; Miyaji, K.; Ishida, K.; Kawai, N.; Ikematsu, H.; Kawakami, C.; et al. Age-Specific Profiles of Antibody Responses against Respiratory Syncytial Virus Infection. EBioMedicine 2017, 16, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Tsagarakis, N.J.; Sideri, A.; Makridis, P.; Triantafyllou, A.; Stamoulakatou, A.; Papadogeorgaki, E. Age-related prevalence of common upper respiratory pathogens, based on the application of the FilmArray Respiratory panel in a tertiary hospital in Greece. Medicine 2018, 97, e10903. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, 15021. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, E.; Shibata, T.; Hirai, T.; Yoshioka, Y. Non-glycosylated G protein with CpG ODN provides robust protection against respiratory syncytial virus without inducing eosinophilia. Front. Immunol. 2023, 14, 1282016. [Google Scholar] [CrossRef]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.-G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef]
- Moderna. Moderna Announces Mrna-1345, an Investigational Respiratory Syncytial Virus (Rsv) Vaccine, Has Met Primary Efficacy Endpoints in Phase 3 Trial in Older Adults. 2023. Available online: https://investors.modernatx.com/news/news-details/2023/Moderna-Announces-mRNA-1345-an-Investigational-Respiratory-Syncytial-Virus-RSV-Vaccine-Has-Met-Primary-Efficacy-Endpoints-in-Phase-3-Trial-in-Older-Adults/default.aspx (accessed on 17 January 2023).
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simões, E.A.; Pahud, B.A.; Llapur, C.; Baker, J.; Marc, G.P.; Radley, D.; Shittu, E.; et al. Bivalent Prefusion F Vaccine in Pregnancy to Prevent RSV Illness in Infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef]
- Jenkins, V.A.; Hoet, B.; Hochrein, H.; De Moerlooze, L. The Quest for a Respiratory Syncytial Virus Vaccine for Older Adults: Thinking beyond the F Protein. Vaccines 2023, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Kim, H.Y.; Lee, M.; Ahn, J.G.; Baek, J.Y.; Kim, M.Y.; Huh, K.; Jung, J.; Kang, J.-M. Respiratory Syncytial Virus Outbreak Without Influenza in the Second Year of the Coronavirus Disease 2019 Pandemic: A National Sentinel Surveillance in Korea, 2021–2022 Season. J. Korean Med Sci. 2022, 37, e258. [Google Scholar] [CrossRef] [PubMed]
- Ujiie, M.; Tsuzuki, S.; Nakamoto, T.; Iwamoto, N. Resurgence of Respiratory Syncytial Virus Infections during COVID-19 Pandemic, Tokyo, Japan. Emerg. Infect. Dis. 2021, 27, 2969–2970. [Google Scholar] [CrossRef] [PubMed]
- Risk of Severe Pressure on Healthcare Systems Due to RSV, Flu and COVID-19 Co-Circulation; European Centre for Disease Prevention and Control (ECDC): Solna, Sweden, 2022.



| Antigen | DF * | Aged < 5 Years | Aged 5–18 Years | Aged 19–49 Years | Aged 50–64 Years | Aged ≥ 65 Years | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| EU/mL (Mean ± SD) | CV (%) | EU/mL (Mean ± SD) | CV (%) | EU/mL (Mean ± SD) | CV (%) | EU/mL (Mean ± SD) | CV (%) | EU/mL (Mean ± SD) | CV (%) | ||
| DS-Cav1 | 1:200 | 58.0 ± 0.6 | 0.99 | 67.0 ± 2.6 | 3.91 | 74.1 ± 2.1 | 2.89 | 30.0 ± 0.9 | 2.94 | 68.4 ± 2.0 | 2.88 |
| 1:400 | 46.5 ± 1.0 | 2.15 | 73.5 ± 3.4 | 4.63 | 85.3 ± 2.3 | 2.69 | 31.0 ± 0.9 | 2.87 | 74.2 ± 3.6 | 4.84 | |
| 1:800 | 50.0 ± 1.2 | 2.31 | 89.9 ± 2.3 | 2.61 | 97.8 ± 2.2 | 2.20 | 40.4 ± 0.6 | 1.54 | 85.8 ± 2.9 | 3.40 | |
| 1:1600 | 70.8 ± 0.3 | 0.36 | 106.0 ± 3.1 | 2.95 | 115.7 ± 4.1 | 3.57 | 65.6 ± 0.4 | 0.56 | 112.3 ± 4.3 | 3.86 | |
| SC-TM | 1:200 | 60.4 ± 1.3 | 2.10 | 81.7 ± 1.0 | 1.24 | 75.8 ± 3.4 | 4.54 | 40.4 ± 1.1 | 2.63 | 26.7 ± 0.8 | 2.94 |
| 1:400 | 47.6 ± 2.5 | 5.28 | 104.0 ± 2.8 | 2.67 | 84.3 ± 3.4 | 4.00 | 40.4 ± 0.6 | 1.57 | 22.1 ± 0.7 | 2.97 | |
| 1:800 | 49.9 ± 0.9 | 1.71 | 132.1 ± 6.0 | 4.53 | 94.7 ± 4.7 | 4.95 | 45.3 ± 0.5 | 1.21 | 30.3 ± 0.3 | 1.05 | |
| 1:1600 | 62.8 ± 1.3 | 2.02 | 152.7 ± 4.1 | 2.69 | 112.8 ± 2.5 | 2.26 | 61.0 ± 1.2 | 2.02 | 50.0 ± 0.5 | 0.90 | |
| G protein | 1:200 | 127.9 ± 7.3 | 5.72 | 16.1 ± 1.9 | 11.55 | 266.9 ± 17.1 | 6.40 | 163.3 ± 13.1 | 8.00 | 610.0 ± 22.0 | 3.60 |
| 1:400 | 98.7 ± 4.5 | 4.51 | 15.9 ± 1.2 | 7.83 | 215.9 ± 15.6 | 7.24 | 117.9 ± 11.1 | 9.38 | 891.8 ± 51.1 | 5.73 | |
| 1:800 | 83.0 ± 4.1 | 4.99 | 24.2 ± 1.2 | 4.88 | 231.7 ± 14.3 | 6.19 | 112.5 ± 15.4 | 13.72 | 1224.9 ± 100.3 | 8.19 | |
| 1:1600 | 79.7 ± 1.9 | 2.43 | 45.2 ± 0.7 | 1.70 | 201.9 ± 10.8 | 5.37 | 104.4 ± 10.5 | 10.11 | 1572.2 ± 168.1 | 10.69 | |
| Coated Protein | DS-Cav1 | SC-TM | G Protein | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor (10 μg/mL) | None | DS-Cav1 | SC-TM | G Protein | None | DS-Cav1 | SC-TM | G Protein | None | DS-Cav1 | SC-TM | G Protein | |
| Aged < 5 years | EU/mL | 115.0 | 83.3 | 78.2 | 122.9 | 153.3 | 35.8 | 30.8 | 113.7 | 117.3 | 53.5 | 49.2 | 22.4 |
| % | - | 72.5 | 68.0 | 106.9 | - | 23.3 | 20.1 | 74.2 | - | 45.6 | 42.0 | 19.1 | |
| Aged 5–18 years | EU/mL | 193.8 | 73.0 | 67.3 | 121.6 | 148.4 | 69.3 | 65.7 | 122.5 | 78.8 | 44.1 | 41.9 | 17.3 |
| % | - | 37.7 | 34.7 | 62.8 | - | 46.7 | 44.2 | 82.5 | - | 56.0 | 53.2 | 22.0 | |
| Aged 19–49 years | EU/mL | 118.8 | 45.2 | 60.5 | 114.0 | 121.1 | 73.4 | 60.6 | 130.7 | 101.9 | 89.0 | 77.3 | 28.2 |
| % | - | 38.1 | 51.0 | 96.0 | - | 60.6 | 50.0 | 108.0 | - | 87.4 | 75.9 | 27.6 | |
| Aged 50–64 years | EU/mL | 122.7 | 65.7 | 84.0 | 107.7 | 107.0 | 88.0 | 61.1 | 120.4 | 134.3 | 145.8 | 132.1 | 42.3 |
| % | - | 53.5 | 68.5 | 87.8 | - | 82.3 | 57.1 | 112.6 | - | 108.6 | 98.4 | 31.5 | |
| Aged ≥ 65 years | EU/mL | 113.7 | 62.2 | 75.6 | 127.2 | 84.1 | 71.4 | 43.5 | 95.2 | 165.8 | 172.4 | 157.0 | 36.1 |
| % | - | 54.7 | 66.5 | 111.9 | - | 84.8 | 51.8 | 113.2 | - | 104.0 | 94.7 | 21.8 | |
| Average | EU/mL | 132.8 | 65.9 | 73.1 | 118.7 | 122.8 | 67.6 | 52.3 | 116.5 | 119.6 | 101.0 | 91.5 | 29.3 |
| % | - | 49.6 | 55.1 | 89.4 | - | 55.0 | 42.6 | 94.9 | - | 84.4 | 76.5 | 24.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nham, E.; Jang, A.-Y.; Ji, H.J.; Ahn, K.B.; Bae, J.-Y.; Park, M.-S.; Yoon, J.G.; Seong, H.; Noh, J.Y.; Cheong, H.J.; et al. Development and Validation of an Enzyme-Linked Immunosorbent Assay-Based Protocol for Evaluation of Respiratory Syncytial Virus Vaccines. Viruses 2024, 16, 952. https://doi.org/10.3390/v16060952
Nham E, Jang A-Y, Ji HJ, Ahn KB, Bae J-Y, Park M-S, Yoon JG, Seong H, Noh JY, Cheong HJ, et al. Development and Validation of an Enzyme-Linked Immunosorbent Assay-Based Protocol for Evaluation of Respiratory Syncytial Virus Vaccines. Viruses. 2024; 16(6):952. https://doi.org/10.3390/v16060952
Chicago/Turabian StyleNham, Eliel, A-Yeung Jang, Hyun Jung Ji, Ki Bum Ahn, Joon-Yong Bae, Man-Seong Park, Jin Gu Yoon, Hye Seong, Ji Yun Noh, Hee Jin Cheong, and et al. 2024. "Development and Validation of an Enzyme-Linked Immunosorbent Assay-Based Protocol for Evaluation of Respiratory Syncytial Virus Vaccines" Viruses 16, no. 6: 952. https://doi.org/10.3390/v16060952
APA StyleNham, E., Jang, A.-Y., Ji, H. J., Ahn, K. B., Bae, J.-Y., Park, M.-S., Yoon, J. G., Seong, H., Noh, J. Y., Cheong, H. J., Kim, W. J., Seo, H. S., & Song, J. Y. (2024). Development and Validation of an Enzyme-Linked Immunosorbent Assay-Based Protocol for Evaluation of Respiratory Syncytial Virus Vaccines. Viruses, 16(6), 952. https://doi.org/10.3390/v16060952






