Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Setting
2.2. Study Population
2.3. Assessment of HCV Infection
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. HCV-Positivity Assessment and HCV Cascade of Care
3.3. Outcome and HCV Reinfection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hill, A.M.; Nath, S.; Simmons, B. The road to elimination of hepatitis C: Analysis of cures versus new infections in 91 countries. J. Virus. Erad. 2017, 3, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Trickey, A.; Fraser, H.; Lim, A.G.; Peacock, A.; College, S.; Walker, J.G.; Leung, J.; Grebely, J.; Larney, S.; Martin, N.K.; et al. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: A modelling study. Lancet Gastroenterol. Hepatol. 2019, 4, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Bourliere, M.; Pietri, O. Hepatitis C virus therapy: No one will be left behind. Int. J. Antimicrob. Agents 2019, 53, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Safreed-Harmon, K.; Blach, S.; Aleman, S.; Bollerup, S.; Cooke, G.; Dalgard, O.; Dillon, J.F.; Dore, G.J.; Duberg, A.-S.; Grebely, J.; et al. The Consensus Hepatitis C Cascade of Care: Standardized reporting to monitor progress toward elimination. Clin. Infect. Dis. 2019, 69, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.D.; Mirzazadeh, A.; Evans, J.L.; Briceno, A.; Coffin, P.; Hahn, J.A.; Page, K.A. Treatment cascade for hepatitis C virus in young adult people who inject drugs in San Francisco: Low number treated. Drug Alcohol Depend. 2019, 198, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Socías, M.E.; Ti, L.; Wood, E.; Nosova, E.; Hull, M.; Hayashi, K.; Debeck, K.; Milloy, M.J. Disparities in uptake of direct-acting antiviral therapy for hepatitis C among people who inject drugs in a Canadian setting. Liver Int. 2019, 39, 1400–1407. [Google Scholar] [CrossRef] [PubMed]
- Tsui, J.I.; Miller, C.M.; Scott, J.D.; Corcorran, M.A.; Dombrowski, J.C.; Glick, S.N. Hepatitis C continuum of care and utilization of healthcare and harm reduction services among persons who inject drugs in Seattle. Drug Alcohol Depend. 2019, 195, 114–120. [Google Scholar] [CrossRef]
- EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 2020, 73, 1170–1218. [CrossRef]
- Socías, M.E.; Karamouzian, M.; Parent, S.; Barletta, J.; Bird, K.; Ti, L. Integrated models of care for people who inject drugs and live with hepatitis C virus: A systematic review. Int. J. Drug Policy 2019, 72, 146–159. [Google Scholar] [CrossRef]
- Platt, L.; Minozzi, S.; Reed, J.; Vickerman, P.; Hagan, H.; French, C.; Jordan, A.; Degenhardt, L.; Hope, V.; Hutchinson, S.; et al. Needle and syringe programmes and opioid substitution therapy for preventing HCV transmission among people who inject drugs: Findings from a Cochrane Review and meta-analysis. Addiction 2018, 113, 545–563. [Google Scholar] [CrossRef]
- Winetsky, D.; Burack, D.; Antoniou, P.; Garcia, B.; Gordon, P.; Scherer, M. Psychosocial Factors and the Care Cascade for Hepatitis C Treatment Colocated at a Syringe Service Program. J. Infect. Dis. 2020, 222 (Suppl. S5), S392–S400. [Google Scholar] [CrossRef]
- Lens, S.; Miralpeix, A.; Gálvez, M.; Martró, E.; González, N.; Rodríguez-Tajes, S.; Mariño, Z.; Saludes, V.; Reyes-Urueña, J.; Majó, X.; et al. HCV microelimination in harm reduction centres has benefits beyond HCV cure but is hampered by high reinfection rates. JHEP Rep. 2022, 4, 100580. [Google Scholar] [CrossRef]
- Betty Ford Institute Consensus Panel. What is recovery? A working definition from the Betty Ford Institute. J. Subst. Abuse Treat. 2007, 33, 221–228. [Google Scholar] [CrossRef]
- Malivert, M.; Fatséas, M.; Denis, C.; Langlois, E.; Auriacombe, M. Effectiveness of therapeutic communities a systematic review. Eur. Addict. Res. 2012, 18, 1–11. [Google Scholar] [CrossRef]
- Hubbard, R.L.; Craddock, S.G.; Anderson, J. Overview of 5-year follow up outcomes in the drug abuse treatment outcome studies (DATOS). J. Subst. Abus. Treat. 2003, 25, 125–134. [Google Scholar] [CrossRef]
- De Leon, G. The Therapeutic Community: Theory, Model and Method; Springer Publishing Company: New York, NY, USA, 2000. [Google Scholar]
- Broekaert, E.; Vandevelde, S.; Soyez, V.; Yates, R.; Slater, A. The third generation of therapeutic communities: The early development of the TC for addictions in Europe. Eur. Addict. Res. 2006, 12, 1–11. [Google Scholar] [CrossRef]
- De Leon, G. Is the therapeutic community an evidence-based treatment? What the evidence says. Int. J. Ther. Commun. 2010, 31, 104–128. [Google Scholar]
- Smith, L.A.; Gates, S.; Foxcroft, D. Therapeutic communities for substance related disorder. Cochrane Database Syst. Rev. 2006, 1, CD005338. [Google Scholar] [CrossRef] [PubMed]
- Fiore, V.; De Matteis, G.; Ranieri, R.; Saderi, L.; Pontali, E.; Muredda, A.; Ialungo, A.M.; Caruso, R.; Madeddu, G.; Sotgiu, G.; et al. HCV testing and treatment initiation in an Italian prison setting: A step-by-step model to micro-eliminate hepatitis C. Int. J. Drug Policy 2021, 90, 103055. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Zarębska-Michaluk, D.; Ciupkeviciene, E.; Drazilova, S.; Frankova, S.; Grgurevic, I.; Hunyady, B.; Jarcuska, P.; Kupčinskas, L.; Makara, M.; et al. HCV Elimination in Central Europe with Particular Emphasis on Microelimination in Prisons. Viruses 2022, 14, 482. [Google Scholar] [CrossRef] [PubMed]
- Lafferty, L.; Sheehan, Y.; Cochrane, A.; Grebely, J.; Lloyd, A.R.; Treloar, C. Reducing barriers to the hepatitis C care cascade in prison via point-of-care RNA testing: A qualitative exploration of men in prison using an integrated framework. Addiction 2023, 118, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- San Patrignano Therapeutic Community. Available online: www.sanpatrignano.org (accessed on 30 November 2023).
- Devlin, A.M.; Wight, D. Mechanisms and context in the San Patrignano drug recovery community, Italy: A qualitative study to inform transfer to Scotland. Drugs Educ. Prev. Policy 2021, 28, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Boschini, A.; Smacchia, C.; Di Fine, M.; Schiesari, A.; Ballarini, P.; Arlotti, M.; Gabrielli, C.; Castellani, G.; Genova, M.; Pantani, P.; et al. Community-acquired pneumonia in a cohort of former injection drug users with and without human immunodeficiency virus infection: Incidence, etiologies, and clinical aspects. Clin. Infect. Dis. 1996, 23, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Vergori, A.; Boschini, A.; Notari, S.; Lorenzini, P.; Castilletti, C.; Colavita, F.; Matusali, G.; Tartaglia, E.; Gagliardini, R.; Boschi, A.; et al. SARS-CoV-2 Specific Immune Response and Inflammatory Profile in Advanced HIV-Infected Persons during a COVID-19 Outbreak. Viruses 2022, 14, 1575. [Google Scholar] [CrossRef]
- Sala, I.; Jarach, C.M.; Bagnardi, V.; Cattaruzza, M.S.; Morri, M.; Ottogalli, P.; Zagà, V.; Gallus, S.; Boschini, A. SARS-CoV-2 Infection in San Patrignano, the Largest European Drug Rehabilitation Community. Int. J. Environ. Res. Public Health 2023, 20, 2136. [Google Scholar] [CrossRef] [PubMed]
- Bedossa, P.; Poynard, T. The METAVIR Cooperative Study Group. An algorithm for the grading of activity in chronic hepatitis C. Hepatology 1996, 24, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yehia, B.R.; Herati, R.S.; Fleishman, J.A.; Gallant, J.E.; Agwu, A.L.; Berry, S.A.; Korthuis, P.T.; Moore, R.D.; Metlay, J.P.; Gebo, K.A.; et al. Hepatitis C virus testing in adults living with HIV: A need for improved screening efforts. PLoS ONE 2014, 9, e102766. [Google Scholar] [CrossRef]
- Breslow, N.E.; Day, N.E. Statistical methods in cancer research. In The Design and Analysis of Cohort Studies; IARC Scientific Publications No. 82; IARC Press: Lyon, France, 1987; Volume 2. [Google Scholar]
- Schwarz, T.; Horváth, I.; Fenz, L.; Schmutterer, I.; Rosian-Schikuta, I.; Mårdh, O. Interventions to increase linkage to care and adherence to treatment for hepatitis C among people who inject drugs: A systematic review and practical considerations from an expert panel consultation. Int. J. Drug Policy 2022, 102, 103588. [Google Scholar] [CrossRef]
- Howell, J.; Traeger, M.W.; Williams, B.; Layton, C.; Doyle, J.S.; Latham, N.; Draper, B.; Bramwell, F.; Membrey, D.; McPherson, M.; et al. The impact of point-of-care hepatitis C testing in needle and syringe exchange programs on linkage to care and treatment uptake among people who inject drugs: An Australian pilot study. J. Viral Hepat. 2022, 29, 375–384. [Google Scholar] [CrossRef]
- Annual Report to Parliament on the Phenomenon of Drug Addiction in Italy Year 2022 (2021 Data). Department of Drug Policies. Available online: https://www.iss.it/en/-/relazione-annuale-al-parlamento-sul-fenomeno-delle-tossicodipendenze-in-italia-anno-2022-dati-2021- (accessed on 30 November 2023).
- Graf, C.; Mücke, M.M.; Dultz, G.; Peiffer, K.H.; Kubesch, A.; Ingiliz, P.; Zeuzem, S.; Herrmann, E.; Vermehren, J. Efficacy of direct-acting antivirals for chronic hepatitis C virus infection in people who inject drugs or receive opioid substitution therapy: A systematic review and meta-analysis. Clin. Infect. Dis. 2020, 70, 2355–2365. [Google Scholar] [CrossRef]
- Aspinall, E.J.; Corson, S.; Doyle, J.S.; Grebely, J.; Hutchinson, S.J.; Dore, G.J.; Goldberg, D.J.; Hellard, M.E. Treatment of hepatitis C virus infection among people who are actively injecting drugs: A systematic review and meta analysis. Clin. Infect. Dis. 2013, 57 (Suppl. S2), S80–S89. [Google Scholar] [CrossRef]
- Falade-Nwulia, O.; Irvin, R.; Merkow, A.; Sulkowski, M.; Niculescu, A.; Olsen, Y.; Stoller, K.; Thomas, D.L.; Latkin, C.; Mehta, S.H. Barriers and facilitators of hepatitis C treatment uptake among people who inject drugs enrolled in opioid treatment programs in Baltimore. J. Subst. Abuse Treat. 2019, 100, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Frankova, S.; Jandova, Z.; Jinochova, G.; Kreidlova, M.; Merta, D.; Sperl, J. Therapy of chronic hepatitis C in people who inject drugs: Focus on adherence. Harm Reduct. J. 2021, 18, 69. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.S.; Pericot-Valverde, I.; Arnsten, J.; Lum, P.J.; Taylor, L.E.; Mehta, S.H.; Tsui, J.I.; Feinberg, J.; Kim, A.Y.; Norton, B.L.; et al. Self-reported and measured adherence to hepatitis C direct-acting antiviral therapy and sustained virologic response among people who inject drugs: The HERO study. Int. J. Drug Policy 2024, 123, 104288. [Google Scholar] [CrossRef] [PubMed]

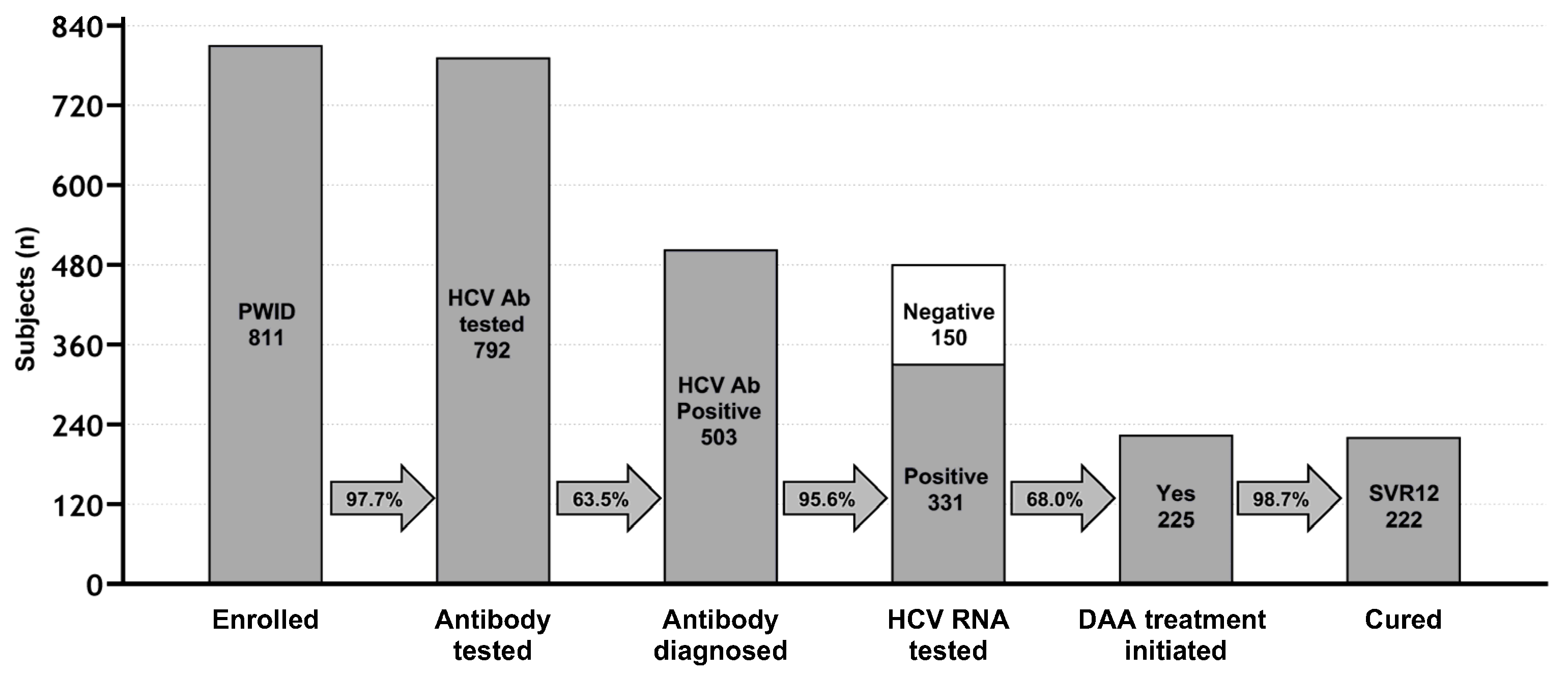
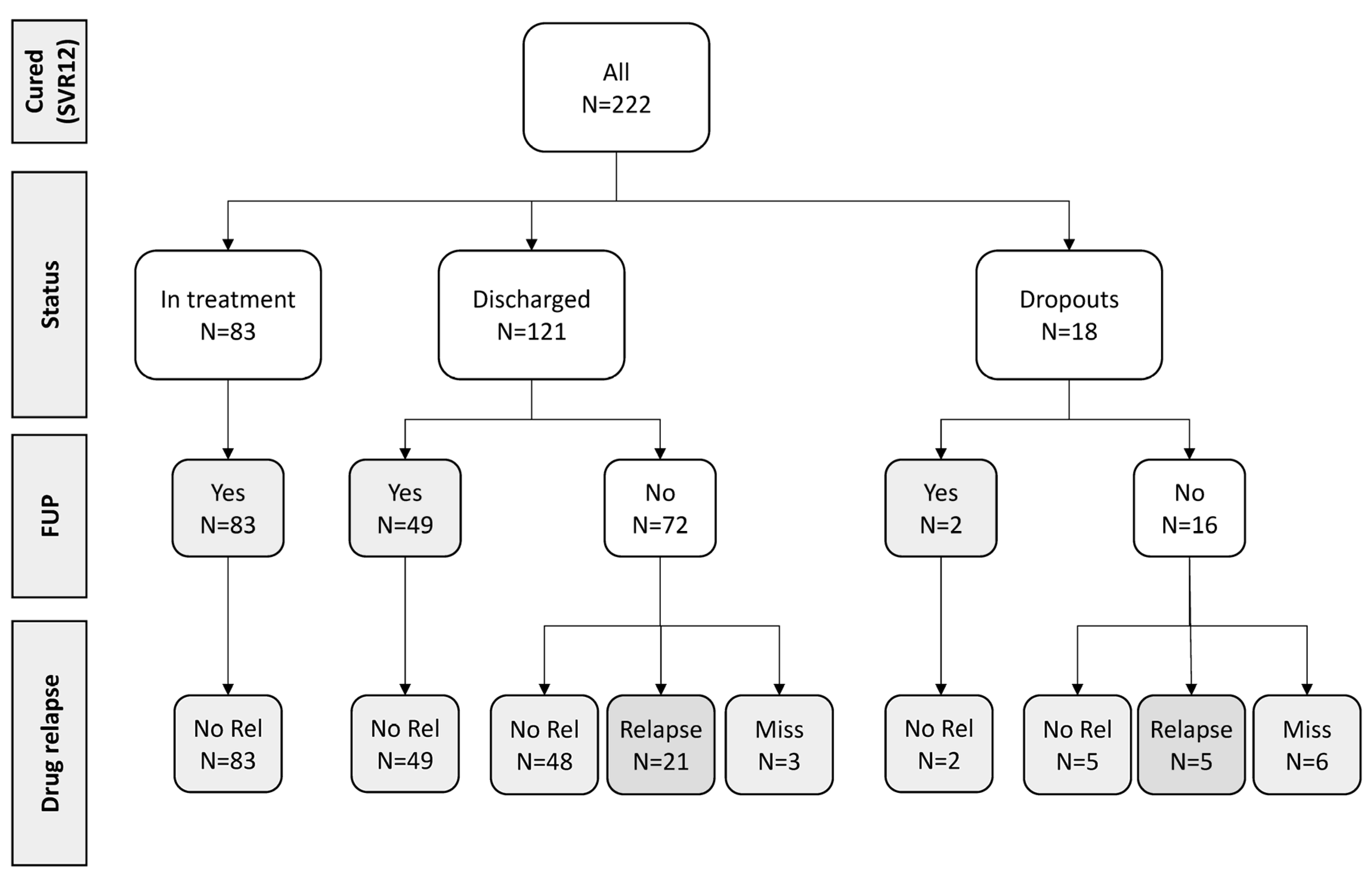
| Characteristics | N (%) | |
|---|---|---|
| All | 811 (100) | |
| Gender | ||
| Male | 625 (77.1) | |
| Female | 186 (22.9) | |
| Age at start of drug injection | Median (IQR) | 20 (18–24) |
| Age at last SPTC entry | Median (IQR) | 32 (26–39) |
| <30 | 321 (39.6) | |
| 30–39 | 292 (36.0) | |
| 40+ | 198 (24.4) | |
| Year of last entry | ||
| <2018 | 409 (50.4) | |
| 2018–2019 | 286 (35.3) | |
| 2020+ | 116 (14.3) | |
| Nationality | ||
| Italians | 751 (92.6) | |
| Not Italians | 60 (7.4) | |
| Use of injectable substance | ||
| Heroin + Cocaine | 740 (91.2) | |
| Only Heroin | 49 (6.0) | |
| Only Cocaine | 20 (2.5) | |
| Other | 2 (0.2) | |
| Incarceration | ||
| No | 595 (73.4) | |
| Yes | 216 (26.6) | |
| HIV | ||
| Neg | 756 (93.2) | |
| Pos | 55 (6.8) | |
| Previous HCV testing | ||
| No | 169 (20.8) | |
| Yes, negative | 265 (32.7) | |
| Yes, positive | 377 (46.5) |
| Characteristics | Total N (%) | Neg N (%) | Pos N (%) | p | |
|---|---|---|---|---|---|
| All | 792 | 289 (36.5) | 503 (63.5) | ||
| Gender | 0.276 | ||||
| Male | 609 | 216 (35.5) | 393 (64.5) | ||
| Female | 183 | 73 (39.9) | 110 (60.1) | ||
| Age at start of drug injection | Median (IQR) | 32 (26–39) | 29 (23–36) | 35 (28–42) | <0.001 2 |
| Age at SPTC entry | <0.001 1 | ||||
| <30 | 310 | 154 (49.7) | 156 (50.3) | ||
| 30–39 | 286 | 99 (34.6) | 187 (65.4) | ||
| 40+ | 196 | 36 (18.4) | 160 (81.6) | ||
| Years of addiction | <0.001 1 | ||||
| 0–4 | 279 | 151 (54.1) | 128 (45.9) | ||
| 5–9 | 167 | 69 (41.3) | 98 (58.7) | ||
| 10+ | 346 | 69 (19.9) | 277 (80.1) | ||
| Year of last entry | <0.001 1 | ||||
| <2018 | 408 | 120 (29.4) | 288 (70.6) | ||
| 2018–2019 | 275 | 110 (40.0) | 165 (60.0) | ||
| 2020+ | 109 | 59 (54.1) | 50 (45.9) | ||
| Nationality | 0.732 | ||||
| Italians | 735 | 267 (36.3) | 468 (63.7) | ||
| Not Italians | 57 | 22 (38.6) | 35 (61.4) | ||
| Incarceration | <0.001 | ||||
| No | 580 | 240 (41.4) | 340 (58.6) | ||
| Yes | 212 | 49 (23.1) | 163 (76.9) | ||
| HIV | <0.001 | ||||
| Neg | 737 | 284 (38.5) | 453 (61.5) | ||
| Pos | 55 | 5 (9.1) | 50 (90.9) | ||
| Previous HCV testing | <0.001 | ||||
| No | 156 | 65 (41.7) | 91 (58.3) | ||
| Yes, negative | 259 | 224 (86.5) | 35 (13.5) | ||
| Yes, positive | 377 | - | 377 (100) |
| Characteristics | Total N | DAA Treatment N (%) | IR | IRR | 95% CI | p | aIRR | 95% CI | p |
|---|---|---|---|---|---|---|---|---|---|
| All | 331 | 225 (68.0) | 2.04 | ||||||
| Gender | 0.766 | 0.448 | |||||||
| Male | 260 | 168 (64.6) | 2.02 | 1 | 1 | ||||
| Female | 71 | 57 (80.3) | 2.12 | 1.05 | 0.78–1.41 | 1.13 | 0.83–1.54 | ||
| Age (by 10-year increase) | 1.08 | 0.95–1.24 | 0.241 | 0.93 | 0.79–1.09 | 0.360 | |||
| Year of last entry | <0.001 | <0.001 | |||||||
| <2018 | 202 | 121 (59.9) | 1.57 | 1 | 1 | ||||
| 2018–2019 | 96 | 75 (78.1) | 2.59 | 1.65 | 1.23–2.20 | 1.48 | 1.09–1.99 | ||
| 2020+ | 33 | 29 (87.9) | 7.06 | 4.49 | 2.99–6.73 | 4.25 | 2.81–6.44 | ||
| Nationality | 0.034 | 0.080 | |||||||
| Italians | 312 | 212 (67.9) | 2.14 | 1 | 1 | ||||
| Non-Italians | 19 | 13 (68.4) | 1.17 | 0.55 | 0.31–0.96 | 0.60 | 0.34–1.06 | ||
| METAVIR score | <0.001 | <0.001 | |||||||
| F0–F1 | 208 | 133 (63.9) | 1.69 | 1 | 1 | ||||
| F2 | 74 | 61 (82.4) | 2.66 | 1.57 | 1.16–2.13 | 1.58 | 1.15–2.16 | ||
| F3–F4 | 26 | 22 (84.6) | 4.65 | 2.75 | 1.75–4.31 | 2.43 | 1.50–3.95 | ||
| Missing | 23 | 9 (39.1) | 2.37 | 1.40 | 0.71–2.75 | 1.16 | 0.58–2.29 | ||
| HIV | <0.001 | <0.001 | |||||||
| Neg | 302 | 201 (66.6) | 1.90 | 1 | 1 | ||||
| Pos | 29 | 24 (82.8) | 5.59 | 2.94 | 1.93–4.49 | 2.39 | 1.43–4.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piselli, P.; Boschini, A.; Gianfreda, R.; Nappo, A.; Cimaglia, C.; Scarfò, G.; Smacchia, C.; Paoletti, R.; Duehren, S.; Girardi, E. Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience. Viruses 2024, 16, 375. https://doi.org/10.3390/v16030375
Piselli P, Boschini A, Gianfreda R, Nappo A, Cimaglia C, Scarfò G, Smacchia C, Paoletti R, Duehren S, Girardi E. Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience. Viruses. 2024; 16(3):375. https://doi.org/10.3390/v16030375
Chicago/Turabian StylePiselli, Pierluca, Antonio Boschini, Romina Gianfreda, Alessandra Nappo, Claudia Cimaglia, Gianpaolo Scarfò, Camillo Smacchia, Raffaella Paoletti, Sarah Duehren, and Enrico Girardi. 2024. "Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience" Viruses 16, no. 3: 375. https://doi.org/10.3390/v16030375
APA StylePiselli, P., Boschini, A., Gianfreda, R., Nappo, A., Cimaglia, C., Scarfò, G., Smacchia, C., Paoletti, R., Duehren, S., & Girardi, E. (2024). Integration of Hepatitis C and Addiction Treatment in People Who Inject Drugs: The San Patrignano HCV-Free and Drug-Free Experience. Viruses, 16(3), 375. https://doi.org/10.3390/v16030375






