Metabolic Alterations in Mothers Living with HIV and Their HIV-Exposed, Uninfected Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Metabolomics Sub-Study
2.2. Demographic and Anthropometric Data
2.3. Experimental Procedures
2.3.1. Sample Processing
2.3.2. Statistical Analyses
3. Results
4. Discussion
4.1. Clinical Parameters
4.2. Altered Ketosis in Pregnant Mothers Living with HIV
4.3. Metabolic Profile of HEU Infants at Birth Linked to Compromised Glucose Metabolism with Possible Aberrant Neurological Development
4.4. Risk of Increased Inflammation in HEU at 6–10 Weeks and Altered Glucose Metabolism at 6 Months Post-Partum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filteau, S. The HIV-exposed, uninfected african child. Trop. Med. Int. Health 2009, 14, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.; Jones, C.E.; Prendergast, A.J. HIV-exposed, uninfected infants: New global challenges in the era of paediatric HIV elimination. Lancet Infect. Dis. 2016, 16, e92–e107. [Google Scholar] [CrossRef] [PubMed]
- Slogrove, A.; Reikie, B.; Naidoo, S.; De Beer, C.; Ho, K.; Cotton, M.; Bettinger, J.; Speert, D.; Esser, M.; Kollmann, T. HIV-exposed uninfected infants are at increased risk for severe infections in the first year of life. J. Trop. Pediatr. 2012, 58, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Uduman, S.A.; Khurranna, A.K. Impact of maternal HIV-1 infection on perinatal outcome. Int. J. Gynecol. Obstet. 1995, 49, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Powis, K.M.; Smeaton, L.; Hughes, M.D.; Tumbare, E.A.; Souda, S.; Jao, J.; Wirth, K.E.; Makhema, J.; Lockman, S.; Fawzi, W. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS 2016, 30, 211. [Google Scholar] [CrossRef] [PubMed]
- Briand, N.; Mandelbrot, L.; Le Chenadec, J.; Tubiana, R.; Teglas, J.-P.; Faye, A.; Dollfus, C.; Rouzioux, C.; Blanche, S.; Warszawski, J. No relation between in-utero exposure to HAART and intrauterine growth retardation. AIDS 2009, 23, 1235–1243. [Google Scholar] [CrossRef] [PubMed]
- Tuomala, R.E.; Shapiro, D.E.; Mofenson, L.M.; Bryson, Y.; Culnane, M.; Hughes, M.D.; O’sullivan, M.; Scott, G.; Stek, A.M.; Wara, D. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N. Engl. J. Med. 2002, 346, 1863–1870. [Google Scholar] [CrossRef]
- du Toit, L.D.; Prinsloo, A.; Steel, H.C.; Feucht, U.; Louw, R.; Rossouw, T.M. Immune and Metabolic Alterations in Children with Perinatal HIV Exposure. Viruses 2023, 15, 279. [Google Scholar] [CrossRef]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics—A review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef]
- Pendyala, G.; Want, E.J.; Webb, W.; Siuzdak, G.; Fox, H.S. Biomarkers for NeuroAIDS: The Widening Scope of Metabolomics. J. Neuroimmune Pharmacol. 2007, 2, 72–80. [Google Scholar] [CrossRef]
- Moutloatse, G.P.; Bunders, M.J.; van Reenen, M.; Mason, S.; Kuijpers, T.W.; Engelke, U.F.H.; Wevers, R.A.; Reinecke, C.J. Metabolic risks at birth of neonates exposed in utero to HIV-antiretroviral therapy relative to unexposed neonates: An NMR metabolomics study of cord blood. Metabolomics 2016, 12, 175. [Google Scholar] [CrossRef]
- De la Luz-Hdez, K. Metabolomics and Mammalian Cell Culture. In Metabolomics; Roessner, U., Ed.; InTech: Rijeka, Croatia, 2012; pp. 3–18. [Google Scholar]
- Griffin, J.L. Metabonomics: NMR spectroscopy and pattern recognition analysis of body fluids and tissues for characterisation of xenobiotic toxicity and disease diagnosis. Curr. Opin. Chem. Biol. 2003, 7, 648–654. [Google Scholar] [CrossRef]
- White, M.; Feucht, U.D.; du Toit, L.d.V.; Rossouw, T.; Connor, K.L. Understanding the Impact of Maternal HIV Infection on the Health and Well-Being of Mothers and Infants in South Africa: Siyakhula Collaborative Workshop Report. J. Multidiscip. Healthc. 2021, 14, 1987–1999. [Google Scholar] [CrossRef]
- Prentice, A.M.; Ward, K.A.; Goldberg, G.R.; Jarjou, L.M.; Moore, S.E.; Fulford, A.J.; Prentice, A. Critical windows for nutritional interventions against stunting. Am. Clin. Nutr. 2013, 97, 911–918. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, L.; Wu, Y.; Wilson, R.; Weston, C.; Fawole, O.; Bleich, S.N.; Cheskin, L.J.; Showell, N.N.; Lau, B. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes. Rev. 2015, 16, 547–565. [Google Scholar] [CrossRef] [PubMed]
- De Onis, M.; Blössner, M. The World Health Organization global database on child growth and malnutrition: Methodology and applications. Int. J. Epidemiol. 2003, 32, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.; Terburgh, K.; Louw, R. Miniaturized 1 H-NMR method for analyzing limited-quantity samples applied to a mouse model of Leigh disease. Metabolomics 2018, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Field, A. Discovering Statistics Using IBM SPSS Statistics; Sage: Newcastle upon Tyne, UK, 2013. [Google Scholar]
- Li, J.C.-H. Effect size measures in a two-independent-samples case with nonnormal and nonhomogeneous data. Behav. Res. Methods 2016, 48, 1560–1574. [Google Scholar] [CrossRef] [PubMed]
- Van Reenen, M.; Westerhuis, J.A.; Reinecke, C.J.; Venter, J.H. Metabolomics variable selection and classification in the presence of observations below the detection limit using an extension of ERp. BMC Bioinform. 2017, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.; Sherman, G.; Bamford, L.; Makua, M.; Ntloana, M.; Nuttall, J.; Pillay, Y.; Goga, A.; Feucht, U. The updated South African national guideline for the prevention of mother to child transmission of communicable infections (2019). S. Afr. J. HIV Med. 2020, 21, 1079. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; WHO: Geneva, Switzerland, 1995. [Google Scholar]
- Ramlal, R.T.; Tembo, M.; Soko, A.; Chigwenembe, M.; Ellington, S.; Kayira, D.; King, C.C.; Chasela, C.; Jamieson, D.; Van Der Horst, C. Maternal Mid–Upper Arm Circumference Is Associated With Birth Weight Among HIV-Infected Malawians. Nutr. Clin. Pract. 2012, 27, 416–421. [Google Scholar] [CrossRef]
- Salih, Y.; Omar, S.M.; AlHabardi, N.; Adam, I. The Mid-Upper Arm Circumference as a Substitute for Body Mass Index in the Assessment of Nutritional Status among Pregnant Women: A Cross-Sectional Study. Medicina 2023, 59, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ntozi, J.P. Impact of HIV/AIDS on fertility in sub-Saharan Africa. Afr. Popul. Stud. 2002, 17, 103–124. [Google Scholar]
- Ramokolo, V.; Goga, A.E.; Lombard, C.; Doherty, T.; Jackson, D.J.; Engebretsen, I.M. In utero ART exposure and birth and early growth outcomes among HIV-exposed uninfected infants attending immunization services: Results from national PMTCT surveillance, South Africa. Open Forum Infect. Dis. 2017, 4, ofx187. [Google Scholar] [CrossRef] [PubMed]
- Dirajlal-Fargo, S.; Mussi-Pinhata, M.M.; Weinberg, A.; Yu, Q.; Cohen, R.; Harris, D.R.; Bowman, E.; Gabriel, J.; Kulkarni, M.; Funderburg, N. HIV-exposed uninfected infants have increased inflammation and monocyte activation. AIDS 2019, 33, 845. [Google Scholar] [CrossRef] [PubMed]
- Abu-Raya, B.; Kollmann, T.R.; Marchant, A.; MacGillivray, D.M. The Immune System of HIV-Exposed Uninfected Infants. Front. Immunol. 2016, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- McPherson, P.A.C. Ketone Bodies. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 483–489. [Google Scholar]
- Dhillon, K.K.; Gupta, S. Biochemistry, ketogenesis. In StatPearls [Internet]; StatPearls Publishing: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Yamashita, H.; Kaneyuki, T.; Tagawa, K. Production of acetate in the liver and its utilization in peripheral tissues. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2001, 1532, 79–87. [Google Scholar] [CrossRef]
- Laffel, L. Ketone bodies: A review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes/Metab. Res. Rev. 1999, 15, 412–426. [Google Scholar] [CrossRef]
- Levitt, N.S.; Peer, N.; Steyn, K.; Lombard, C.; Maartens, G.; Lambert, E.V.; Dave, J.A. Increased risk of dysglycaemia in South Africans with HIV; especially those on protease inhibitors. Diabetes Res. Clin. Pract. 2016, 119, 41–47. [Google Scholar] [CrossRef]
- Munshi, S.U.; Rewari, B.B.; Bhavesh, N.S.; Jameel, S. Nuclear Magnetic Resonance Based Profiling of Biofluids Reveals Metabolic Dysregulation in HIV-Infected Persons and Those on Anti-Retroviral Therapy. PLoS ONE 2013, 8, e64298. [Google Scholar] [CrossRef]
- Gonzalez-Uarquin, F.; Rodehutscord, M.; Huber, K. Myo-inositol: Its metabolism and potential implications for poultry nutrition—A review. Poult. Sci. 2020, 99, 893–905. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. Myo-inositol for insulin resistance, metabolic syndrome, polycystic ovary syndrome and gestational diabetes. Arch. Dis. Child. 2022, 9, e001989. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, M.; Grasso, E.A.; Tripodi, R.; Chiarelli, F. Impact of glucose metabolism on the developing brain. Front. Endocrinol. 2022, 13, 1047545. [Google Scholar] [CrossRef] [PubMed]
- Hemachandra, A.H.; Cowett, R.M. Neonatal hyperglycemia. Pediatr. Rev. 1999, 20, e16–e24. [Google Scholar] [CrossRef]
- Hantzidiamantis, P.; Lappin, S. Physiology, Glucose; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: www.ncbi.nlm.nih.gov/books/NBK545201/ (accessed on 17 November 2023).
- Tibbetts, A.S.; Appling, D.R. Compartmentalization of Mammalian folate-mediated one-carbon metabolism. Annu. Rev. Nutr. 2010, 30, 57–81. [Google Scholar] [CrossRef]
- Meiser, J.; Vazquez, A. Give it or take it: The flux of one-carbon in cancer cells. FEBS J. 2016, 283, 3695–3704. [Google Scholar] [CrossRef]
- Lamarre, S.G.; Morrow, G.; Macmillan, L.; Brosnan, M.E.; Brosnan, J.T. Formate: An essential metabolite, a biomarker, or more? Clin. Chem. Lab. Med. 2013, 51, 571–578. [Google Scholar] [CrossRef]
- Kavitha, K.; Saharia, G.K.; Singh, A.K.; Mangaraj, M. Association of serum vitamin B12 with immuno-hematological parameters in treatment-naive HIV positive cases. J. Fam. Med. Prim. Care 2022, 11, 3784. [Google Scholar]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in inflammation: Mechanistic aspects and applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Zangerle, R.; Kurz, K.; Neurauter, G.; Kitchen, M.; Sarcletti, M.; Fuchs, D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav. Immun. 2010, 24, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, D.; Hausen, A.; Reibnegger, G.; Werner, E.R.; Werner-Felmayer, G.; Dierich, M.P.; Wachter, H. Interferon-γ concentrations are increased in sera from individuals infected with human immunodeficiency virus type 1. JAIDS J. Acquir. Immune Defic. Syndr. 1989, 2, 158–162. [Google Scholar]
- Fuchs, D.; Jäger, H.; Popescu, M.; Reibnegger, G.; Werner, E.R.; Dierich, M.P.; Kaboth, W.; Tilz, G.P.; Wachter, H. Immune activation markers to predict AIDS and survival in HIV-1 seropositives. Immunol. Lett. 1990, 26, 75–79. [Google Scholar] [CrossRef]
- Sokoya, T.; Steel, H.C.; Nieuwoudt, M.; Rossouw, T.M. HIV as a cause of immune activation and immunosenescence. Mediat. Inflamm. 2017, 2017, 6825493. [Google Scholar] [CrossRef]
- Reinecke, C.J.; Koekemoer, G.; Van der Westhuizen, F.H.; Louw, R.; Lindeque, J.Z.; Mienie, L.J.; Smuts, I. Metabolomics of urinary organic acids in respiratory chain deficiencies in children. Metabolomics 2012, 8, 264–283. [Google Scholar] [CrossRef]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef]
- Foo, J.; Bellot, G.; Pervaiz, S.; Alonso, S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022, 30, 679–692. [Google Scholar] [CrossRef]
- Riederer, M.; Schweighofer, N.; Trajanoski, S.; Stelzer, C.; Zehentner, M.; Fuchs-Neuhold, B.; Kashofer, K.; Mayr, J.A.; Hörmann-Wallner, M.; Holasek, S. Free threonine in human breast milk is related to infant intestinal microbiota composition. Amino Acids 2022, 54, 365–383. [Google Scholar] [CrossRef]
- Paquette, A.F.; Carbone, B.E.; Vogel, S.; Israel, E.; Maria, S.D.; Patil, N.P.; Sah, S.; Chowdhury, D.; Kondratiuk, I.; Labhart, B. The human milk component myo-inositol promotes neuronal connectivity. Proc. Natl. Acad. Sci. USA 2023, 120, e2221413120. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Suh, Y.; Chung, Y.-J. Choline and betaine concentrations in breast milk of Korean lactating women and the choline and betaine intakes of their infants. Korean J. Nutr. 2010, 43, 588–596. [Google Scholar] [CrossRef][Green Version]
- Feng, P.; Gao, M.; Holley, T.; Zhou, T.; Burgher, A.; Trabulsi, J.; Pramuk, K.; Nazzario, J. Amino acid composition and protein content of mature human milk from nine countries. FASEB J. 2009, 23, LB448. [Google Scholar] [CrossRef]
- Alexandre-Gouabau, M.-C.; Moyon, T.; David-Sochard, A.; Fenaille, F.; Cholet, S.; Royer, A.-L.; Guitton, Y.; Billard, H.; Darmaun, D.; Rozé, J.-C. Comprehensive preterm breast milk metabotype associated with optimal infant early growth pattern. Nutrients 2019, 11, 528. [Google Scholar] [CrossRef] [PubMed]
- LeMieux, M.J.; Aljawadi, A.; Moustaid-Moussa, N. Nutrimetabolomics. Adv. Nutr. 2014, 5, 792–794. [Google Scholar] [CrossRef] [PubMed]

| Time Point | 28 Weeks’ Gestation | Birth | ||||
|---|---|---|---|---|---|---|
| Group | PWLWH (n = 29) | HIV-Uninfected (n = 31) | p-Value | MLWH (n = 29) | HIV-Uninfected (n = 31) | p-Value |
| Age at time of delivery (years) | 37.0 (32.0–39.0) | 28.0 (24.0–36.0) | 0.0021 | |||
| CD4+ T-cell count (cells/µL) | 317.5 (220.0–538.0) | 351.0 (220.0–587.0) | ||||
| Viral load (copies/mL) | 143.0 (40.0–243.0) | 150.0 (40.0–217.0) | ||||
| Weight (kg) | 72.5 ± 12.4 | 68.7 ± 10.5 | 0.1851 | 69.9 ± 12.1 | 71.6 ± 11.3 | 0.7081 |
| Height (cm) | 160.2 ± 6.0 | 158.1 ± 6.0 | 0.1383 | 160.2 ± 6.0 | 158.1 ± 6.0 | 0.1383 |
| BMI (kg/m2) | 28.1 ± 4.0 | 27.6 ± 4.7 | 0.5227 | 27.3 ± 4.0 | 28.6 ± 5.1 | 0.3682 |
| MUAC (cm) | 29.6 ± 4.2 | 27.5 ± 3.6 | 0.0112 | 29.1 ± 4.4 | 29.1 ± 5.0 | 0.9515 |
| Delivery method | 10 (36%) C/S 18 (64%) NVD 1 information missing | 18 (58%) C/S 13 (42%) NVD | 0.1330 | |||
| Time Point | Birth | 6 Weeks | ||||
|---|---|---|---|---|---|---|
| Group | HEU (n = 29) | HUU (n = 31) | p-Value | HEU (n = 29) | HUU (n = 31) | p-Value |
| Weight (kg) | 2.9 ± 0.5 | 3.0 ± 0.4 | 0.5199 | 4.3 (3.8–4.7) | 4.6 (4.1–4.9) | 0.0375 |
| Length (cm) | 50.0 ± 3.9 | 49.7 ± 3.4 | 0.6348 | 54.5 (52.6–55.6) | 53.7 (52.5–56.0) | 0.8673 |
| BMI (kg/m2) | 11.5 ± 1.6 | 12.1 ± 1.0 | 0.0261 | 14.2 ± 1.9 | 15.6 ± 1.7 | 0.0045 |
| HC (cm) | 34.4 ± 2.0 | 34.0 ± 1.7 | 0.4380 | 37.9 (36.7–38.5) | 38.0 (37.4–39.1) | 0.1275 |
| MUAC | 12.9 (11.9–13.1) | 13.3 (12.7–14.0) | 0.0612 | |||
| Weight z-score | −0.6 ± 1.4 | −0.3 ± 1.1 | 0.4915 | |||
| Length z-score | 0.7 ± 2.1 | 0.5 ± 1.9 | 0.5383 | |||
| HC z-score | 0.7 (−0.2–1.8) | 0.6 (−0.2–1.3) | 0.4030 | |||
| WHZ | −0.5 (−1.4–0.6) | 0.4 (−0.3–1.7) | 0.0123 | |||
| WAZ | −0.6 ± 1.7 | 0.19 ± 1.4 | 0.0539 | |||
| HAZ | −0.4 (−1.5–1.2) | −0.4 (−1.2–0.5) | 0.9033 | |||
| BAZ | −0.5 (−1.2–0.5) | 0.7 (−0.2–1.1) | 0.0096 | |||
| HCZ | 0.5 (−0.4–1.5) | 0.8 (0.1–1.5) | 0.2519 | |||
| Breastfeeding | 27/29 (93.1%) | 31/31 100% | 0.2290 | 21/27 (77.8%) | 30/31 (96.8%) | 0.0330 |
| Time Point | 10 Weeks | 6 Months | ||||
|---|---|---|---|---|---|---|
| Group | HEU (n = 29) | HUU (n = 31) | p-Value | HEU (n = 29) | HUU (n = 31) | p-Value |
| Weight (kg) | 5.3 (4.8–5.7) | 5.6 (5.0–5.9) | 0.1867 | 7.1 (6.8–7.6) | 7.4 (6.8–8.0) | 0.2454 |
| Length (cm) | 58.5 (56.4–59.7) | 57.3 (56.4–58.8) | 0.2841 | 66.4 (64.1–68.0) | 66.5 (64.0–68.8) | 0.6233 |
| BMI (kg/m2) | 15.2 ± 2.1 | 16.9 ± 2.2 | 0.0136 | 16.6 ± 1.1 | 17.2 ± 2.0 | 0.3456 |
| HC (cm) | 39.3 (39.0–40.4) | 39.6 (39.0–40.2) | 0.9362 | 44.0 (43.0–44.6) | 43.1 (42.4–44.0) | 0.1155 |
| MUAC | 13.8 (13.0–14.5) | 13.8 (13.0–15.0) | 0.6518 | 14.2 (14.0–15.0) | 15.0 (14.0–16.0) | 0.0465 |
| WHZ | −0.4 (−1.2–0.8) | 0.6 (−0.6–1.4) | 0.0190 | −0.2 (−0.7–0.4) | 0 (−0.8–0.8) | 0.4086 |
| WAZ | −0.5 ± 1.4 | 0.04 ± 1.1 | 0.1335 | −0.7 ± 0.9 | −0.2 ± 1.3 | 0.1101 |
| HAZ | 0.2 (−1.3–0.9) | −0.4 (−1.1–0.7) | 0.4335 | −0.3 (−1.5–0.5) | −0.5 (−1.1–0.8) | 0.3416 |
| BAZ | −0.5 (−1.0–0.2) | 0.7 (−0.6–1.0) | 0.0053 | −0.4 (−0.8–0.1) | −0.1 (−1.0–0.7) | 0.3577 |
| HCZ | 0.7 (−0.2–1.5) | 0.6 (0.1–1.2) | 0.9745 | 0.7 (−0.2–1.1) | 0.5 (−0.4–1.0) | 0.3869 |
| MUACZ | 0.2 (−0.2–0.7) | 0.7 (0.2–1.5) | 0.0381 | |||
| Breastfeeding | 18/28 (64.3%) | 30/31 (96.8%) | 0.0020 | 16/28 (57.1%) | 22/31 (71.0%) | 0.1680 |
| Time Point | Metabolite | PWLWH/HEU (n = 29) | HIV-Uninfected/HUU (n = 31) | p-Value |
|---|---|---|---|---|
| Mothers at 28 weeks’ gestation | 3-Hydroxybutyric acid | 43.8 (28.5–59.3) | 61.8 (41.8–118.8) | 0.0351 |
| Acetoacetic acid | 17.6 (13.2–23.2) | 25.6 (15.1–58.7) | 0.0241 | |
| Acetic acid | 34.7 (23.6–48.3) | 44.9 (29.8–54.9) | 0.0377 | |
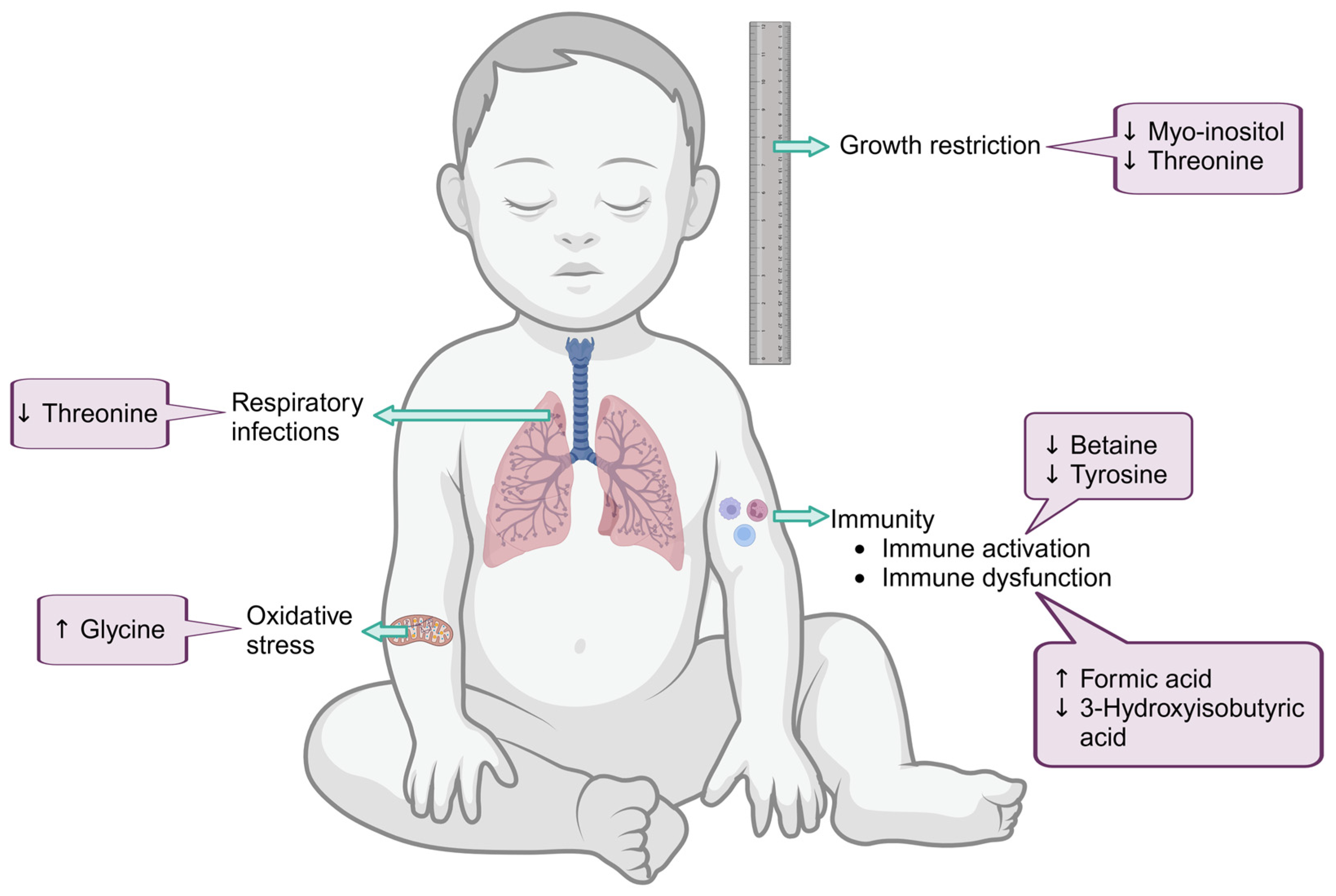
| Infants at Birth | Threonine | 218.3 (170.9–277.5) | 293.5 (245.4–351.3) | 0.0076 |
| Myo-inositol | 196.7 (151.9–238.0) | 252.0 (193.2–307.6) | 0.0500 | |
| Formic acid | 32.8 (16.8–52.1) | 19.6 (13.2–24.7) | 0.0500 | |
| Infants at 6/10 weeks post-partum | Betaine | 143.0 (108.2–164.3) | 162.5 (148.1–213.6) | 0.0176 |
| Tyrosine | 94.9 (76.9–135.9) | 134.1 (94.4–151.7) | 0.0483 | |
| Infants at 6 months post-partum | 3-Hydroxyisobutyric acid | 12.7 (6.9–14.3) | 15.3 (7.1–18.2) | 0.0390 |
| Glycine | 324.6 (292.6–371.3) | 264.3 (230.6–323.3) | 0.0078 | |
| Excluding infants not breastfed | ||||
| Infants at 6/10 weeks post-partum | Betaine | 147.6 (119.0–193.7) | 166.1 (148.6–213.6) | 0.0453 |
| Tyrosine | 94.5 (74.6–125.0) | 132.3 (94.4–144.0) | 0.0431 | |
| Glycine | 332.3 (290.2–362.8) | 283.9 (239.3–348.0) | 0.0315 | |
| Infants at 6 months post-partum | 3-Hydroxyisobutyric acid | 12.0 (6.2–14.8) | 13.4 (4.8–17.9) | 0.4643 |
| Glycine | 329.6 (297.2–378.8) | 265.8 (233.2–323.3) | 0.0205 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
du Toit, L.D.V.; Mason, S.; van Reenen, M.; Rossouw, T.M.; Louw, R. Metabolic Alterations in Mothers Living with HIV and Their HIV-Exposed, Uninfected Infants. Viruses 2024, 16, 313. https://doi.org/10.3390/v16020313
du Toit LDV, Mason S, van Reenen M, Rossouw TM, Louw R. Metabolic Alterations in Mothers Living with HIV and Their HIV-Exposed, Uninfected Infants. Viruses. 2024; 16(2):313. https://doi.org/10.3390/v16020313
Chicago/Turabian Styledu Toit, Louise D. V., Shayne Mason, Mari van Reenen, Theresa M. Rossouw, and Roan Louw. 2024. "Metabolic Alterations in Mothers Living with HIV and Their HIV-Exposed, Uninfected Infants" Viruses 16, no. 2: 313. https://doi.org/10.3390/v16020313
APA Styledu Toit, L. D. V., Mason, S., van Reenen, M., Rossouw, T. M., & Louw, R. (2024). Metabolic Alterations in Mothers Living with HIV and Their HIV-Exposed, Uninfected Infants. Viruses, 16(2), 313. https://doi.org/10.3390/v16020313








