Insights from Avian Influenza: A Review of Its Multifaceted Nature and Future Pandemic Preparedness
Abstract
1. Introduction

2. Virus Dissemination
2.1. Global Spread of AIVs
2.2. Regional and Local Spread of AIVs
2.3. Antigenic Drift
2.4. Antigenic Shift
2.5. The Mechanism of Human Infections
3. Clinical Features and Severity of Disease
4. Characteristics of AIVs
5. Role of Immune System in the Pathology of AIVs
5.1. Innate Immunity
5.2. Adaptive Immunity
5.2.1. Humoral Immunity
5.2.2. Cell-Mediated Immunity
6. Control Measures
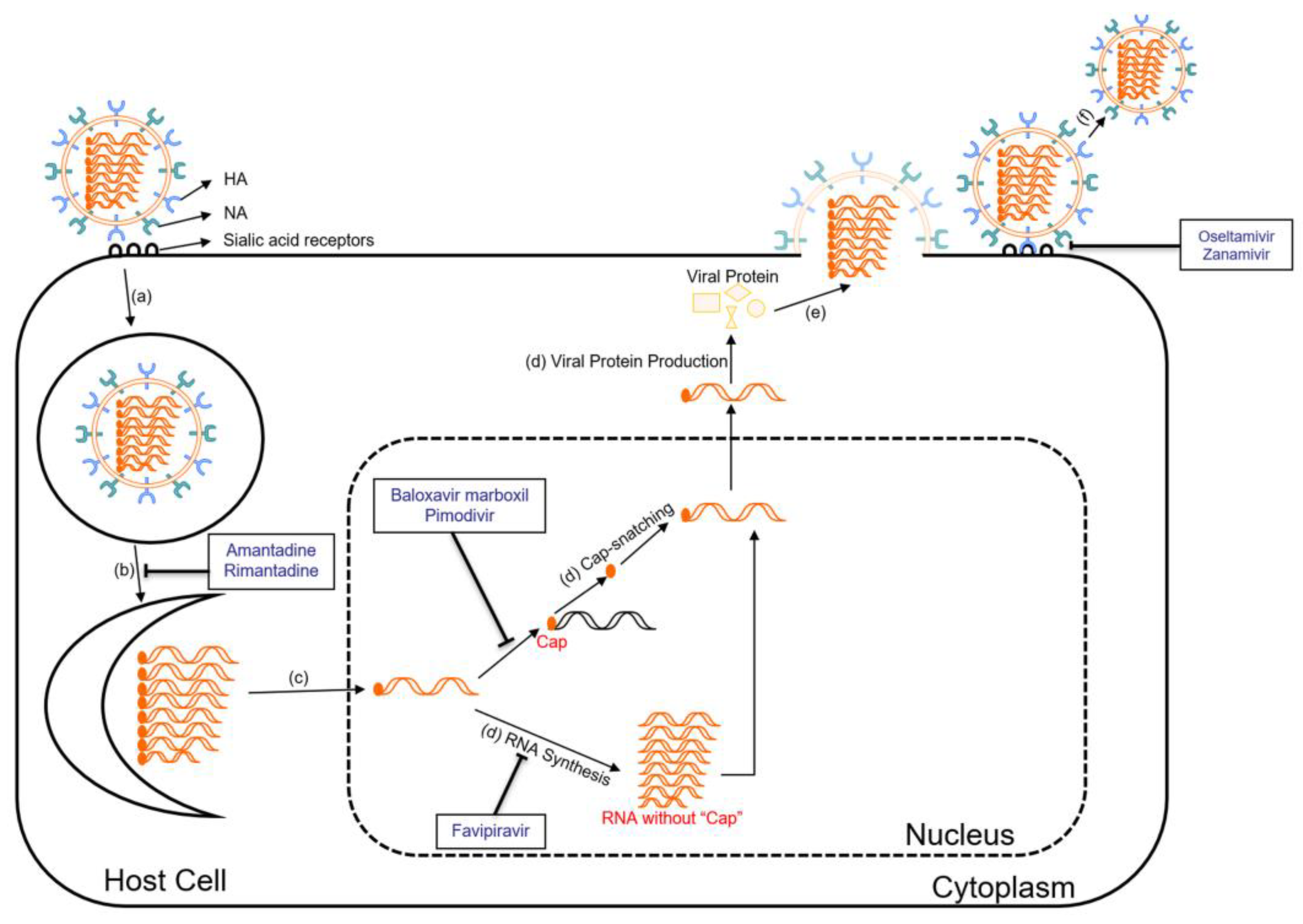
6.1. Antiviral Drugs
6.1.1. Neuraminidase Inhibitors
6.1.2. M2 Ion Channel Inhibitors
6.1.3. Polymerase Inhibitors
6.2. Vaccines
6.2.1. Live Attenuated Influenza Vaccines
6.2.2. Inactivated Influenza Vaccines
6.2.3. Subunit Influenza Vaccines
6.2.4. Epitope-Based Influenza Vaccines
6.2.5. mRNA Influenza Vaccines
7. Lessons Learned from the Avian Influenza and Strategies for Future Pandemic
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuszewski, K.; Brydak, L. The epidemiology and history of influenza. Biomed. Pharmacother. 2000, 54, 188–195. [Google Scholar] [CrossRef]
- Potter, C.W. A history of influenza. J. Appl. Microbiol. 2001, 91, 572–579. [Google Scholar] [CrossRef]
- Barnett, R. Influenza. Lancet 2019, 393, 396. [Google Scholar] [CrossRef]
- Layne, S.P.; Monto, A.S.; Taubenberger, J.K. Pandemic influenza: An inconvenient mutation. Science 2009, 323, 1560–1561. [Google Scholar] [CrossRef] [PubMed]
- Asha, K.; Kumar, B. Emerging influenza D virus threat: What we know so far! J. Clin. Med. 2019, 8, 192. [Google Scholar] [CrossRef] [PubMed]
- Palese, P.; Young, J.F. Variation of influenza A, B, and C viruses. Science 1982, 215, 1468–1474. [Google Scholar] [CrossRef]
- Hulseberg, C.E.; Fénéant, L.; Szyman´ska, K.M.; Szyman´ska-De Wijs, S.; Kessler, N.P.; Nelson, E.A.; Shoemaker, C.J.; Schmaljohn, C.S.; Polyak, S.J.; White, J.M.; et al. Influenza C and D viruses package eight organized ribonucleoprotein complexes. J. Virol. 2018, 92, e02185-18. [Google Scholar] [CrossRef]
- Cheung, T.K.; Poon, L.L. Biology of influenza a virus. Ann. N. Y. Acad. Sci. 2007, 1102, 90. [Google Scholar] [CrossRef] [PubMed]
- Bavagnoli, L.; Maga, G. The 2009 influenza pandemic: Promising lessons for antiviral therapy for future outbreaks. Curr. Med. Chem. 2011, 18, 5466–5475. [Google Scholar] [CrossRef]
- Suarez, D.L.; Schultz-Cherry, S. Immunology of avian influenza virus: A review. Dev. Comp. Immunol. 2000, 24, 269–283. [Google Scholar] [CrossRef]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann. N. Y. Acad. Sci. 2014, 1323, 115–139. [Google Scholar] [CrossRef]
- Lupiani, B.; Reddy, S.M. The history of avian influenza. Comp. Immunol. Microbiol. Infect. Dis. 2009, 32, 311–323. [Google Scholar] [CrossRef] [PubMed]
- BBC News. Bird flu case confirmed at farm. BBC NEWS, 24 May 2007.
- Szablewski, C.M.; Iwamoto, C.; Olsen, S.J.; Greene, C.M.; Duca, L.M.; Davis, C.T.; Coggeshall, K.C.; Davis, W.W.; Emukule, G.O.; Gould, P.L.; et al. Reported global avian influenza detections among humans and animals during 2013–2022: Comprehensive review and analysis of available surveillance data. JMIR Public Health Surveill. 2023, 9, e46383. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol. Rev. 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Luo, M. Influenza virus entry. Viral Mol. Mach. 2012, 2012, 201–221. [Google Scholar]
- Skowronski, D.; Tweed, S.; Petric, M.; Booth, T.; Li, Y.; Tam, T. Human illness from avian influenza H7N3, British Columbia. Emerg. Infect. Dis. 2004, 10, 219. [Google Scholar]
- Barrera-Badillo, G.; Ramirez-Gonzalez, E.; Aparicio-Antonio, R.; Nuñez-Garcia, T.; Arellano-Suarez, D. Highly Pathogenic Avian Influenza A (H7N3) Virus Infection in Two Poultry Workers—Jalisco, Mexico, July 2012. MMWR 2012, 61, 36. [Google Scholar]
- Stegeman, A.; Bouma, A.; Elbers, A.R.W.; de Jong, M.C.M.; Nodelijk, G.; de Klerk, F.; Koch, G.; van Boven, M. Avian influenza A virus (H7N7) epidemic in The Netherlands in 2003: Course of the epidemic and effectiveness of control measures. J. Infect. Dis. 2004, 190, 2088–2095. [Google Scholar] [CrossRef]
- Technical Report: Highly Pathogenic Avian Influenza A(H5N1) Viruses. 2023. Available online: https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/h5n1-technical-report.htm (accessed on 12 April 2023).
- Reported Human Infections with Avian Influenza A Viruses. 2023. Available online: https://www.cdc.gov/flu/avianflu/reported-human-infections.htm (accessed on 3 April 2023).
- Kullman, G.; Delaney, L.J.; Decker, J.; MacMahon, K. Protecting Poultry Workers from Avian Influenza (Bird Flu); DHHS (NIOSH): Cincinnati, OH, USA, 2008. [Google Scholar]
- Belser, J.A.; Bridges, C.B.; Katz, J.M.; Tumpey, T.M. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg. Infect. Dis. 2009, 15, 859. [Google Scholar] [CrossRef]
- El-Shesheny, R.; Franks, J.; Marathe, B.M.; Hasan, M.K.; Feeroz, M.M.; Krauss, S.; Vogel, P.; McKenzie, P.; Webby, R.J.; Webster, R.G. Genetic characterization and pathogenic potential of H10 avian influenza viruses isolated from live poultry markets in Bangladesh. Sci. Rep. 2018, 8, 10693. [Google Scholar] [CrossRef]
- Blagodatski, A.; Trutneva, K.; Glazova, O.; Mityaeva, O.; Shevkova, L.; Kegeles, E.; Onyanov, N.; Fede, K.; Maznina, A.; Khavina, E.; et al. Avian influenza in wild birds and poultry: Dissemination pathways, monitoring methods, and virus ecology. Pathogens 2021, 10, 630. [Google Scholar] [CrossRef]
- Prosser, D.J.; Chen, J.; Ahlstrom, C.A.; Reeves, A.B.; Poulson, R.L.; Sullivan, J.D.; McAuley, D.; Callahan, C.R.; McGowan, P.C.; Bahl, J.; et al. Maintenance and dissemination of avian-origin influenza A virus within the northern Atlantic Flyway of North America. PLoS Pathog. 2022, 18, e1010605. [Google Scholar] [CrossRef]
- Herfst, S.; Imai, M.; Kawaoka, Y.; Fouchier, R. Avian influenza virus transmission to mammals. Influenza Pathog. Control. Vol. I 2014, 2014, 137–155. [Google Scholar]
- Abdelwhab, E.; Hafez, H.M. An overview of the epidemic of highly pathogenic H5N1 avian influenza virus in Egypt: Epidemiology and control challenges. Epidemiol. Infect. 2011, 139, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Carrat, F.; Flahault, A. Influenza vaccine: The challenge of antigenic drift. Vaccine 2007, 25, 6852–6862. [Google Scholar] [CrossRef]
- Smith, D.J.; Lapedes, A.S.; De Jong, J.C.; Bestebroer, T.M.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E.; Fouchier, R.A.M. Mapping the antigenic and genetic evolution of influenza virus. Science 2004, 305, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.C. Epidemiology and pathogenesis of influenza. J. Antimicrob. Chemother. 1999, 44 (Suppl. S2), 3–9. [Google Scholar] [CrossRef]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.J.D.; Bahl, J.; Vijaykrishna, D.; Zhang, J.; Poon, L.L.M.; Chen, H.; Webster, R.G.; Peiris, J.S.M.; Guan, Y. Dating the emergence of pandemic influenza viruses. Proc. Natl. Acad. Sci. USA 2009, 106, 11709–11712. [Google Scholar] [CrossRef]
- Taubenberger, J.K.; Morens, D.M. The 1918 influenza pandemic and its legacy. Cold Spring Harb. Perspect. Med. 2019, 2019, a038695. [Google Scholar] [CrossRef] [PubMed]
- Philippon, D.A.; Wu, P.; Cowling, B.J.; Lau, E.H. Avian influenza human infections at the human-animal interface. J. Infect. Dis. 2020, 222, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Avian Influenza A Viruses: Clinical Features. 2022. Available online: https://www.cdc.gov/flu/avianflu/avian-in-humans.htm (accessed on 17 June 2023).
- Yuen, K.; Chan, P.; Peiris, M.; Tsang, D.; Que, T.; Shortridge, K.; Cheung, P.; To, W.; Ho, E.; Sung, R.; et al. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 1998, 351, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Mei, Z.; Lu, S.; Wu, X.; Shao, L.; Hui, Y.; Wang, J.; Li, T.; Zhang, H.; Wang, X.; Yang, F.; et al. Avian influenza A (H7N9) virus infections, Shanghai, China. Emerg. Infect. Dis. 2013, 19, 1179. [Google Scholar] [CrossRef]
- Wang, Z.; Loh, L.; Kedzierski, L.; Kedzierska, K. Avian influenza viruses, inflammation, and CD8+ T cell immunity. Front. Immunol. 2016, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Kuiken, T.; Taubenberger, J.K. Pathology of human influenza revisited. Vaccine 2008, 26, D59–D66. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.E.; Koster, F.; Cawthon, A. Neurologic aspects of influenza viruses. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 619–645. [Google Scholar]
- Chen, E.; Wang, F.; Lv, H.; Zhang, Y.; Ding, H.; Liu, S.; Cai, J.; Xie, L.; Xu, X.; Chai, C.; et al. The first avian influenza A (H7N9) viral infection in humans in Zhejiang Province, China: A death report. Front. Med. 2013, 7, 333–344. [Google Scholar] [CrossRef]
- Thompson, A.J.; Paulson, J.C. Adaptation of influenza viruses to human airway receptors. J. Biol. Chem. 2021, 296, 100017. [Google Scholar] [CrossRef]
- Kuchipudi, S.V.; Nelli, R.; White, G.A.; Bain, M.; Chang, K.C.; Dunham, S. Differences in influenza virus receptors in chickens and ducks: Implications for interspecies transmission. J. Mol. Genet. Med. Int. J. Biomed. Res. 2009, 3, 143. [Google Scholar] [CrossRef]
- Bortz, E.; Westera, L.; Maamary, J.; Steel, J.; Albrecht, R.A.; Manicassamy, B.; Chase, G.; Martínez-Sobrido, L.; Schwemmle, M.; García-Sastre, A. Host-and strain-specific regulation of influenza virus polymerase activity by interacting cellular proteins. MBio 2011, 2, 10-128. [Google Scholar]
- Koutsakos, M.; Kedzierska, K.; Subbarao, K. Immune responses to avian influenza viruses. J. Immunol. 2019, 202, 382–391. [Google Scholar] [CrossRef]
- Van de Sandt, C.E.; Kreijtz, J.H.; Rimmelzwaan, G.F. Evasion of influenza A viruses from innate and adaptive immune responses. Viruses 2012, 4, 1438–1476. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.H.; Baumgarth, N. The multifaceted B cell response to influenza virus. J. Immunol. 2019, 202, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.; Weaver, C. Janeway’s Immunobiology; Garland Science: New York, NY, USA, 2016. [Google Scholar]
- van Riet, E.; Ainai, A.; Suzuki, T.; Hasegawa, H. Mucosal IgA responses in influenza virus infections; thoughts for vaccine design. Vaccine 2012, 30, 5893–5900. [Google Scholar] [CrossRef] [PubMed]
- Staneková, Z.; Varečková, E. Conserved epitopes of influenza A virus inducing protective immunity and their prospects for universal vaccine development. Virol. J. 2010, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- What are Flu Antiviral Drugs. 2024. Available online: https://www.cdc.gov/flu/treatment/whatyoushould.htm (accessed on 25 February 2024).
- Gubareva, L.V.; Kaiser, L.; Hayden, F.G. Influenza virus neuraminidase inhibitors. Lancet 2000, 355, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, N.; Poongavanam, V.; Pratheepa, V. Viral M2 ion channel protein: A promising target for anti-influenza drug discovery. Mini Rev. Med. Chem. 2014, 14, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Deyde, V.M.; Xu, X.; Bright, R.A.; Shaw, M.; Smith, C.B.; Zhang, Y.; Shu, Y.; Gubareva, L.V.; Cox, N.J.; Klimov, A.I. Surveillance of resistance to adamantanes among influenza A (H3N2) and A (H1N1) viruses isolated worldwide. J. Infect. Dis. 2007, 196, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Hayden, F.G.; Shindo, N. Influenza virus polymerase inhibitors in clinical development. Curr. Opin. Infect. Dis. 2019, 32, 176. [Google Scholar] [CrossRef]
- Noshi, T.; Kitano, M.; Taniguchi, K.; Yamamoto, A.; Omoto, S.; Baba, K.; Hashimoto, T.; Ishida, K.; Kushima, Y.; Hattori, K.; et al. In vitro characterization of baloxavir acid, a first-in-class cap-dependent endonuclease inhibitor of the influenza virus polymerase PA subunit. Antivir. Res. 2018, 160, 109–117. [Google Scholar] [CrossRef]
- Patel, M.C.; Chesnokov, A.; Jones, J.; Mishin, V.P.; De La Cruz, J.A.; Nguyen, H.T.; Zanders, N.; Wentworth, D.E.; Davis, T.C.; Gubareva, L.V. Susceptibility of widely diverse influenza a viruses to PB2 polymerase inhibitor pimodivir. Antivir. Res. 2021, 188, 105035. [Google Scholar] [CrossRef]
- Shiraki, K.; Daikoku, T. Favipiravir, an anti-influenza drug against life-threatening RNA virus infections. Pharmacol. Ther. 2020, 209, 107512. [Google Scholar] [CrossRef]
- Beigel, J.; Bray, M. Current and future antiviral therapy of severe seasonal and avian influenza. Antivir. Res. 2008, 78, 91–102. [Google Scholar] [CrossRef]
- Nypaver, C.; Dehlinger, C.; Carter, C. Influenza and influenza vaccine: A review. J. Midwifery Women’s Health 2021, 66, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Nayak, J.L.; Caserta, M.T. Cell-Culture–Based Influenza Vaccines: Don’t Put All of Your Influenza Vaccines in the Egg Basket. Pediatrics 2022, 150, e2022058143. [Google Scholar] [CrossRef] [PubMed]
- Bonnafous, P.; Nicolaï, M.-C.; Taveau, J.-C.; Chevalier, M.; Barrière, F.; Medina, J.; Le Bihan, O.; Adam, O.; Ronzon, F.; Lambert, O. Treatment of influenza virus with beta-propiolactone alters viral membrane fusion. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.M.; Patriarca, P.A.; Treanor, J. FluBlok, a recombinant hemagglutinin influenza vaccine. Influenza Other Respir. Viruses 2008, 2, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Holtz, K.M.; Anderson, K.; Chubet, R.; Mahmoud, W.; Cox, M.M. Expression and purification of an influenza hemagglutinin—One step closer to a recombinant protein-based influenza vaccine. Vaccine 2006, 24, 2176–2185. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhu, S.; Govinden, R.; Chenia, H.Y. Multiple Vaccines and Strategies for Pandemic Preparedness of Avian Influenza Virus. Viruses 2023, 15, 1694. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Brokstad, K.A.; Cox, R.J. Influenza vaccination strategies: Comparing inactivated and live attenuated influenza vaccines. Vaccines 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Goncalves, P.; Young, J. Influenza Vaccine. J. Asthma Allergy Educ. 2011, 2, 44–46. [Google Scholar] [CrossRef]
- Gasparini, R.; Amicizia, D.; Lai, P.; Panatto, D. Live attenuated influenza vaccine–a review. J. Prev. Med. Hyg. 2011, 52, 95–101. [Google Scholar]
- Gottlieb, T.; Ben-Yedidia, T. Epitope-based approaches to a universal influenza vaccine. J. Autoimmun. 2014, 54, 15–20. [Google Scholar] [CrossRef]
- Mintaev, R.R.; Glazkova, D.V.; Orlova, O.V.; Bogoslovskaya, E.V.; Shipulin, G.A. Development of a Universal Epitope-Based Influenza Vaccine and Evaluation of Its Effectiveness in Mice. Vaccines 2022, 10, 534. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Rappuoli, R. mRNA, the beginning of a new influenza vaccine game. Proc. Natl. Acad. Sci. USA 2022, 119, e2217533119. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Peeling, R.W.; Sia, S.K. Lessons from COVID-19 for improving diagnostic access in future pandemics. Lab A Chip 2023, 23, 1376–1388. [Google Scholar] [CrossRef]
- Li, L.; Taeihagh, A.; Tan, S.Y. A scoping review of the impacts of COVID-19 physical distancing measures on vulnerable population groups. Nat. Commun. 2023, 14, 599. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Bausch-Jurken, M. The burden of COVID-19 in the immunocompromised patient: Implications for vaccination and needs for the future. J. Infect. Dis. 2023, 228 (Suppl. S1), S4–S12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Kam, Y.-W. Insights from Avian Influenza: A Review of Its Multifaceted Nature and Future Pandemic Preparedness. Viruses 2024, 16, 458. https://doi.org/10.3390/v16030458
He J, Kam Y-W. Insights from Avian Influenza: A Review of Its Multifaceted Nature and Future Pandemic Preparedness. Viruses. 2024; 16(3):458. https://doi.org/10.3390/v16030458
Chicago/Turabian StyleHe, Jianning, and Yiu-Wing Kam. 2024. "Insights from Avian Influenza: A Review of Its Multifaceted Nature and Future Pandemic Preparedness" Viruses 16, no. 3: 458. https://doi.org/10.3390/v16030458
APA StyleHe, J., & Kam, Y.-W. (2024). Insights from Avian Influenza: A Review of Its Multifaceted Nature and Future Pandemic Preparedness. Viruses, 16(3), 458. https://doi.org/10.3390/v16030458






