From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults
Abstract
1. Introduction
2. Research Questions
- (a)
- Is RSV a forgotten pathogen among older adults? Is there a shift in trend in the importance that is being given to it?
- (b)
- Does RSV disease among older adults present a considerable burden?
- (c)
- What new and developing strategies are there to reduce the need for medical care in older adults? Are these relevant?
3. Materials and Methods
4. RSV Microbiological and Epidemiological Overview
5. Literature Overview Output
6. Can We Consider RSV a Forgotten Pathogen in Older Adults?
6.1. Literature Focus of RSV Infection in Older Adults
6.2. Factors Supporting RSV Being a Forgotten Pathogen in Older Adults
6.2.1. Clinical Picture and Disease Management
6.2.2. Diagnostic Challenges
6.2.3. Recent Developments on Surveillance and Case Definition
7. Estimating RSV Disease Burden in Older Adults
7.1. Limitations in Estimating RSV Disease Burden in Older Adults
7.2. Burden of Coinfections
8. Pipeline of Vaccines and New Therapeutics
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chanock, R.; Roizman, B.; Myers, R. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA): Isolation, properties and characterization. Am. J. Epidemiol. 1957, 66, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; Walsh, E.E. Respiratory syncytial virus infection in elderly adults. Drugs Aging 2005, 22, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Falsey, A.R.; McElhaney, J.E.; Beran, J.; van Essen, G.A.; Duval, X.; Esen, M.; Galtier, F.; Gervais, P.; Hwang, S.-J.; Kremsner, P.; et al. Respiratory syncytial virus and other respiratory viral infections in older adults with moderate to severe influenza-like illness. J. Infect. Dis. 2014, 209, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Saiman, L.; Walsh, E.E.; Falsey, A.R.; Sieling, W.D.; Greendyke, W.; Peterson, D.R.; Vargas, C.Y.; Phillips, M.; Finelli, L. Incidence of Respiratory Syncytial Virus Infection among Hospitalized Adults, 2017–2020. Clin. Infect. Dis. 2022, 74, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Colosia, A.D.; Yang, J.; Hillson, E.; Mauskopf, J.; Copley-Merriman, C.; Shinde, V.; Stoddard, J. The epidemiology of medically attended respiratory syncytial virus in older adults in the United States: A systematic review. PLoS ONE 2017, 12, e0182321. [Google Scholar] [CrossRef] [PubMed]
- Wyffels, V.; Kariburyo, F.; Gavart, S.; Fleischhackl, R.; Yuce, H. A Real-World Analysis of Patient Characteristics and Predictors of Hospitalization among US Medicare Beneficiaries with Respiratory Syncytial Virus Infection. Adv. Ther. 2020, 37, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Childs, A.; Zullo, A.R.; Joyce, N.R.; McConeghy, K.W.; van Aalst, R.; Moyo, P.; Bosco, E.; Mor, V.; Gravenstein, S. The burden of respiratory infections among older adults in long-term care: A systematic review. BMC Geriatr. 2019, 19, 210. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L.; Alexander, J.P.; Anderson, L.J. Respiratory syncytial virus pneumonia among the elderly: An assessment of disease burden. J. Infect. Dis. 1999, 179, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Lui, G.C.; Wong, K.T.; Li, T.C.; Tse, E.C.; Chan, J.Y.; Yu, J.; Wong, S.S.; Choi, K.W.; Wong, R.Y.; et al. High morbidity and mortality in adults hospitalized for respiratory syncytial virus infections. Clin. Infect. Dis. 2013, 57, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Tseng, H.F.; Sy, L.S.; Ackerson, B.; Solano, Z.; Slezak, J.; Luo, Y.; Shinde, V. Severe Morbidity and Short- and Mid- to Long-term Mortality in Older Adults Hospitalized with Respiratory Syncytial Virus Infection. J. Infect. Dis. 2020, 222, 1298–1310. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.; Tseng, H.F.; Sy, L.S.; Solano, Z.; Slezak, J.; Luo, Y.; Fischetti, C.A.; Shinde, V. Severe Morbidity and Mortality Associated with Respiratory Syncytial Virus Versus Influenza Infection in Hospitalized Older Adults. Clin. Infect. Dis. 2019, 69, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.M.; Taylor, R.J.; Lustig, R.L.; Schuck-Paim, C.; Haguinet, F.; Webb, D.J.; Logie, J.; Matias, G.; Taylor, S. Modelling estimates of the burden of Respiratory Syncytial virus infection in adults and the elderly in the United Kingdom. BMC Infect. Dis. 2015, 15, 443. [Google Scholar] [CrossRef] [PubMed]
- Domnich, A.; Calabrò, G.E. Epidemiology and burden of respiratory syncytial virus in Italian adults: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0297608. [Google Scholar] [CrossRef]
- Maggi, S.; Veronese, N.; Burgio, M.; Cammarata, G.; Ciuppa, M.E.; Ciriminna, S.; Di Gennaro, F.; Smith, L.; Trott, M.; Dominguez, L.J.; et al. Rate of Hospitalizations and Mortality of Respiratory Syncytial Virus Infection Compared to Influenza in Older People: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 2092. [Google Scholar] [CrossRef] [PubMed]
- ElSherif, M.; Andrew, M.K.; Ye, L.; Ambrose, A.; Boivin, G.; Bowie, W.; David, M.P.; Gruselle, O.; Halperin, S.A.; Hatchette, T.F.; et al. Leveraging Influenza Virus Surveillance From 2012 to 2015 to Characterize the Burden of Respiratory Syncytial Virus Disease in Canadian Adults ≥50 Years of Age Hospitalized with Acute Respiratory Illness. Open Forum Infect. Dis. 2023, 10, ofad315. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, B.; An, J.; Sy, L.S.; Solano, Z.; Slezak, J.; Tseng, H.F. Cost of Hospitalization Associated with Respiratory Syncytial Virus Infection Versus Influenza Infection in Hospitalized Older Adults. J. Infect. Dis. 2020, 222, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Douglas, R.G., Jr.; Schnabel, K.C.; Geiman, J.M. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect. Immun. 1981, 33, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.; Smith, C.M.; Lee, D.H.; Hirst, R.A.; Easton, A.J.; O’Callaghan, C. Evidence of Respiratory Syncytial Virus Spread by Aerosol. Time to Revisit Infection Control Strategies? Am. J. Respir. Crit. Care Med. 2016, 194, 308–316. [Google Scholar] [CrossRef]
- Kestler, M.; Muñoz, P.; Mateos, M.; Adrados, D.; Bouza, E. Respiratory syncytial virus burden among adults during flu season: An underestimated pathology. J. Hosp. Infect. 2018, 100, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.C.; Pirçon, J.Y.; Verheij, T.J.M.; Bont, L.J.; Wildenbeest, J.G.; RESCEU Investigators. Contact with Young Children Increases the Risk of Respiratory Infection in Older Adults in Europe-the RESCEU Study. J. Infect. Dis. 2022, 226, S79–S86. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef] [PubMed]
- Guitart, C.; Bobillo-Perez, S.; Alejandre, C.; Armero, G.; Launes, C.; Cambra, F.J.; Balaguer, M.; Jordan, I.; Hospital Network for R. S. V. surveillance in Catalonia. Bronchiolitis, epidemiological changes during the SARS-CoV-2 pandemic. BMC Infect. Dis. 2022, 22, 84. [Google Scholar] [CrossRef]
- Hamid, S.; Winn, A.; Parikh, R.; Jones, J.M.; McMorrow, M.; Prill, M.M.; Silk, B.J.; Scobie, H.M.; Hall, A.J. Seasonality of Respiratory Syncytial Virus—United States, 2017–2023. Morb. Mortal. Wkly. Rep. (MMWR) 2023, 72, 355–361. [Google Scholar] [CrossRef]
- Walsh, E.E.; Peterson, D.R.; Falsey, A.R. Risk factors for severe respiratory syncytial virus infection in elderly persons. J. Infect. Dis. 2004, 189, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Van-Tam, J.S.; O’Leary, M.; Martin, E.T.; Heijnen, E.; Callendret, B.; Fleischhackl, R.; Comeaux, C.; Tran, T.M.P.; Weber, K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022, 31, 220105. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Denouel, A.; Tietjen, A.K.; Campbell, I.; Moran, E.; Li, X.; Campbell, H.; Demont, C.; Nyawanda, B.O.; Chu, H.Y.; et al. Global Disease Burden Estimates of Respiratory Syncytial Virus-Associated Acute Respiratory Infection in Older Adults in 2015: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 222, S577–S583. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Vennard, S.; Jasiewicz, F.; Brogden, R.; Nair, H.; RESCEU Investigators. Disease Burden Estimates of Respiratory Syncytial Virus related Acute Respiratory Infections in Adults with Comorbidity: A Systematic Review and Meta-Analysis. J. Infect. Dis. 2022, 226, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, D.H.; Arcega, V.; Rao, M. Review of respiratory syncytial virus infection among older adults and transplant recipients. Ther. Adv. Infect. Dis. 2022, 9, 20499361221091413. [Google Scholar] [CrossRef] [PubMed]
- Savic, M.; Penders, Y.; Shi, T.; Branche, A.; Pirçon, J.Y. Respiratory syncytial virus disease burden in adults aged 60 years and older in high-income countries: A systematic literature review and meta-analysis. Influenza Other Respir Viruses 2023, 17, e13031. [Google Scholar] [CrossRef] [PubMed]
- Tin Tin Htar, M.; Yerramalla, M.S.; Moïsi, J.C.; Swerdlow, D.L. The burden of respiratory syncytial virus in adults: A systematic review and meta-analysis. Epidemiol. Infect. 2020, 148, e48. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.H.; Ison, M.G. Respiratory syncytial virus infection in adults. BMJ 2019, 366, l5021. [Google Scholar] [CrossRef] [PubMed]
- Bouzid, D.; Visseaux, B.; Ferré, V.M.; Peiffer-Smadja, N.; Le Hingrat, Q.; Loubet, P. Respiratory syncytial virus in adults with comorbidities: An update on epidemiology, vaccines, and treatments. Clin. Microbiol. Infect. 2023, 29, 1538–1550. [Google Scholar] [CrossRef] [PubMed]
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.; Ravanfar, B.; Rutter, H.; Allen, J.; Falsey, A.; Pirçon, J.Y.; Gruselle, O.; et al. Burden of respiratory syncytial virus infection in community-dwelling older adults in Europe (RESCEU): An international prospective cohort study. Eur. Respir. J. 2021, 57, 2002688. [Google Scholar] [CrossRef] [PubMed]
- Ivey, K.S.; Edwards, K.M.; Talbot, H.K. Respiratory Syncytial Virus and Associations with Cardiovascular Disease in Adults. J. Am. Coll. Cardiol. 2018, 71, 1574–1583. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Walker, T.A.; Waite, B.; Wood, T.; Trenholme, A.A.; Baker, M.G.; McArthur, C.; Wong, C.A.; Grant, C.C.; Huang, Q.S.; et al. Respiratory Syncytial Virus-Associated Hospitalizations among Adults with Chronic Medical Conditions. Clin. Infect. Dis. 2021, 73, e158–e163. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Peterson, D.R.; Kalkanoglu, A.E.; Lee, F.E.; Falsey, A.R. Viral shedding and immune responses to respiratory syncytial virus infection in older adults. J. Infect. Dis. 2013, 207, 1424–1432. [Google Scholar] [CrossRef]
- Waghmare, A.; Campbell, A.P.; Xie, H.; Seo, S.; Kuypers, J.; Leisenring, W.; Jerome, K.R.; Englund, J.A.; Boeckh, M. Respiratory syncytial virus lower respiratory disease in hematopoietic cell transplant recipients: Viral RNA detection in blood, antiviral treatment, and clinical outcomes. Clin. Infect. Dis. 2013, 57, 1731–1741. [Google Scholar] [CrossRef] [PubMed]
- Blunck, B.N.; Angelo, L.S.; Henke, D.; Avadhanula, V.; Cusick, M.; Ferlic-Stark, L.; Zechiedrich, L.; Gilbert, B.E.; Piedra, P.A. Adult Memory T Cell Responses to the Respiratory Syncytial Virus Fusion Protein During a Single RSV Season (2018–2019). Front. Immunol. 2022, 13, 823652. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Lower Respiratory Infections Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 2018, 18, 1191–1210. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Abrysvo. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/abrysvo (accessed on 20 December 2023).
- European Medicines Agency. Arexvy. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/arexvy (accessed on 20 December 2023).
- International Committee on Taxonomy of Viruses. Family: Paramysoviridae. Chapter Version: ICTV Ninth Report; 2009 Taxonomy Release. Available online: https://ictv.global/report_9th/RNAneg/Mononegavirales/Paramyxoviridae (accessed on 24 January 2024).
- Schobel, S.A.; Stucker, K.M.; Moore, M.L.; Anderson, L.J.; Larkin, E.K.; Shankar, J.; Bera, J.; Puri, V.; Shilts, M.H.; Rosas-Salazar, C.; et al. Respiratory Syncytial Virus whole-genome sequencing identifies convergent evolution of sequence duplication in the C-terminus of the G gene. Sci. Rep. 2016, 6, 26311. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, M. Respiratory syncytial virus infection in the modern era. Curr. Opin. Infect. Dis. 2023, 36, 155–163. [Google Scholar] [CrossRef] [PubMed]
- McLellan, J.S.; Ray, W.C.; Peeples, M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104. [Google Scholar] [CrossRef] [PubMed]
- CDC. RSV Surveillance & Research. October 2022. Available online: https://www.cdc.gov/rsv/research/index.html (accessed on 29 January 2024).
- Mangtani, P.; Hajat, S.; Kovats, S.; Wilkinson, P.; Armstrong, B. The association of respiratory syncytial virus infection and influenza with emergency admissions for respiratory disease in London: An analysis of routine surveillance data. Clin. Infect. Dis. 2006, 42, 640–646. [Google Scholar] [CrossRef] [PubMed]
- McCracken, J.P.; Prill, M.M.; Arvelo, W.; Lindblade, K.A.; López, M.R.; Estevez, A.; Müller, M.L.; Muñoz, F.; Bernart, C.; Cortez, M.; et al. Respiratory syncytial virus infection in Guatemala, 2007–2012. J. Infect. Dis. 2013, 208 (Suppl. 3), S197–S206. [Google Scholar] [CrossRef] [PubMed]
- Volling, C.; Hassan, K.; Mazzulli, T.; Green, K.; Al-Den, A.; Hunter, P.; Mangat, R.; Ng, J.; McGeer, A. Respiratory syncytial virus infection-associated hospitalization in adults: A retrospective cohort study. BMC Infect. Dis. 2014, 14, 665. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, M.E.; Meece, J.K.; Sifakis, F.; Gasser, R.A., Jr.; Belongia, E.A. Medically attended respiratory syncytial virus infections in adults aged ≥ 50 years: Clinical characteristics and outcomes. Clin. Infect. Dis. 2014, 58, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Malosh, R.E.; Martin, E.T.; Callear, A.P.; Petrie, J.G.; Lauring, A.S.; Lamerato, L.; Fry, A.M.; Ferdinands, J.; Flannery, B.; Monto, A.S. Respiratory syncytial virus hospitalization in middle-aged and older adults. J. Clin. Virol. 2017, 96, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Belongia, E.A.; King, J.P.; Kieke, B.A.; Pluta, J.; Al-Hilli, A.; Meece, J.K.; Shinde, V. Clinical Features, Severity, and Incidence of RSV Illness During 12 Consecutive Seasons in a Community Cohort of Adults ≥60 Years Old. Open Forum Infect. Dis. 2018, 5, ofy316. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Das, A.; Nam, H.; Yang, A.; Ison, M.G. Epidemiology and outcomes of hospitalized adults with respiratory syncytial virus: A 6-year retrospective study. Influenza Other Respir. Viruses 2019, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chuaychoo, B.; Ngamwongwan, S.; Kaewnaphan, B.; Athipanyasilp, N.; Horthongkham, N.; Kantakamalakul, W.; Muangman, N. Clinical manifestations and outcomes of respiratory syncytial virus infection in adult hospitalized patients. J. Clin. Virol. 2019, 117, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.G.; Noh, J.Y.; Choi, W.S.; Park, J.J.; Suh, Y.B.; Song, J.Y.; Cheong, H.J.; Kim, W.J. Clinical characteristics and disease burden of respiratory syncytial virus infection among hospitalized adults. Sci. Rep. 2020, 10, 12106. [Google Scholar] [CrossRef] [PubMed]
- Begley, K.M.; Monto, A.S.; Lamerato, L.E.; Malani, A.N.; Lauring, A.S.; Talbot, H.K.; Gaglani, M.; McNeal, T.; Silveira, F.P.; Zimmerman, R.K.; et al. Prevalence and Clinical Outcomes of Respiratory Syncytial Virus vs Influenza in Adults Hospitalized With Acute Respiratory Illness From a Prospective Multicenter Study. Clin. Infect. Dis. 2023, 76, 1980–1988. [Google Scholar] [CrossRef] [PubMed]
- Vos, L.M.; Oosterheert, J.J.; Hoepelman, A.I.M.; Bont, L.J.; Coenjaerts, F.E.J.; Naaktgeboren, C.A. External validation and update of a prognostic model to predict mortality in hospitalized adults with RSV: A retrospective Dutch cohort study. J. Med. Virol. 2019, 91, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Loubet, P.; Lenzi, N.; Valette, M.; Foulongne, V.; Krivine, A.; Houhou, N.; Lagathu, G.; Rogez, S.; Alain, S.; Duval, X.; et al. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin. Microbiol. Infect. 2017, 23, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Zuber, B.; Garrigues, E.; Gros, A.; Vandueren, C.; Epaillard, N.; Voiriot, G.; Tandjaoui-Lambiotte, Y.; Lascarrou, J.B.; Boissier, F.; et al. Characteristics and Outcomes of Patients in the ICU with Respiratory Syncytial Virus Compared with Those with Influenza Infection: A Multicenter Matched Cohort Study. Chest 2022, 161, 1475–1484. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Christaki, E.; Marques, T.M.; Tosatto, V.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Karagiannis, C.; Maikanti, P.; et al. Severity of RSV infection in Southern European elderly patients during two consecutive winter seasons (2017–2018). J. Med. Virol. 2021, 93, 5152–5157. [Google Scholar] [CrossRef] [PubMed]
- Chorazka, M.; Flury, D.; Herzog, K.; Albrich, W.C.; Vuichard-Gysin, D. Clinical outcomes of adults hospitalized for laboratory confirmed respiratory syncytial virus or influenza virus infection. PLoS ONE 2021, 16, e0253161. [Google Scholar] [CrossRef] [PubMed]
- Celante, H.; Oubaya, N.; Fourati, S.; Beaune, S.; Khellaf, M.; Casalino, E.; Ricard, J.D.; Vieillard-Baron, A.; Heming, N.; Mekontso Dessap, A.; et al. Prognosis of hospitalised adult patients with respiratory syncytial virus infection: A multicentre retrospective cohort study. Clin. Microbiol. Infect. 2023, 29, e1–e943. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, A.; Savinainen, E.; Hämäläinen, S.; Sivenius, K.; Kauppinen, J.; Koivula, I.; Patovirta, R.L. Disease burden caused by respiratory syncytial virus compared with influenza among adults: A retrospective cohort study from Eastern Finland in 2017–2018. BMJ Open 2022, 12, e060805. [Google Scholar] [CrossRef] [PubMed]
- Njue, A.; Nuabor, W.; Lyall, M.; Margulis, A.; Mauskopf, J.; Curcio, D.; Kurosky, S.; Gessner, B.D.; Begier, E. Systematic Literature Review of Risk Factors for Poor Outcomes Among Adults with Respiratory Syncytial Virus Infection in High-Income Countries. Open Forum Infect. Dis. 2023, 10, ofad513. [Google Scholar] [CrossRef] [PubMed]
- Schubert, L.; Steininger, J.; Lötsch, F.; Herdina, A.N.; Redlberger-Fritz, M.; Tobudic, S.; Kundi, M.; Strassl, R.; Steininger, C. Surveillance of respiratory syncytial virus infections in adults, Austria, 2017 to 2019. Sci. Rep. 2021, 11, 8939. [Google Scholar] [CrossRef] [PubMed]
- Nolen, L.D.; Seeman, S.; Desnoyers, C.; DeByle, C.; Klejka, J.; Bruden, D.; Rudolph, K.; Gerber, S.I.; Kim, L.; Langley, G.; et al. Respiratory syncytial virus and influenza hospitalizations in Alaska native adults. J. Clin. Virol. 2020, 127, 104347. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Chan, M.C.; Lui, G.C.; Li, R.; Wong, R.Y.; Yung, I.M.; Cheung, C.S.; Chan, E.C.; Hui, D.S.; Chan, P.K. High Viral Load and Respiratory Failure in Adults Hospitalized for Respiratory Syncytial Virus Infections. J. Infect. Dis. 2015, 212, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Chatzis, O.; Darbre, S.; Pasquier, J.; Meylan, P.; Manuel, O.; Aubert, J.D.; Beck-Popovic, M.; Masouridi-Levrat, S.; Ansari, M.; Kaiser, L.; et al. Burden of severe RSV disease among immunocompromised children and adults: A 10 year retrospective study. BMC Infect. Dis. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Holmen, J.E.; Kim, L.; Cikesh, B.; Kirley, P.D.; Chai, S.J.; Bennett, N.M.; Felsen, C.B.; Ryan, P.; Monroe, M.; Anderson, E.J.; et al. Relationship between neighborhood census-tract level socioeconomic status and respiratory syncytial virus-associated hospitalizations in U.S. adults, 2015–2017. BMC Infect. Dis. 2021, 21, 293. [Google Scholar] [CrossRef] [PubMed]
- Pilie, P.; Werbel, W.A.; Riddell, J., 4th; Shu, X.; Schaubel, D.; Gregg, K.S. Adult patients with respiratory syncytial virus infection: Impact of solid organ and hematopoietic stem cell transplantation on outcomes. Transpl. Infect. Dis. 2015, 17, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Azzi, J.M.; Kyvernitakis, A.; Shah, D.P.; El Haddad, L.; Mahajan, S.N.; Ghantoji, S.S.; Heredia-Ariza, E.; Chemaly, R.F. Leukopenia and lack of ribavirin predict poor outcomes in patients with haematological malignancies and respiratory syncytial virus infection. J. Antimicrob. Chemother. 2018, 73, 3162–3169. [Google Scholar] [CrossRef] [PubMed]
- Goldman, C.R.; Sieling, W.D.; Alba, L.R.; Silverio Francisco, R.A.; Vargas, C.Y.; Barrett, A.E.; Phillips, M.; Finelli, L.; Saiman, L. Severe Clinical Outcomes among Adults Hospitalized with Respiratory Syncytial Virus Infections, New York City, 2017–2019. Public Health Rep. 2022, 137, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Lui, G.; Wong, C.K.; Chan, M.; Chong, K.C.; Wong, R.; Chu, I.; Zhang, M.; Li, T.; Hui, D.; Lee, N.; et al. Host inflammatory response is the major marker of severe respiratory syncytial virus infection in older adults. J. Infect. 2021, 83, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Smithgall, M.; Maykowski, P.; Zachariah, P.; Oberhardt, M.; Vargas, C.Y.; Reed, C.; LaRussa, P.; Saiman, L.; Stockwell, M.S. Epidemiology, clinical features, and resource utilization associated with respiratory syncytial virus in the community and hospital. Influenza Other Respir. Viruses 2020, 14, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.; Griffin, M.R.; Zhu, Y.; Williams, J.V.; Talbot, H.K. Respiratory syncytial virus- and human metapneumovirus-associated emergency department and hospital burden in adults. Influenza Other Respir. Viruses 2014, 8, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.B.; Walsh, E.E.; Peterson, D.R.; Lee, F.E.; Falsey, A.R. Risk factors for respiratory failure associated with respiratory syncytial virus infection in adults. J. Infect. Dis. 2009, 200, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Widmer, K.; Zhu, Y.; Williams, J.V.; Griffin, M.R.; Edwards, K.M.; Talbot, H.K. Rates of hospitalizations for respiratory syncytial virus, human metapneumovirus, and influenza virus in older adults. J. Infect. Dis. 2012, 206, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Walsh, E.E.; Mahadevia, P.J.; Falsey, A.R. Risk factors for respiratory syncytial virus illness among patients with chronic obstructive pulmonary disease. COPD 2013, 10, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.W.; Binnicker, M.J.; Harris, D.M.; Chirila, R.M.; Brumble, L.; Mandrekar, J.; Hata, D.J. Morbidity and mortality among patients with respiratory syncytial virus infection: A 2-year retrospective review. Diagn. Microbiol. Infect. Dis. 2016, 85, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Presser, L.D.; van den Akker, W.M.R.; Meijer, A.; PROMISE investigators. Respiratory Syncytial Virus European Laboratory Network 2022 Survey: Need for Harmonization and Enhanced Molecular Surveillance. J. Infect. Dis. 2023, 229, S34–S39. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Begier, E.; Kurosky, S.K.; Whelan, J.; Bem, D.; Pouwels, K.B.; Postma, M.; Bont, L. Incidence of Respiratory Syncytial Virus Infection in Older Adults: Limitations of Current Data. Infect. Dis. Ther. 2023, 12, 1487–1504. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, J.J.; Avellaneda, A.M.; Salazar-Ardiles, C.; Maya, J.E.; Kalergis, A.M.; Lay, M.K. Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Front. Immunol. 2019, 10, 2152. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B. Respiratory Syncytial Viruses. In Mandell, Dougals and Bennet’s Principles and Practice of Infectious Diseases, 7th ed.; Churchill Livingstone: London, UK, 2010; pp. 2207–2227. [Google Scholar]
- Colosia, A.; Costello, J.; McQuarrie, K.; Kato, K.; Bertzos, K. Systematic literature review of the signs and symptoms of respiratory syncytial virus. Influenza Other Respir. Viruses 2023, 17, e13100. [Google Scholar] [CrossRef] [PubMed]
- Riccò, M.; Corrado, S.; Palmieri, S.; Marchesi, F. Respiratory Syncytial Virus: A Systematic Review and Meta-Analysis of Tomographic Findings (2000–2022). Children 2023, 10, 1169. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.P.; Ghantoji, S.S.; Shah, J.N.; El Taoum, K.K.; Jiang, Y.; Popat, U.; Hosing, C.; Rondon, G.; Tarrand, J.J.; Champlin, R.E.; et al. Impact of aerosolized ribavirin on mortality in 280 allogeneic haematopoietic stem cell transplant recipients with respiratory syncytial virus infections. J. Antimicrob. Chemother. 2013, 68, 1872–1880. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.P.; Whalen, M.; Hilts-Horeczko, A.; Doernberg, S.B.; Liu, C. Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single-center retrospective cohort study and review of the literature. Transpl. Infect. Dis. 2018, 20, e12844. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.E.; Walsh, E.E.; Falsey, A.R. The effect of steroid use in hospitalized adults with respiratory syncytial virus-related illness. Chest 2011, 140, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Jeannoël, M.; Lina, G.; Rasigade, J.P.; Lina, B.; Morfin, F.; Casalegno, J.S. Microorganisms associated with respiratory syncytial virus pneumonia in the adult population. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, J.; Zou, X.; Fan, Y.; Xiong, Z.; Li, B.; Wang, C.; Li, H.; Han, J.; Liu, X.; et al. Severity of influenza virus and respiratory syncytial virus coinfections in hospitalized adult patients. J. Clin. Virol. 2020, 133, 104685. [Google Scholar] [CrossRef] [PubMed]
- Godefroy, R.; Giraud-Gatineau, A.; Jimeno, M.T.; Edouard, S.; Meddeb, L.; Zandotti, C.; Chaudet, H.; Colson, P.; Raoult, D.; Cassir, N. Respiratory Syncytial Virus Infection: Its Propensity for Bacterial Coinfection and Related Mortality in Elderly Adults. Open Forum Infect. Dis. 2020, 7, ofaa546. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, T.; Jang, Y.R.; Kim, M.C.; Chong, Y.P.; Lee, S.O.; Choi, S.H.; Kim, Y.S.; Woo, J.H.; Kim, S.H. Factors predicting life-threatening infections with respiratory syncytial virus in adult patients. Infect. Dis. 2017, 49, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Osei-Yeboah, R.; Johannesen, C.K.; Egeskov-Cavling, A.M.; Chen, J.; Lehtonen, T.; Fornes, A.U.; Paget, J.; Fischer, T.K.; Wang, X.; Nair, H.; et al. Respiratory syncytial virus-associated hospitalisation in adults with comorbidities in two European countries: A modelling study. J. Infect. Dis. 2023, 229, S70–S77. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Boattini, M.; Christaki, E.; Moreira Marques, T.; Moreira, I.; Cruz, L.; Tosatto, V.; Antão, D.; Bianco, G.; Iannaccone, M.; et al. Comparative virulence of seasonal viruses responsible for lower respiratory tract infections: A southern European multi-centre cohort study of hospital admissions. Infection 2021, 49, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Boattini, M.; Almeida, A.; Christaki, E.; Cruz, L.; Antão, D.; Moreira, M.I.; Bianco, G.; Iannaccone, M.; Tsiolakkis, G.; Khattab, E.; et al. Influenza and respiratory syncytial virus infections in the oldest-old continent. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2085–2090. [Google Scholar] [CrossRef] [PubMed]
- Casiano-Colón, A.E.; Hulbert, B.B.; Mayer, T.K.; Walsh, E.E.; Falsey, A.R. Lack of sensitivity of rapid antigen tests for the diagnosis of respiratory syncytial virus infection in adults. J. Clin. Virol. 2003, 28, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, C.; Tremblay, N.; Renaud, C.; Papenburg, J. Diagnostic Accuracy of Rapid Antigen Detection Tests for Respiratory Syncytial Virus Infection: Systematic Review and Meta-analysis. J. Clin. Microbiol. 2015, 53, 3738–3749. [Google Scholar] [CrossRef] [PubMed]
- Onwuchekwa, C.; Moreo, L.M.; Menon, S.; Machado, B.; Curcio, D.; Kalina, W.; Atwell, J.E.; Gessner, B.D.; Siapka, M.; Agarwal, N.; et al. Underascertainment of Respiratory Syncytial Virus Infection in Adults Due to Diagnostic Testing Limitations: A Systematic Literature Review and Meta-analysis. J. Infect. Dis. 2023, 228, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance (accessed on 20 December 2023).
- Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/case-definitions (accessed on 20 December 2023).
- Available online: https://www.who.int/teams/global-influenza-programme/surveillance-and-monitoring/case-definitions-for-ili-and-sari (accessed on 20 December 2023).
- Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/sampling-strategy-for-rsv-testing (accessed on 20 December 2023).
- Available online: https://www.who.int/teams/global-influenza-programme/global-respiratory-syncytial-virus-surveillance/collection-transport-and-storage (accessed on 20 December 2023).
- Osei-Yeboah, R.; Spreeuwenberg, P.; Del Riccio, M.; Fischer, T.K.; Egeskov-Cavling, A.M.; Bøås, H.; van Boven, M.; Wang, X.; Lehtonen, T.; Bangert, M.; et al. Estimation of the Number of Respiratory Syncytial Virus-Associated Hospitalizations in Adults in the European Union. J. Infect. Dis. 2023, 228, 1539–1548. [Google Scholar] [CrossRef] [PubMed]
- Rozenbaum, M.H.; Judy, J.; Tran, D.; Yacisin, K.; Kurosky, S.K.; Begier, E. Low Levels of RSV Testing Among Adults Hospitalized for Lower Respiratory Tract Infection in the United States. Infect. Dis. Ther. 2023, 12, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Korsten, K.; Adriaenssens, N.; Coenen, S.; Butler, C.C.; Verheij, T.J.M.; Bont, L.J.; Wildenbeest, J.G.; RESCEU Investigators. World Health Organization Influenza-Like Illness Underestimates the Burden of Respiratory Syncytial Virus Infection in Community-Dwelling Older Adults. J. Infect. Dis. 2022, 226, S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Pebody, R.; Moyes, J.; Hirve, S.; Campbell, H.; Jackson, S.; Moen, A.; Nair, H.; Simões, E.A.F.; Smith, P.G.; Wairagkar, N.; et al. Approaches to use the WHO respiratory syncytial virus surveillance platform to estimate disease burden. Influenza Other Respir. Viruses 2020, 14, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Cawcutt, K.; Kalil, A.C. Pneumonia with bacterial and viral coinfection. Curr. Opin. Crit. Care 2017, 23, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, G.A.; Gálvez, N.M.S.; Soto, J.A.; Andrade, C.A.; Kalergis, A.M. Bacterial and Viral Coinfections with the Human Respiratory Syncytial Virus. Microorganisms 2021, 9, 1293. [Google Scholar] [CrossRef] [PubMed]
- Mazur, N.I.; Terstappen, J.; Baral, R.; Bardají, A.; Beutels, P.; Buchholz, U.J.; Cohen, C.; Crowe, J.E., Jr.; Cutland, C.L.; Eckert, L.; et al. Respiratory syncytial virus prevention within reach: The vaccine and monoclonal antibody landscape. Lancet Infect. Dis. 2023, 23, e2–e21. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.path.org/our-impact/resources/rsv-vaccine-and-mab-snapshot/ (accessed on 29 January 2024).
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory Syncytial Virus Prefusion F Protein Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Pérez Marc, G.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Falsey, A.R.; Scott, D.A.; Gurtman, A.; Zareba, A.M.; Jansen, K.U.; Gruber, W.C.; Dormitzer, P.R.; Swanson, K.A.; Radley, D.; et al. A Randomized Phase 1/2 Study of a Respiratory Syncytial Virus Prefusion F Vaccine. J. Infect. Dis. 2022, 225, 1357–1366. [Google Scholar] [CrossRef] [PubMed]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Rämet, M.; et al. Efficacy and Safety of an mRNA-Based RSV PreF Vaccine in Older Adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Available online: https://clinicaltrials.gov/study/NCT05585632?cond=mRNA-1230&rank=1#study-overview (accessed on 29 January 2024).
- Cockerill, G.S. JNJ-5371678, Defining a Role for Fusion Inhibitors in the Treatment of Respiratory Syncytial Virus. J. Med. Chem. 2020, 63, 8043–8045. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Eze, K.; Noulin, N.; Horvathova, V.; Murray, B.; Baillet, M.; Grey, L.; Mori, J.; Adda, N. EDP-938, a Respiratory Syncytial Virus Inhibitor, in a Human Virus Challenge. N. Engl. J. Med. 2022, 386, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Melgar, M.; Britton, A.; Roper, L.E.; Talbot, H.K.; Long, S.S.; Kotton, C.N.; Havers, F.P. Use of Respiratory Syncytial Virus Vaccines in Older Adults: Recommendations of the Advisory Committee on Immunization Practices—United States, 2023. Morb. Mortal. Wkly. Rep. (MMWR) 2023, 72, 793–801. [Google Scholar] [CrossRef] [PubMed]
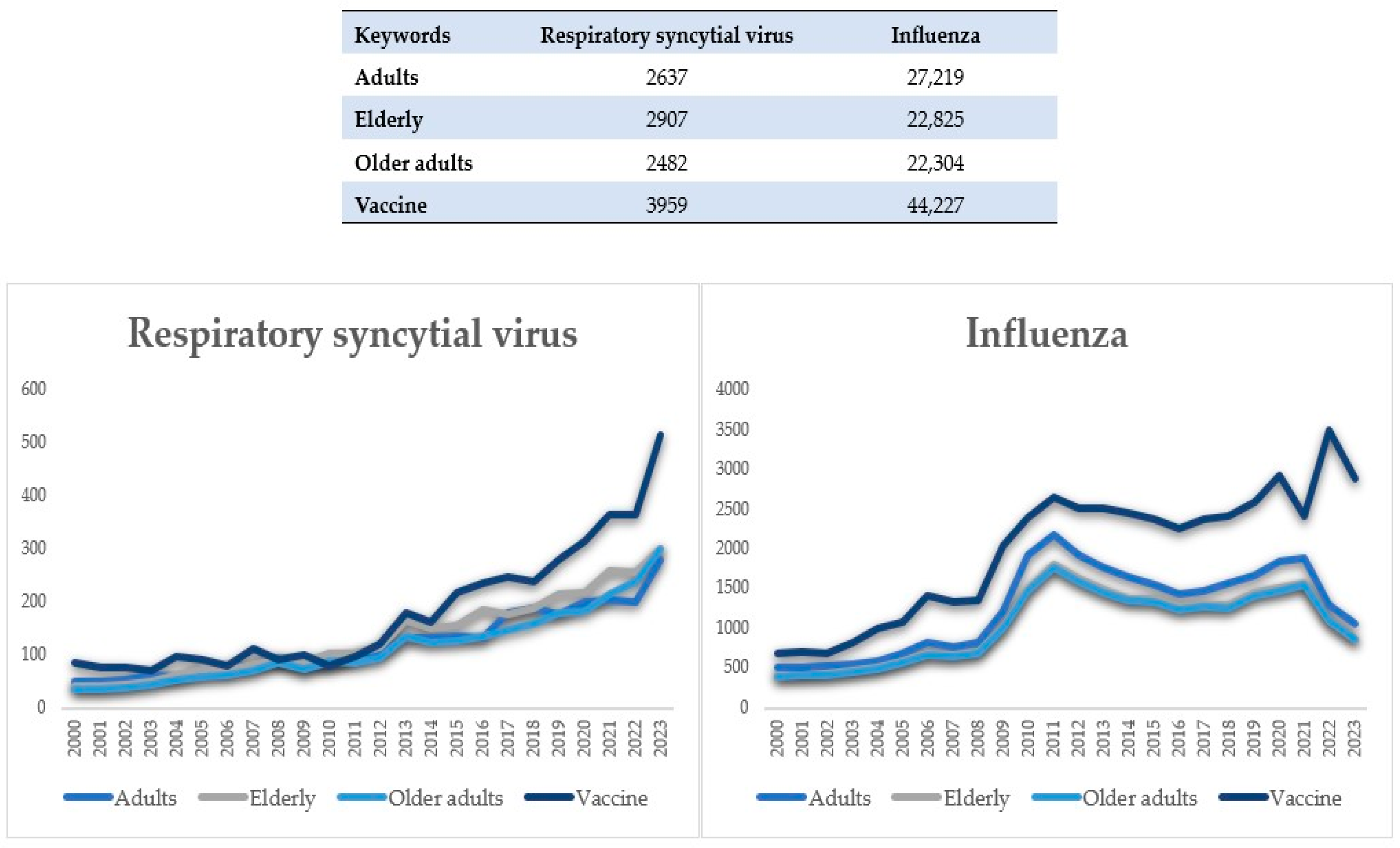
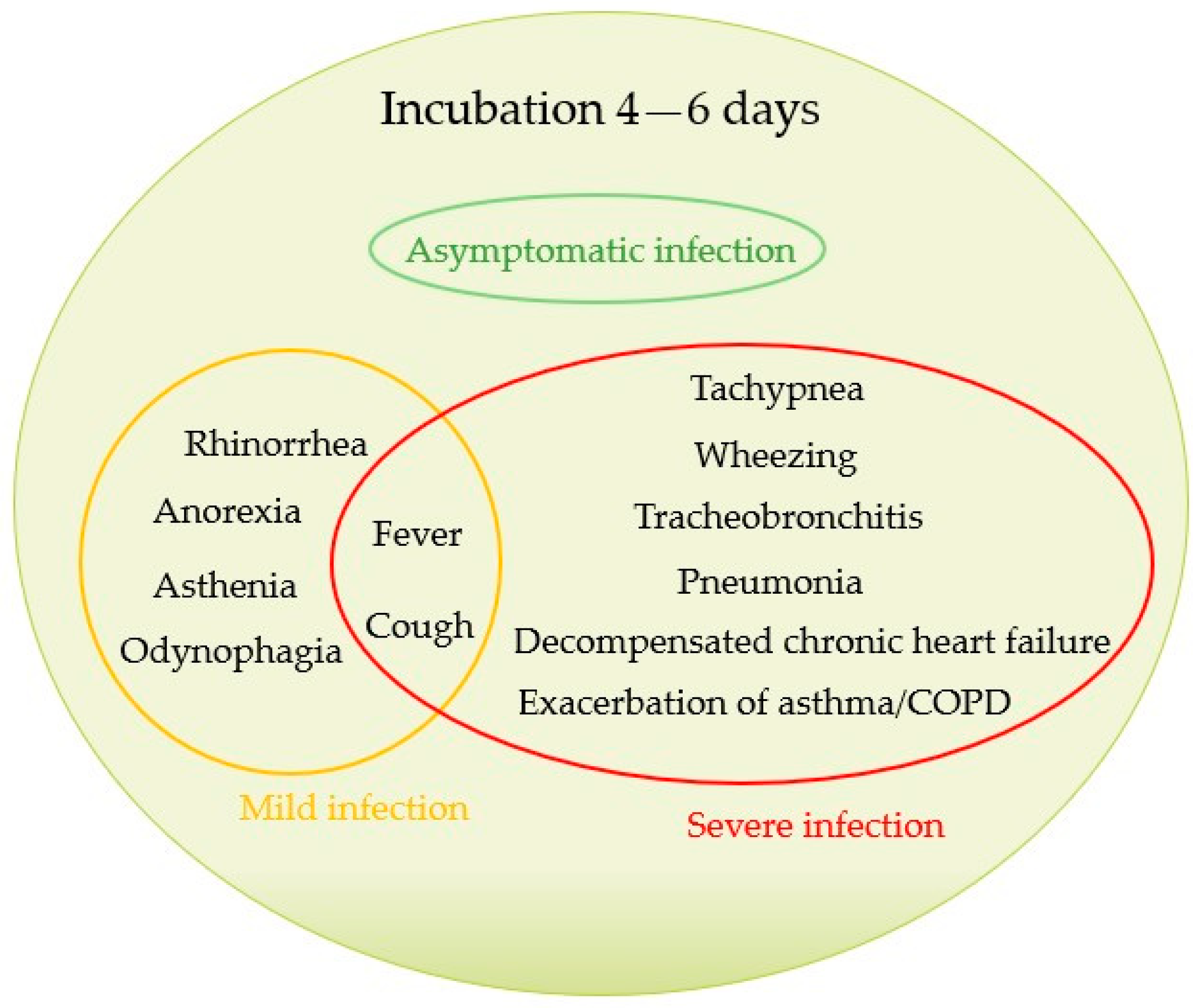
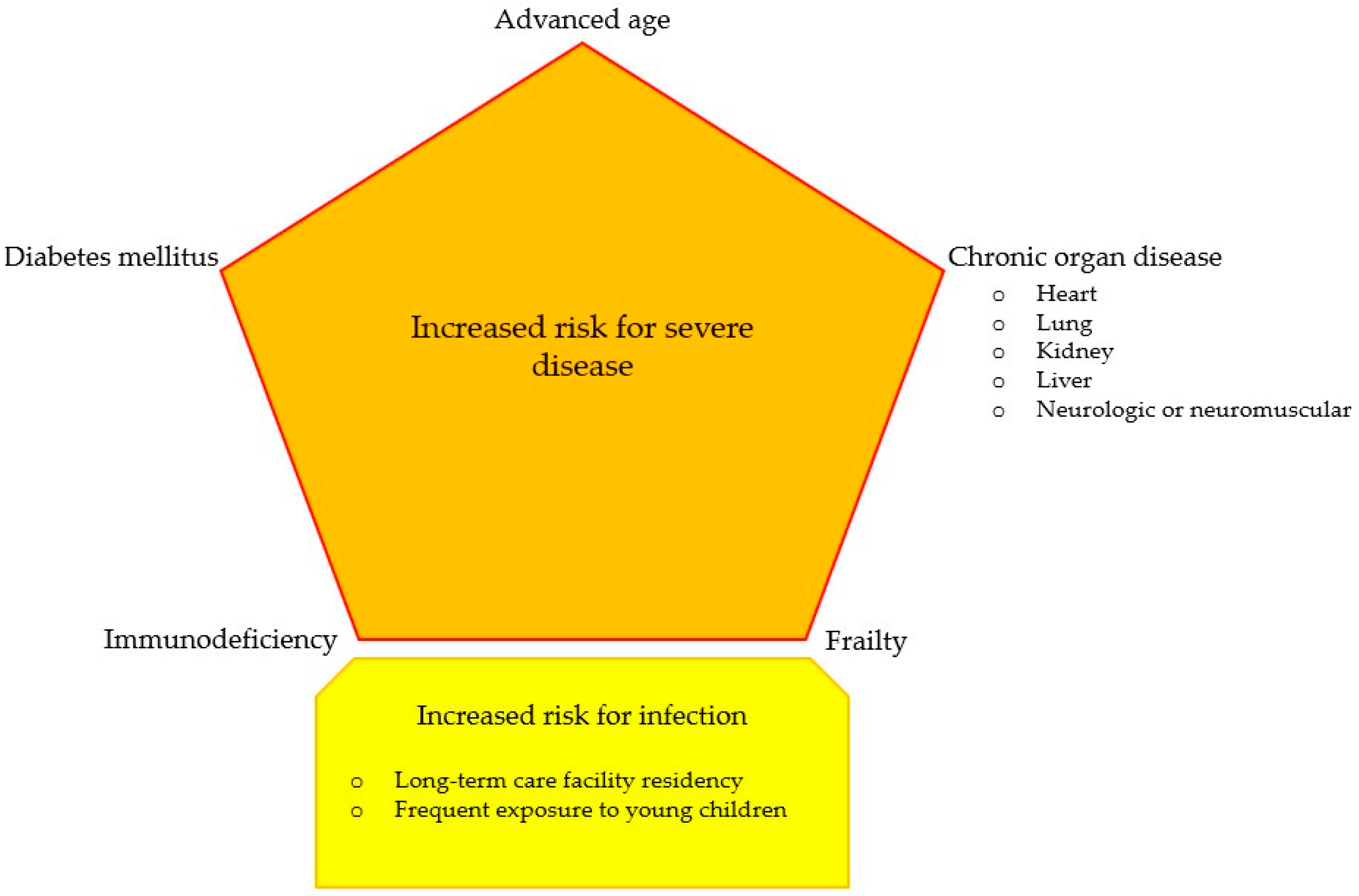
| Study | Period | Country | Patients (n) | Age ± SD or (Range) or [IQR], Years | Critically Ill Patients % (n) | Pneumonia % (n) | Coinfection % (n) | Mortality Rate % (n) |
|---|---|---|---|---|---|---|---|---|
| [2] | 1999–2003 | USA | 46 | Not reported | Not reported | 2 (1) | Not reported | 0 |
| 56 | Not reported | Not reported | 7 (4) | 4 (2) | ||||
| 132 | 76 ± 13 | 15 (20) | 31 (41) | 8 (10) | ||||
| [78] | 2007–2008 | USA | 26 | 65 ± 14 | 0 | Not reported | Not reported | 0 |
| 32 | 71 ± 13 | 34 (11) | 19 (6) | |||||
| [90] | 2005–2008 | USA | 33 | 69.8 ± 14.9 | 18 (6) | 15 (5) | Not reported | 6 (2) |
| 17 | 72.0 ± 14.8 | 29 (5) | 24 (4) | 0 | ||||
| [79] | 2006–2009 | USA | 31 | 68 [56–78] | 9.7 (3) | Not reported | Not reported | 6.5 (2) |
| [50] | 2007–2012 | Guatemala | 65 | ≥50 | 9 (6) | 59 (23) | Not reported | 13 (8) |
| [11] | 2009–2011 | Hong Kong, China | 607 | 75.1 ± 16.4 | Not reported | 42.3 | 12.5 | 9.1 |
| [77] | 2009–2010 | USA | 32 | 60.8 [44.8–68.9] | 16.7 (4) | Not reported | Not reported | 4.2 (1) |
| [51] | 2012–2013 | Canada | 86 | 74 (19–102) | 15 (13) | 40 (34) | 13 (11) | 6 (5) |
| [4] | 2008–2009 | 15 countries | 41 | Not reported | Not reported | 4.9 (2) | 4.9 (2) | Not reported |
| [72] | 2009–2012 | USA | 41 | 53.8 ± 11.8 | 14.6 (6) | Not reported | Not reported | 4.9 (2) |
| 28 | 55 ± 15.1 | 17.9 (5) | 10.7 (3) | |||||
| 106 | 62.1 ± 19.8 | 24.5 (26) | 6.6 (7) | |||||
| [69] | 2013 | Hong Kong, China | 123 | 78 ± 15 | 12.2 (15) | 67.5 (83) | Not reported | 8.9 (11) |
| [81] | 2012–2013 | USA | 75 | >65 | Not reported | 34.7 | Not reported | 4 |
| 39 | Not reported | 38.5 | 10.3 | |||||
| [60] | 2012–2015 | France | 53 | 74 (61–84) | 15 (8) | 44 (23) | Not reported | 8 (4) |
| [54] | 2004–2016 | USA | 243 | ≥60 | 0 | 9.5 (23) | Not reported | 0 |
| [21] | 2015–2016 | Spain | 95 | 57.7 | Not reported | 33.6 (32) | Not reported | 14.7 (14) |
| [73] | 2000–2013 | USA | 181 | 59 (18–87) | 13 (24) | Not reported | 8 (14) | 7 (13) |
| [70] | 2005–2014 | Switzerland | 107 | 60.5 [48–70.6] | 29.3 (17) | 36.9 (62) | 23.4 (25) | 19 (11) |
| 68 | 50.8 [37.3–59.4] | |||||||
| [55] | 2009–2015 | USA | 489 | 60 ± 17 | 27 (132) | 38.8 (190) | 8.2 (40) | 3.9 (19) |
| [59] | 2005–2018 | The Netherlands | 192 | 60.7 [50.8–69.2] | 16 (30) | Not reported | 42.2 (81) | 8 (16) |
| [56] | 2014–2015 | Thailand | 69 | 72 [58–81] | 36.2 (25) | 79.7 (55) | 8.7 (6) | 15.9 (11) |
| [91] | 2013–2016 | France | 27 | 70 [56–82] | 66.7 (18) | 100 (27) | 100 (27) | 25.9 (7) |
| 62 | 76 [59–85] | 21 (13) | 100 (62) | 0 | 17.7 (11) | |||
| [57] | 2012–2015 | Republic of Korea | 132 | ≥65 | 25 (33) | 56.8 (75) | Not reported | 10.6 (14) |
| [12] | 2011–2015 | USA | 664 | 78 (60–103) | 18 | 66 | Not reported | 5.6 |
| [92] | 2017–2019 | China | 113 | 64.2 ± 16.3 | 22.1 (25) | Not reported | Not reported | 11.5 (13) |
| [93] | 2014–2019 | France | 616 | 70.4 ± 19.4 | Not reported | Not reported | 0 | 4.9 (30) |
| 85 | 66.6 ± 18.6 | 100 (85) | 12.9 (11) | |||||
| [68] | 2016–2018 | Alaska, USA | 8 | 68 [52–77] | Not reported | 75(6) | 0 | 0 |
| [76] | 2013–2015 | USA | 192 | ≥65 | 13 (25) | Not reported | 20.3 (39) | 5.9 (11) |
| [67] | 2019–2019 | Austria | 103 | 57 [40–73] | 6.8 (7) | 17.5 (18) | Not reported | 2.9 (3) |
| [37] | 2012–2015 | New Zealand | 281 | (18–80) | 2.8 (8) | Not reported | Not reported | 1.4 (4) |
| [35] | 2017–2019 | Belgium, UK, The Netherlands | 59 | 75 (70–79) | 0 | Not reported | Not reported | 0 |
| [62] | 2017–2019 | Italy, Portugal, Cyprus | 166 | 80.9 ± 8.7 | Not reported | 29.6 (49) | Not reported | 12.1 (20) |
| [63] | 2017–2019 | Switzerland | 79 | 78 [65–84] | 19 (15) | 40.5 (32) | Not reported | 10.1 (8) |
| [71] | 2015–2017 | USA | 1713 | ≥65 (60%) | 20 (344) | Not reported | Not reported | 5 (86) |
| [75] | 2016–2018 | China | 71 | 77 [67–83] | 4.2 (3) | 46.5 (33) | 21.1 (15) | 7 (5) |
| [65] | 2017–2018 | Finland | 152 | 73 [65–86] | 3.9 (6) | 37.5 (57) | Not reported | 8.6 (13) |
| [61] | 2011–2018 | France, Belgium | 309 | 67.2 ± 15 | 100 (309) | Not reported | 27.2 (84) | 23.9 (74) |
| [74] | 2017–2019 | USA | 403 | 69.0 [57.2–82.1] | 16.4 (66) | Not reported | Not reported | 7.7 (31) |
| [64] | 2015–2019 | France | 1168 | 75 [63–85] | 24.6 (288) | Not reported | 18.2 (213) | 6.6 (77) |
| [58] | 2016–2019 | USA | 622 | ≥65 | 12.4 | Not reported | Not reported | 1.5 (9) |
| Study | Acute Lower Respiratory Infection | Hospitalization | Requirement for Ventilatory Support or ICU Admission | Short-Term Mortality |
|---|---|---|---|---|
| [26] | - Chronic pulmonary disease; | |||
| - Functional disability; | ||||
| - Low serum neutralizing antibody titre; | ||||
| [38] | - Underlying medical conditions; | |||
| - Female sex; | ||||
| - Increased mucosal IL-6 level; | ||||
| - Longer duration of virus shedding; | ||||
| [11] | - Chronic lung disease; - Pneumonia; - Elevated urea and ALT; | - Advanced age; | ||
| - Pneumonia; | ||||
| - Requirement for ventilation; | ||||
| - Bacterial superinfection; | ||||
| - Elevated urea and WBC count; | ||||
| [80] | - Congestive heart failure; | |||
| - Exposure to children; | ||||
| [50] | - Cardiovascular disease; | |||
| [51] | - Need for ICU and mechanical ventilation; | |||
| [69] | - Older age; | |||
| - Major comorbidities; | ||||
| - Bacterial superinfection; | ||||
| - Requirement for ventilation; | ||||
| [72] | - Age > 60 years (vs. age ≤ 60) | |||
| [94] | - Lower respiratory infection, chronic respiratory disease, bacterial coinfection, and fever; | |||
| [60] | - Cancer - Immunosuppressive treatment; | |||
| [54] | - Age ≥ 75 years (vs. 60–64 years); - COPD or congestive heart failure; | |||
| [73] | - Neutropenia and lymphocytopenia and not receiving ribavirin-based therapy during RSV upper respiratory tract infection; | - Neutropenia and lymphocytopenia at RSV diagnosis; | ||
| [70] | - Solid tumours or leukaemia, chronic immunosuppression (vs. HSCT recipients); | |||
| [59] | - Lower respiratory tract infection, chronic pulmonary disease, temperature, confusion, and elevated urea; | |||
| [8] | - Older age; | |||
| - COPD; | ||||
| - Congestive heart failure; | ||||
| - Chronic kidney disease; | ||||
| - Previous pneumonia; | ||||
| - Haematological malignancies; | ||||
| - Stroke; | ||||
| - Baseline healthcare resource use; | ||||
| [12] | - ≥ two hospitalizations in the prior six months; | |||
| - Tachypnoea; | ||||
| - Altered consciousness; | ||||
| - Lymphoma; | ||||
| - During hospitalization: | ||||
| - Acute renal failure; | ||||
| - Atrial fibrillation; | ||||
| - Neurovascular complication; | ||||
| [76] | - Neurologic disease; - Respiratory disease; - Congestive heart failure; | |||
| [62] | - OSA/OHS; - Chronic kidney disease; | - Male gender; - Solid neoplasm; - OSA/OHS; | ||
| [67] | - Age > 65 years; | - Respiratory disease; - Complications; - Pneumonia; - Superinfection; | - Age > 65 years; | |
| - Smoking; | ||||
| - Cardiac disease; | ||||
| - Diabetes mellitus; | ||||
| - Pneumonia; | ||||
| [37] | - Age 65–80 and diabetes mellitus; - Age ≥ 50 years and chronic heart failure or COPD; | |||
| [71] | - Higher census tract-level poverty and crowding; | |||
| [75] | - IL-6 concentration; | |||
| [5] | - COPD; | |||
| - Coronary artery disease; | ||||
| - Congestive heart failure; | ||||
| [64] | - Chronic heart or respiratory failure; - Coinfection; | - Age ≥ 85 years; | ||
| - Neutropenia; | ||||
| - Acute respiratory failure; | ||||
| - Need for ventilation support; | ||||
| - Withdrawing of life-sustaining therapies; | ||||
| [95] | - COPD or asthma; | |||
| - Ischemic heart disease; | ||||
| - Stroke; | ||||
| - Diabetes; | ||||
| - Chronic kidney disease; | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boattini, M.; Almeida, A.; Comini, S.; Bianco, G.; Cavallo, R.; Costa, C. From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults. Viruses 2024, 16, 531. https://doi.org/10.3390/v16040531
Boattini M, Almeida A, Comini S, Bianco G, Cavallo R, Costa C. From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults. Viruses. 2024; 16(4):531. https://doi.org/10.3390/v16040531
Chicago/Turabian StyleBoattini, Matteo, André Almeida, Sara Comini, Gabriele Bianco, Rossana Cavallo, and Cristina Costa. 2024. "From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults" Viruses 16, no. 4: 531. https://doi.org/10.3390/v16040531
APA StyleBoattini, M., Almeida, A., Comini, S., Bianco, G., Cavallo, R., & Costa, C. (2024). From Forgotten Pathogen to Target for New Vaccines: What Clinicians Need to Know about Respiratory Syncytial Virus Infection in Older Adults. Viruses, 16(4), 531. https://doi.org/10.3390/v16040531








