Intensive Care Unit Mortality Trends during the First Two Years of the COVID-19 Pandemic in Greece: A Multi-Center Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Patient Selection and Data Collection
2.3. Ethical Statement
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Demographics
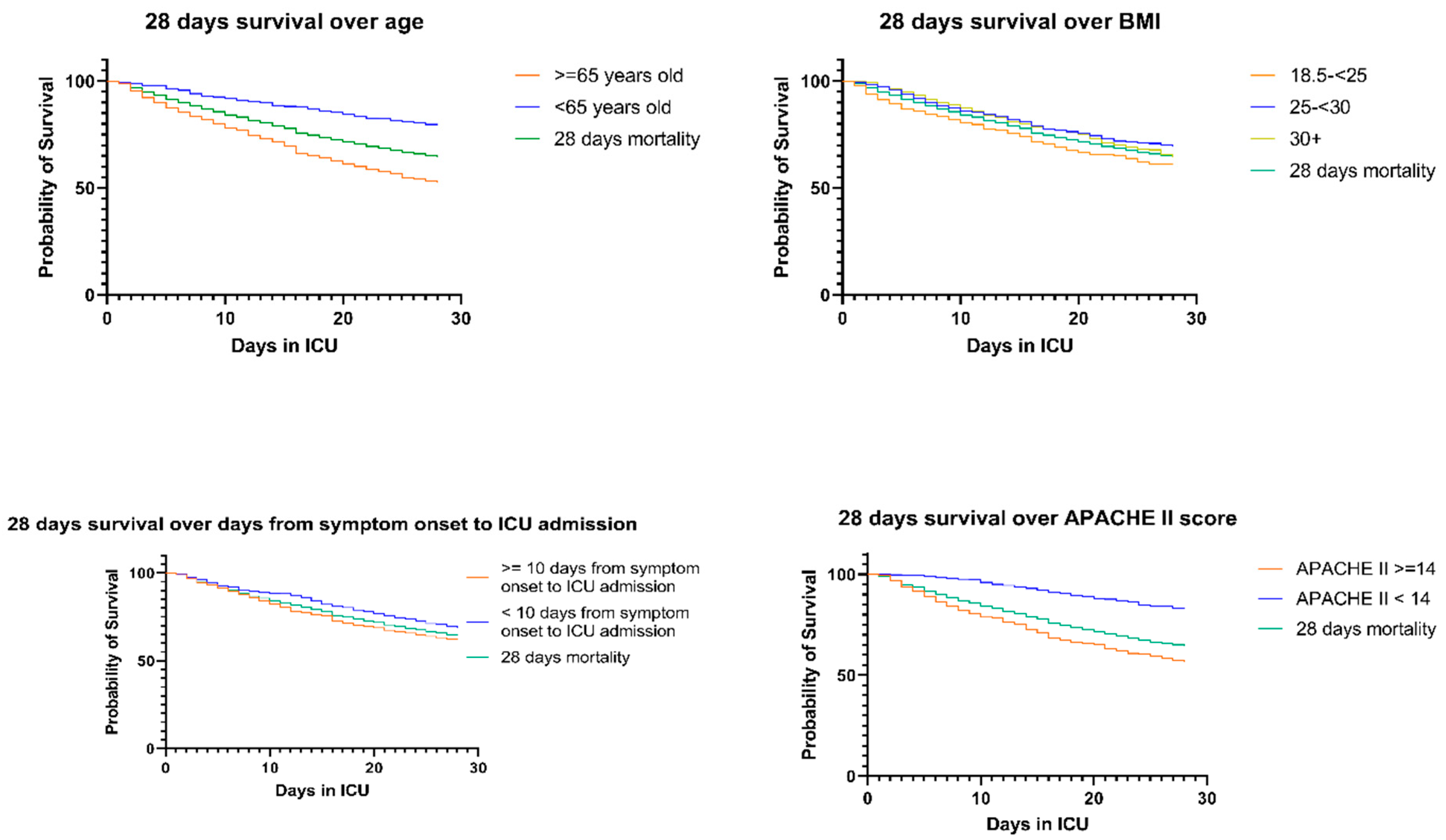
3.2. 28-Day Mortality
3.3. Overall In-Hospital Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Joy, M.; Hobbs, F.R.; Bernal, J.L.; Sherlock, J.; Amirthalingam, G.; McGagh, D.; Akinyemi, O.; Byford, R.; Dabrera, G.; Dorward, J.; et al. Excess mortality in the first COVID pandemic peak: Cross-sectional analyses of the impact of age, sex, ethnicity, household size, and long-term conditions in people of known SARS-CoV-2 status in England. Br. J. Gen. Pract. 2020, 70, e890–e898. [Google Scholar] [CrossRef] [PubMed]
- Richards-Belle, A.; Orzechowska, I.; Gould, D.W.; Thomas, K.; Doidge, J.C.; Mouncey, P.R.; Christian, M.D.; Shankar-Hari, M.; Harrison, D.A.; Rowan, K.M.; et al. COVID-19 in critical care: Epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020, 46, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, G.; Greco, M.; Zanella, A.; Albano, G.; Antonelli, M.; Bellani, G.; Bonanomi, E.; Cabrini, L.; Carlesso, E.; Castelli, G.; et al. Risk Factors Associated with Mortality Among Patients With COVID-19 in Intensive Care Units in Lombardy, Italy. JAMA Intern. Med. 2020, 180, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.G.; Amin, A.B.; Ali, A.R.; Hoots, B.; Cadwell, B.L.; Arora, S.; Avoundjian, T.; Awofeso, A.O.; Barnes, J.; Bayoumi, N.S.; et al. COVID-19 Incidence and Death Rates Among Unvaccinated and Fully Vaccinated Adults with and Without Booster Doses During Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4–December 25, 2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 132–138. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; Molina, K.C.; Beaty, L.E.; Bennett, T.D.; Carlson, N.E.; Mayer, D.A.; Peers, J.L.; Russell, S.; Wynia, M.K.; Ginde, A.A. Real-world use of nirmatrelvir&ritonavir in outpatients with COVID-19 during the era of omicron variants including BA.4 and BA.5 in Colorado, USA: A retrospective cohort study. Lancet Infect. Dis. 2023, 23, 696–705. [Google Scholar] [CrossRef]
- Agrawal, U.; Agrawal, U.; Bedston, S.; Bedston, S.; McCowan, C.; McCowan, C.; Oke, J.; Oke, J.; Patterson, L.; Patterson, L.; et al. Severe COVID-19 outcomes after full vaccination of primary schedule and initial boosters: Pooled analysis of national prospective cohort studies of 30 million individuals in England, Northern Ireland, Scotland, and Wales. Lancet 2022, 400, 1305–1320. [Google Scholar] [CrossRef]
- Schwartz, K.L.; Wang, J.; Tadrous, M.; Langford, B.J.; Daneman, N.; Leung, V.; Gomes, T.; Friedman, L.; Daley, P.; Brown, K.A. Population-based evaluation of the effectiveness of nirmatrelvir–ritonavir for reducing hospital admissions and mortality from COVID-19. Can. Med. Assoc. J. 2023, 195, E220. [Google Scholar] [CrossRef]
- Demombynes, G.; de Walque, D.; Gubbins, P.; Urdinola, P.; Veillard, J. Are COVID-19 age-mortality curves for 2020 flatter in developing countries? Evidence from a cross-sectional observational study of population-level official death counts and excess deaths estimates. BMJ Open 2022, 12, e061589. [Google Scholar] [CrossRef]
- Ahmed, T.; Roberton, T.; Vergeer, P.; Hansen, P.M.; Peters, M.A.; Ofosu, A.A.; Mwansambo, C.; Nzelu, C.; Wesseh, C.S.; Smart, F.; et al. Healthcare utilization and maternal and child mortality during the COVID-19 pandemic in 18 low- and middle-income countries: An interrupted time-series analysis with mathematical modeling of administrative data. PLoS Med. 2022, 19, e1004070. [Google Scholar] [CrossRef]
- Wang, H.; Paulson, K.R.; Pease, S.A.; Watson, S.; Comfort, H.; Zheng, P.; Aravkin, A.Y.; Bisignano, C.; Barber, R.M.; Alam, T.; et al. Estimating excess mortality due to the COVID-19 pandemic: A systematic analysis of COVID-19-related mortality, 2020–2021. Lancet 2022, 399, 1513–1536. [Google Scholar] [CrossRef]
- Chew, M.S.; Kattainen, S.; Haase, N.; Buanes, E.A.; Kristinsdottir, L.B.; Hofsø, K.; Laake, J.H.; Kvåle, R.; Hästbacka, J.; Reinikainen, M.; et al. A descriptive study of the surge response and outcomes of ICU patients with COVID-19 during first wave in Nordic countries. Acta Anaesthesiol. Scand. 2022, 66, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.D.W.; Fumeaux, T.; Guerci, P.; Heuberger, D.M.; Montomoli, J.; Roche-Campo, F.; Schuepbach, R.A.; Hilty, M.P.; Farias, M.A.; Margarit, A.; et al. Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine 2020, 25, 100449. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A.; Kane, A.D.; Kursumovic, E.; Oglesby, F.C.; Cook, T.M. Mortality in patients admitted to intensive care with COVID-19: An updated systematic review and meta-analysis of observational studies. Anaesthesia 2021, 76, 537–548. [Google Scholar] [CrossRef]
- Florescu, S.; Stanciu, D.; Zaharia, M.; Kosa, A.; Codreanu, D.; Kidwai, A.; Masood, S.; Kaye, C.; Coutts, A.; MacKay, L.; et al. Investigators WC for the RC. Long-term (180-Day) Outcomes in Critically Ill Patients With COVID-19 in the REMAP-CAP Randomized Clinical Trial. JAMA 2023, 329, 39–51. [Google Scholar] [CrossRef]
- COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021, 47, 60–73. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Nyasulu, P.S.; Ayele, B.T.; Koegelenberg, C.F.; Irusen, E.; Lalla, U.; Davids, R.; Chothia, Y.; Retief, F.; Johnson, M.; Venter, S.; et al. Clinical characteristics associated with mortality of COVID-19 patients admitted to an intensive care unit of a tertiary hospital in South Africa. PLoS ONE 2022, 17, e0279565. [Google Scholar] [CrossRef]
- Van Do, T.; Manabe, T.; Van Vu, G.; Nong, V.M.; Fujikura, Y.; Phan, D.; Pham, T.T.; Do, C.D.; Doan, T.T.; Nguyen, N.T.; et al. Clinical characteristics and mortality risk among critically ill patients with COVID-19 owing to the B.1.617.2 (Delta) variant in Vietnam: A retrospective observational study. PLoS ONE 2023, 18, e0279713. [Google Scholar] [CrossRef]
- Malli, F.; Lampropoulos, I.C.; Perlepe, G.; Papagiannis, D.; Gourgoulianis, K.I. Analysis of SARS-CoV-2 Cases, COVID-19 Outcomes and Vaccinations, during the Different SARS-CoV-2 Variants in Greece. Vaccines 2023, 11, 126. [Google Scholar] [CrossRef]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, S.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Tabatabai, M.; Juarez, P.D.; Matthews-Juarez, P.; Wilus, D.M.; Ramesh, A.; Alcendor, D.J.; Tabatabai, N.; Singh, K.P. An Analysis of COVID-19 Mortality During the Dominancy of Alpha, Delta, and Omicron in the USA. J. Prim. Care Community Health 2023, 14, 21501319231170164. [Google Scholar] [CrossRef] [PubMed]
| Survivors N = 717 | Non-Survivors N = 745 | p-Value | |
|---|---|---|---|
| Demographics | |||
| Age, median (IQR) [years] | 61 (52–69) | 71 (64–78) | <0.001 |
| Sex, n (%) (1 missing) | Male: 475 (66.34%) | Male: 495 (66.44%) | 0.967 |
| Wave, n (%) (0 missing) | 1st: 25 (3.49%) 2nd: 170 (23.71%) 3rd: 399 (55.65%) 4th: 123 (17.15%) | 1st: 18 (2.42%) 2nd: 154 (20.67%) 3rd: 324 (43.49%) 4th: 249 (33.42%) | <0.001 |
| Comorbidities | |||
| BMI (kg/m2) n (%) (0 missing) | 18.5–24: 304 (42.40%) 25–29: 197 (27.48%) 30–34: 109 (15.20%) 35–39: 64 (8.93%) ≥40: 43 (6.00%) | 18.8- < 25: 298 (40.00%) 25- < 30: 203 (27.25%) 30- < 35: 143 (19.19%) 35- < 40: 47 (6.31%) 40+: 54 (7.25%) | 0.090 |
| Smoking Status, n (%) (129 missing) | Never: 458 (66.38%) Former: 159 (23.04%) Current: 73 (10.58%) | Never: 401 (61.50%) Former: 164 (25.15%) Current: 87 (13.34%) | 0.135 |
| Hypothyroidism, n (%) (53 missing) | 106 (14.91%) | 65 (9.19%) | 0.001 |
| Immunosuppression, n (%) (50 missing) | 25 (3.52%) | 49 (6.90%) | 0.004 |
| Diabetes Mellitus, n (%) (47 missing) | 159 (22.33%) | 210 (29.49%) | 0.002 |
| Coronary Artery Disease, n (%) (47 missing) | 61 (8.57%) | 144 (20.22%) | <0.001 |
| Hypertension, n (%) (47 missing) | 332 (46.63%) | 405 (56.88%) | <0.001 |
| Cancer/Hematologic Malignancy, n (%) (47 missing) | 74 (10.39%) | 138 (19.38%) | <0.001 |
| Chronic Obstructive Pulmonary Disease, n (%) (47 missing) | 34 (4.77%) | 99 (13.90%) | <0.001 |
| Chronic Kidney Disease on Renal Replacement Therapy, n (%) (110 missing) | 19 (2.76%) | 68 (10.10%) | <0.001 |
| Disease status | |||
| Days from symptom onset to hospital admission, median (IQR) (51 missing) | 7 (5–9) | 5 (3–8) | <0.001 |
| Days from symptom onset to ICU, median (IQR) (36 missing) | 10 (7–13) | 11 (7–15.5) | 0.017 |
| SOFA at ICU admission, median (IQR) (269 missing) | 4 (2–7) | 8 (5–9) | <0.001 |
| APACHEII at ICU admission, median (IQR) (249 missing) | 11 (8–16) | 18 (13–24) | <0.001 |
| Complications during ICU stay | |||
| Intubation, n(%) (11 missing) | 446 (62.20%) | 734 (98,79%) | <0.001 |
| Acute Kidney Injury, n (%) (52 missing) | Stage 1: 75 (10.58%) Stage 2: 23 (3.24%) Stage 3: 57 (8.04%) | Stage 1: 97 (13.66%) Stage 2: 98 (13.80%) Stage 3: 281 (39.58%) | <0.001 |
| Continuous Venovenous Hemodiafiltration, n (%) (52 missing) | 60 (8.42%) | 276 (39.09%) | <0.001 |
| Bacteremia, n (%) (9 missing) | 206 (28.73%) | 456 (61.21%) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Age | ||||
| ≥65 years old | 2.74 (2.54, 3.34) | <0.001 | 2.26 (1.77, 2.89) | <0.001 |
| Sex | ||||
| Female | 1.06 (0.89, 1.27) | 0.514 | 0.83 (0.66, 1.05) | 0.123 |
| Wave of admission | ||||
| 2nd wave | 0.99 (0.56, 1.77) | 0.994 | 1.01 (0.45, 2.29) | 0.973 |
| 3rd wave | 0.90 (0.51, 1.58) | 0.723 | 1.01 (0.46, 2.23) | 0.979 |
| 4th wave | 1.92 (1.09, 3.36) | 0.023 | 1.64 (0.74, 3.60) | 0.220 |
| BMI | ||||
| 25–29 | 0.72 (0.58, 0.89) | 0.004 | 0.71 (0.54, 0.93) | 0.012 |
| ≥30+ | 0.84 (0.68, 1.02) | 0.084 | 0.76 (0.58, 0.98) | 0.037 |
| Total number of comorbidities | ||||
| ≥2 | 1.70 (1.41, 2.04) | <0.001 | 1.14 (0.91, 1.42) | 0.255 |
| Smoking status | ||||
| Former | 1.03 (0.82, 1.29) | 0.803 | 0.98 (0.76, 1.26) | 0.880 |
| Current | 1.36 (1.04, 1.77) | 0.026 | 1.36 (0.99, 1.87) | 0.058 |
| Days from symptom onset to ICU admission | 1.03 (1.02, 1.04) | <0.001 | 1.02 (1.01, 10.4) | <0.001 |
| APACHE II score upon ICU admission | 1.02 (1.01, 1.03) | <0.001 | 1.04 (1.03, 1.04) | <0.001 |
| Steroids | ||||
| Dexamethasone | 0.81 (0.66, 0.99) | 0.040 | 0.92 (0.70, 1.21) | 0.549 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| Variables | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Age | ||||
| ≥65 years old | 2.16 (1.83, 2.54) | <0.001 | 1.79 (1.47, 2.20) | <0.001 |
| Sex | ||||
| Female | 1.18 (1.01, 1.37) | 0.037 | 0.98 (0.80, 1.19) | 0.835 |
| Wave of admission | ||||
| 2nd wave | 1.61 (0.99, 2.63) | 0.056 | 1.57 (0.77, 3.22) | 0.216 |
| 3rd wave | 1.36 (0.85, 2.19) | 0.206 | 1.45 (0.72, 2.89) | 0.296 |
| 4th wave | 2.14 (1.32, 3.46) | 0.002 | 1.91 (0.96, 3.82) | 0.068 |
| BMI | ||||
| 25–29 | 0.94 (0.79, 1.23) | 0.508 | 0.89 (0.71, 1.12) | 0.322 |
| ≥30 | 1.05 (0.88, 1.24) | 0.582 | 0.96 (0.77, 1.20) | 0.740 |
| Total number of comorbidities | ||||
| ≥2 | 1.41 (1.21, 1.64) | <0.001 | 1.08 (0.89, 1.29) | 0.434 |
| Smoking status | ||||
| Former | 1.05 (0.88, 1.27) | 0.538 | 1.00 (0.81, 1.24) | 0.970 |
| Current | 1.20 (0.95, 1.52) | 0.120 | 1.17 (0.90, 1.54) | 0.243 |
| Days from symptom onset to ICU admission | 1.01 (1.01, 1.02) | <0.001 | 1.02 (1.01, 1.03) | <0.001 |
| APACHE II score upon ICU admission | 1.04 (1.03, 1.05) | <0.001 | 1.03 (1.02, 1.04) | <0.001 |
| Steroids | ||||
| Dexamethasone | 0.93 (0.79, 1.11) | 0.457 | 1.06 (0.85, 1.33) | 0.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fragkou, P.C.; Karagiannis, S.P.; Dimopoulou, D.; Kefala, S.; Fligou, F.; Gallos, P.; Jahaj, E.; Bellou, A.; Koukaki, E.; Magira, E.; et al. Intensive Care Unit Mortality Trends during the First Two Years of the COVID-19 Pandemic in Greece: A Multi-Center Retrospective Study. Viruses 2024, 16, 488. https://doi.org/10.3390/v16040488
Fragkou PC, Karagiannis SP, Dimopoulou D, Kefala S, Fligou F, Gallos P, Jahaj E, Bellou A, Koukaki E, Magira E, et al. Intensive Care Unit Mortality Trends during the First Two Years of the COVID-19 Pandemic in Greece: A Multi-Center Retrospective Study. Viruses. 2024; 16(4):488. https://doi.org/10.3390/v16040488
Chicago/Turabian StyleFragkou, Paraskevi C., Sotirios P. Karagiannis, Dimitra Dimopoulou, Sotiria Kefala, Fotini Fligou, Parisis Gallos, Edison Jahaj, Angeliki Bellou, Evangelia Koukaki, Eleni Magira, and et al. 2024. "Intensive Care Unit Mortality Trends during the First Two Years of the COVID-19 Pandemic in Greece: A Multi-Center Retrospective Study" Viruses 16, no. 4: 488. https://doi.org/10.3390/v16040488
APA StyleFragkou, P. C., Karagiannis, S. P., Dimopoulou, D., Kefala, S., Fligou, F., Gallos, P., Jahaj, E., Bellou, A., Koukaki, E., Magira, E., Orfanos, P., Papathanakos, G., Papathanasiou, A., Pediaditis, E., Pontikis, K., Rovina, N., Vaporidi, K., Xenikakis, M., Theodorakopoulou, M., & Kotanidou, A. (2024). Intensive Care Unit Mortality Trends during the First Two Years of the COVID-19 Pandemic in Greece: A Multi-Center Retrospective Study. Viruses, 16(4), 488. https://doi.org/10.3390/v16040488









