Association between Human Papillomavirus 16 Viral Load in Pregnancy and Preterm Birth
Abstract
1. Introduction
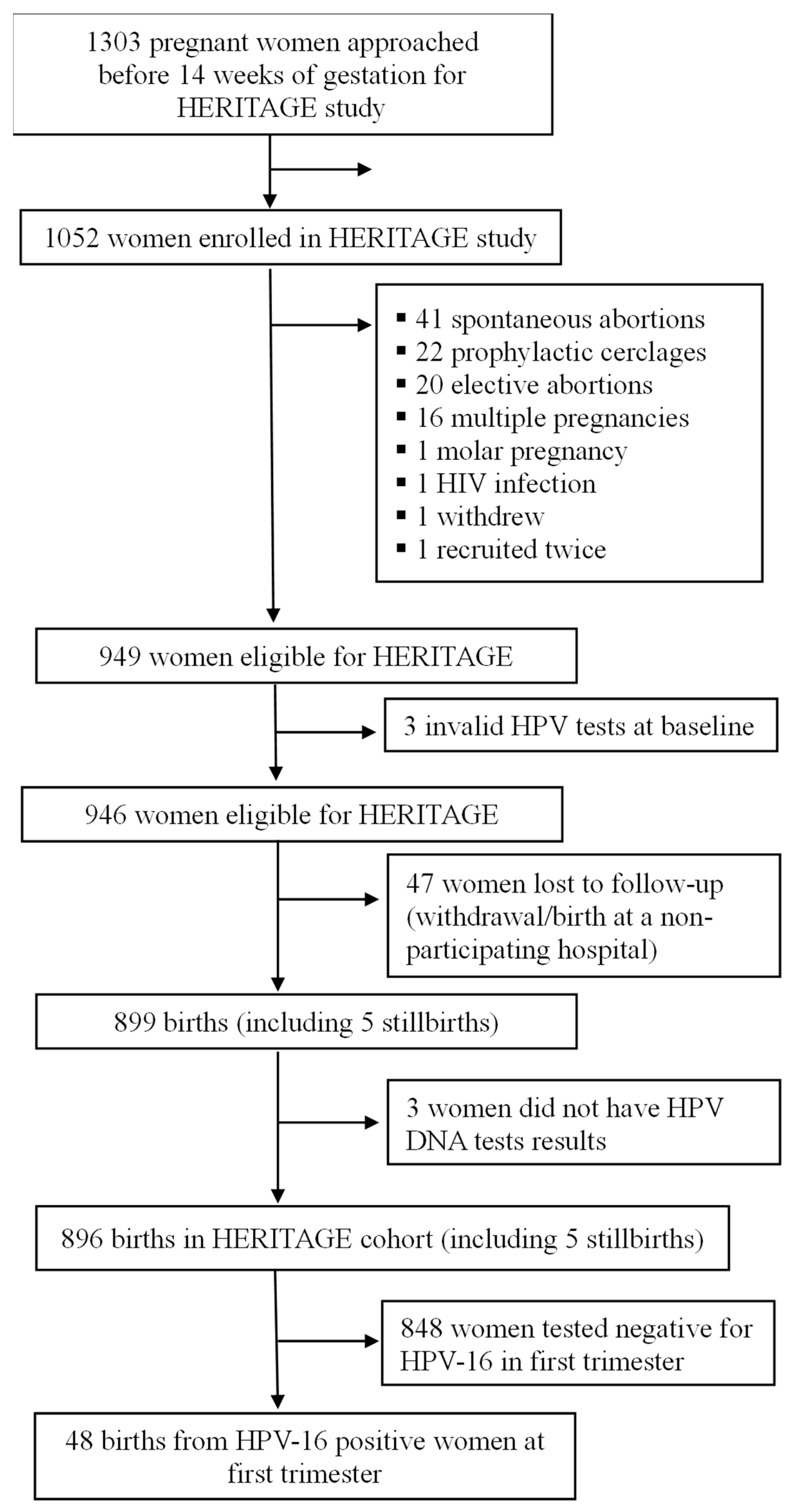
2. Material and Methods
2.1. Design and Participants
2.2. Sample Collection
2.3. HPV Testing
2.4. Exposure, Outcome, and Covariates
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 10 January 2024).
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.-B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef]
- Government of Canada. Preterm Birth Initiative. Available online: https://www.canada.ca/en/institutes-health-research/news/2017/05/preterm_birth_researchinitiative.html (accessed on 10 January 2024).
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef]
- Romero, R.; Dey, S.K.; Fisher, S.J. Preterm labor: One syndrome, many causes. Science 2014, 345, 760–765. [Google Scholar] [CrossRef]
- Racicot, K.; Kwon, J.Y.; Aldo, P.; Silasi, M.; Mor, G. Understanding the complexity of the immune system during pregnancy. Am. J. Reprod. Immunol. 2014, 72, 107–116. [Google Scholar] [CrossRef]
- Butler, A.S.; Behrman, R.E. Preterm Birth: Causes, Consequences, and Prevention; National Academies Press: Washington, DC, USA, 2007. [Google Scholar]
- Muglia, L.J.; Katz, M. The Enigma of Spontaneous Preterm Birth. N. Engl. J. Med. 2010, 362, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.E.; Rubens, C.E.; Darmstadt, G.L.; Gravett, M.G. Preventing Preterm Birth and Neonatal Mortality: Exploring the Epidemiology, Causes, and Interventions. Semin. Perinatol. 2010, 34, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Zanré, N.; Mayrand, M.H.; Trottier, H. Association Between Maternal Human Papillomavirus Infection and Adverse Pregnancy Outcomes: Systematic Review and Meta-Analysis. J. Infect. Dis. 2020, 221, 1925–1937. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.-T.; Zhong, M.; Gao, Y.-F.; Huang, L.-P.; Huang, Q.; Wang, W.; Wang, Z.-J.; Yu, Y.-H. Can HPV vaccine have other health benefits more than cancer prevention? A systematic review of association between cervical HPV infection and preterm birth. J. Clin. Virol. 2014, 61, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.Q.; Mo, Y.; Luo, Q.M.; Huo, S.T.; He, W.Q.; Chen, Q. The Risk of Human Papillomavirus Infection for Spontaneous Abortion, Spontaneous Preterm Birth, and Pregnancy Rate of Assisted Reproductive Technologies: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2018, 83, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Niyibizi, J.; Mayrand, M.-H.; Audibert, F.; Monnier, P.; Brassard, P.; Laporte, L.; Lacaille, J.; Zahreddine, M.; Bedard, M.-J.; Girard, I.; et al. Association between Human Papillomavirus Infection Among Pregnant Women and Preterm Birth. JAMA Netw. Open 2021, 4, e2125308. [Google Scholar] [CrossRef] [PubMed]
- Yuill, S.; Egger, S.; Smith, M.; Velentzis, L.; Wrede, C.D.; Bateson, D.; Canfell, K. Has Human Papillomavirus (HPV) Vaccination Prevented Adverse Pregnancy Outcomes? Population-Level Analysis After 8 Years of a National HPV Vaccination Program in Australia. J. Infect. Dis. 2020, 222, 499–508. [Google Scholar] [CrossRef]
- McClymont, E.; Faber, M.T.; Belmonte, F.; Kjaer, S.K. Spontaneous preterm birth risk among HPV-vaccinated and -unvaccinated women: A nationwide retrospective cohort study of over 240,000 singleton births. BJOG 2023, 130, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Kalliala, I.; Eriksson, T.; Aro, K.; Hokkanen, M.; Lehtinen, M.; Gissler, M.; Nieminen, P. Preterm birth rate after bivalent HPV vaccination: Registry-based follow-up of a randomized clinical trial. Prev. Med. 2021, 146, 106473. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, L.; Xing, Z. Impact of HPV infection on vaginal microecology and maternal and neonatal outcomes. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2021, 46, 497–502. [Google Scholar] [PubMed]
- Wiik, J.; Nilsson, S.; Kärrberg, C.; Strander, B.; Jacobsson, B.; Sengpiel, V. Associations of treated and untreated human papillomavirus infection with preterm delivery and neonatal mortality: A Swedish population-based study. PLoS Med. 2021, 18, e1003641. [Google Scholar] [CrossRef]
- Mosbah, A.; Barakat, R.; Nabiel, Y.; Barakat, G. High-risk and low-risk human papilloma virus in association to spontaneous preterm labor: A case-control study in a tertiary center, Egypt. J. Matern.-Fetal Neonatal Med. 2018, 31, 720–725. [Google Scholar] [CrossRef]
- Ambühl, L.M.; Baandrup, U.; Dybkær, K.; Blaakær, J.; Uldbjerg, N.; Sørensen, S. Human Papillomavirus Infection as a Possible Cause of Spontaneous Abortion and Spontaneous Preterm Delivery. Infect. Dis. Obstet. Gynecol. 2016, 2016, 3086036. [Google Scholar] [CrossRef]
- Slatter, T.L.; Hung, N.G.; Clow, W.M.; Royds, J.A.; Devenish, C.J.; Hung, N.A. A clinicopathological study of episomal papillomavirus infection of the human placenta and pregnancy complications. Mod. Pathol. 2015, 28, 1369–1382. [Google Scholar] [CrossRef]
- Kaur, H.; Schmidt-Grimminger, D.; Remmenga, S.W.; Chen, B.; Islam, K.M.M.; Watanabe-Galloway, S. Does human papillomavirus affect pregnancy outcomes? an analysis of hospital data 2012–2014. Int. J. Women’s Health Wellness 2015, 1, 158. [Google Scholar] [CrossRef]
- Zuo, Z.; Goel, S.; Carter, J.E. Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm birth. Am. J. Clin. Pathol. 2011, 136, 260–265. [Google Scholar] [CrossRef]
- Mammas, I.N.; Sourvinos, G.; Spandidos, D.A. Maternal human papillomavirus (HPV) infection and its possible relationship with neonatal prematurity. Br. J. Biomed. Sci. 2010, 67, 222–224. [Google Scholar] [CrossRef]
- Bánhidy, F.; Acs, N.; Puhó, E.H.; Czeizel, A.E. Birth outcomes among pregnant women with genital warts. Int. J. Gynaecol. Obstet. 2010, 108, 153–154. [Google Scholar] [CrossRef]
- Gomez, L.M.; Ma, Y.; Ho, C.; McGrath, C.M.; Nelson, D.B.; Parry, S. Placental infection with human papillomavirus is associated with spontaneous preterm delivery. Hum. Reprod. 2008, 23, 709–715. [Google Scholar] [CrossRef]
- Wu, D.; Chen, L.; Zhen, J.; Jin, X.J.; Ao, P.M. Systematic review and meta-analysis on influence of human papillomavirus infection during pregnancy on premature rupture of membranes and premature delivery. Ann. Palliat. Med. 2021, 10, 10735–10743. [Google Scholar] [CrossRef]
- Hooda, R.; Baghla, N.; Malik, N.; Kaushik, S. To evaluate the role of placental human papilloma virus (HPV) infection as a risk factor for spontaneous preterm birth: A prospective case control study. J. Perinat. Med. 2022, 50, 427–432. [Google Scholar] [CrossRef]
- Trottier, H.; Mayrand, M.H.; Coutlée, F.; Monnier, P.; Laporte, L.; Niyibizi, J.; Carceller, A.-M.; Fraser, W.D.; Brassard, P.; Lacroix, J.; et al. Human papillomavirus (HPV) perinatal transmission and risk of HPV persistence among children: Design, methods and preliminary results of the HERITAGE study. Papillomavirus Res. 2016, 2, 145–152. [Google Scholar] [CrossRef]
- Khayargoli, P.; Niyibizi, J.; Mayrand, M.H.; Audibert, F.; Monnier, P.; Brassard, P.; Laporte, L.; Lacaille, J.; Zahreddine, M.; Bedard, M.-J.; et al. Human Papillomavirus Transmission and Persistence in Pregnant Women and Neonates. JAMA Pediatr. 2023, 177, 684–692. [Google Scholar] [CrossRef]
- Niyibizi, J.; Mayrand, M.H.; Audibert, F.; Monnier, P.; Brassard, P.; Laporte, L.; Lacaille, J.; Zahreddine, M.; Bedard, M.-J.; Girard, I.; et al. Risk factors for placental human papillomavirus infection. Sex Transm. Infect. 2022, 98, 575–581. [Google Scholar] [CrossRef]
- Coutlée, F.; Rouleau, D.; Petignat, P.; Ghattas, G.; Kornegay, J.R.; Schlag, P.; Boyle, S.; Hankins, C.; Vezina, S.; Cote, P.; et al. Enhanced detection and typing of human papillomavirus (HPV) DNA in anogenital samples with PGMY primers and the Linear array HPV genotyping test. J. Clin. Microbiol. 2006, 44, 1998–2006. [Google Scholar] [CrossRef]
- Wissing, M.D.; Louvanto, K.; Comète, E.; Burchell, A.N.; El-Zein, M.; Rodrigues, A.; Tellier, P.-P.; Coutlee, F.; Franco, E.L. Human Papillomavirus Viral Load and Transmission in Young, Recently Formed Heterosexual Couples. J. Infect. Dis. 2019, 220, 1152–1161. [Google Scholar] [CrossRef]
- Malagón, T.; Louvanto, K.; Ramanakumar, A.V.; Koushik, A.; Coutlée, F.; Franco, E.L. Viral load of human papillomavirus types 16/18/31/33/45 as a predictor of cervical intraepithelial neoplasia and cancer by age. Gynecol. Oncol. 2019, 155, 245–253. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, X.; Liu, J.; Zhang, L. Correlation between human papillomavirus viral load and cervical lesions classification: A review of current research. Front. Med. 2023, 10, 1111269. [Google Scholar] [CrossRef]
- Taheri, M. The Association Between Human Papillomavirus Infection and Preterm Labor: A Literature Mini-review. Fertil. Gynecol. Androl. 2022, 2, e129015. [Google Scholar] [CrossRef]
- Nimrodi, M.; Kleitman, V.; Wainstock, T.; Gemer, O.; Meirovitz, M.; Maymon, E.; Benshalom-Tirosh, N.; Erez, O. The association between cervical inflammation and histologic evidence of HPV in PAP smears and adverse pregnancy outcome in low risk population. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 225, 160–165. [Google Scholar] [CrossRef]
- Ambühl, L.M.M.; Leonhard, A.K.; Widen Zakhary, C.; Jorgensen, A.; Blaakaer, J.; Dybkaer, K.; Baandrup, U.; Uldbjerg, N.; Sorensen, S. Human papillomavirus infects placental trophoblast and Hofbauer cells, but appears not to play a causal role in miscarriage and preterm labor. Acta Obstet. Gynecol. Scand. 2017, 96, 1188–1196. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, Z.; Wang, W.; Gu, L.; Gao, H.; Qiu, L.; Di, W. Association between the vaginal microbiome and high-risk human papillomavirus infection in pregnant Chinese women. BMC Infect. Dis. 2019, 19, 677. [Google Scholar] [CrossRef]
| Low HPV16 Viral Load (≤1 Copy/Cell) n = 40 | High HPV16 Viral Load (>1 Copy/Cell) n = 8 | Total Sample n = 48 | |
|---|---|---|---|
| Characteristics at baseline | |||
| Mean age (SD); median [25–75%] | 31.4 (4.6); 31 [28–34.5] | 30.1 (5.5); 29 [26–33.5] | 31.2 (4.7); 31 [28–34.5] |
| Completed years of education, median [25–75%] | 17 [16–19] | 17 [15.5–17] | 17 [16–18.5] |
| Ethnicity, n (%) | |||
| White | 34 (85.0) | 6 (75.0) | 40 (83.3) |
| Arabic-West Asian | 3 (7.5) | 0 | 3 (6.3) |
| Native African | 0 | 2 (25.0) | 2 (4.2) |
| East Asian | 1 (2.5) | 0 | 1 (2.1) |
| Others a | 2 (5.0) | 0 | 2 (4.2) |
| Smoker, n (%) | |||
| Yes | 3 (7.5) | 1 (12.5) | 4 (8.3) |
| No | 36 (90.0) | 7 (87.5) | 43 (89.6) |
| Missing | 1 (2.5) | 0 | 1 (2.1) |
| Alcohol consumption (number of days since the beginning of pregnancy) b, n (%) | |||
| None | 21 (52.5) | 6 (75.0) | 27 (56.3) |
| 1–4 | 13 (32.5) | 2 (25.0) | 15 (31.3) |
| ≥5 | 6 (15.0) | 0 | 6 (12.5) |
| Nulliparous, n (%) | |||
| Yes | 25 (62.5) | 4 (50) | 29 (60.4) |
| No | 15 (37.5) | 4 (50) | 19 (39.6) |
| History of preterm birth among parous women (n = 19), n (%) | |||
| Yes | 1 (6.7) | 0 | 1 (5.3) |
| No | 14 (93.3) | 4 (100) | 18 (94.7) |
| History of cervical intraepithelial neoplasia treatment, n (%) | |||
| Yes | 5 (12.5) | 1 (12.5) | 6 (12.5) |
| No | 31 (77.5) | 5 (62.5) | 36 (75) |
| Missing | 4 (10.0) | 2 (25.0) | 6 (12.5) |
| HPV16 viral load (copies/cell) | |||
| Mean (SD) | 5.9 × 10−2 (0.2) | 9.5 (11.4) | 1.6 (5.6) |
| Min–Max | 4.0 × 10−5–0.77 | 1.15–31.46 | 4.0 × 10−5–31.46 |
| Median [25–75%] | 3.2 × 10−3 [6.9 × 10−4–3.8 × 10−2] | 3.3 [1.7–16.6] | 8.0 × 10−3 [1.2 × 10−3–0.1] |
| Characteristics during pregnancy | |||
| Gestational diabetes, n (%) | |||
| Yes | 3 (7.5) | 2 (25.0) | 5 (10.4) |
| No | 35 (87.5) | 6 (75.0) | 41 (85.4) |
| Missing | 2 (5.0) | 0 | 2 (4.2) |
| Pregnancy-induced hypertensive disorders, n (%) | |||
| Yes | 1 (2.5) | 0 | 1 (2.1) |
| No | 38 (95.0) | 8 (100) | 46 (95.8) |
| Missing | 1 (2.5) | 0 | 1 (2.1) |
| Urinary tract or genital infections c, n (%) | |||
| Yes | 0 | 1 (12.5) | 1 (2.1) |
| No | 40 (100) | 7 (87.5) | 47 (97.9) |
| Pregnancy outcome, n (%) | |||
| Preterm birth | 2 (5.0) | 3 (37.5) | 5 (10.4) |
| Term birth | 38 (95.0) | 5 (62.5) | 43 (89.6) |
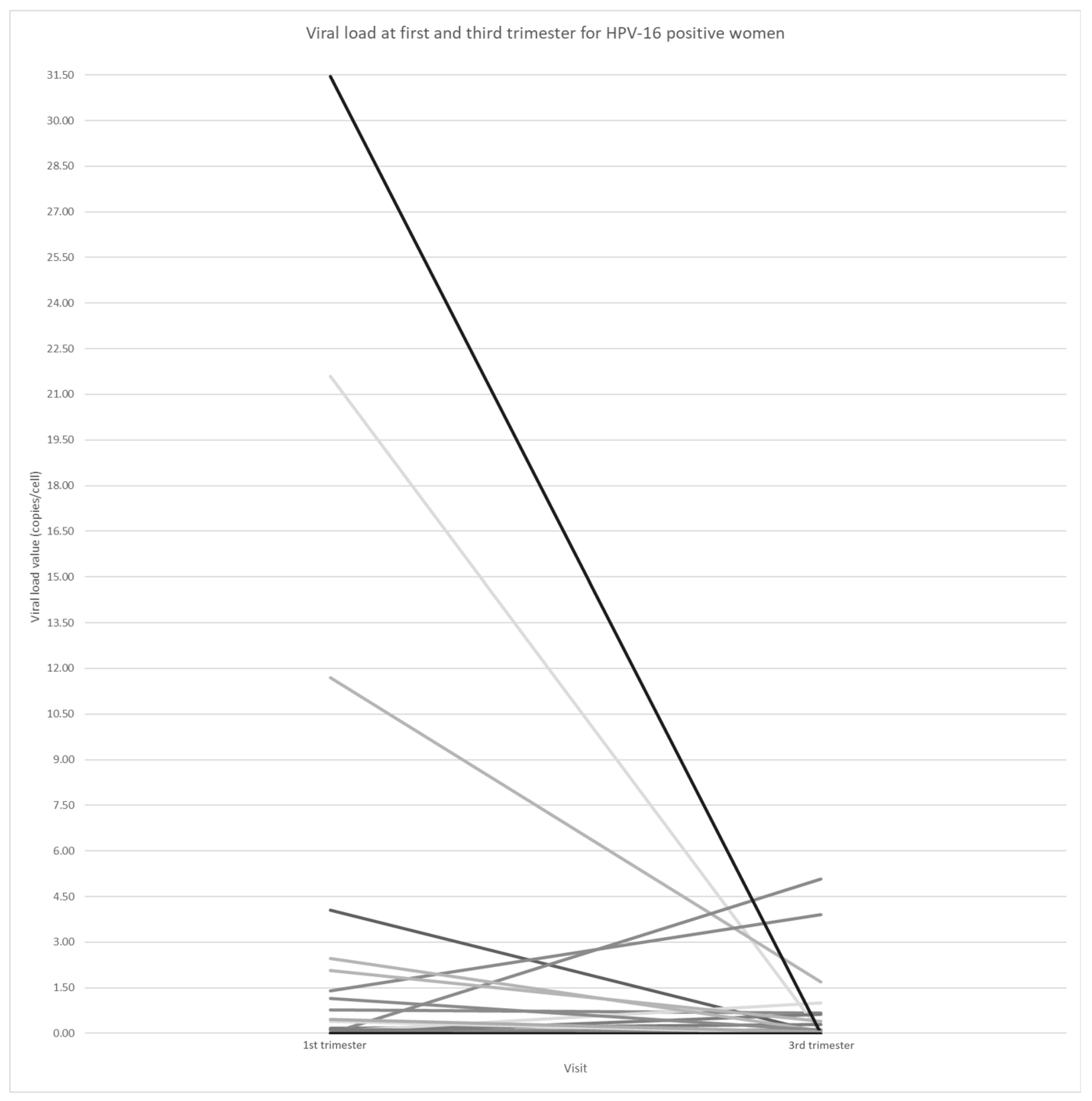
| Sequential Number | Age | 1st-Trimester HPV16 Loads (Copies/Cell) | 3rd-Trimester HPV16 Loads (Copies/Cell) | Difference in Viral Loads between 1st and 3rd Trimester | Preterm Birth | Gestational Age (Weeks) | History of Cervical Treatment * |
|---|---|---|---|---|---|---|---|
| 1 | 28 | 11.69219 | 1.69494 | −9.99725 | Yes | 36 | No |
| 2 | 40 | 1.40437 | 3.90897 | 2.5046 | Yes | 36 | No |
| 3 | 30 | 0.03511 | 0.00529 | −0.02982 | Yes | 36 | No |
| 4 | 31 | 0.03989 | 0.00774 | −0.03215 | Yes | 35 | Missing |
| 5 | 36 | 31.4574 | 0.00114 | −31.45626 | Yes | 36 | Yes |
| 6 | 26 | 0.00284 | 0.00009 | −0.00275 | No | 38 | No |
| 7 | 26 | 0.01402 | 0.00166 | −0.01236 | No | 40 | No |
| 8 | 36 | 0.7735 | 0.65959 | −0.11391 | No | 37 | No |
| 9 | 34 | 0.00049 | 0 | −0.00049 | No | 39 | No |
| 10 | 22 | 0.05343 | Missing | NA | No | 38 | No |
| 11 | 26 | 21.59379 | 0.01448 | −21.57932 | No | 39 | No |
| 12 | 31 | 0.09955 | 0.08503 | −0.01451 | No | 38 | No |
| 13 | 36 | 0.00165 | 0.00589 | 0.00424 | No | 38 | Yes |
| 14 | 30 | 4.05012 | 0.02146 | −4.02866 | No | 41 | No |
| 15 | 28 | 0.00579 | Missing | NA | No | 38 | No |
| 16 | 27 | 0.01189 | 0.00415 | −0.00774 | No | 40 | No |
| 17 | 29 | 0.00051 | Missing | NA | No | 41 | Missing |
| 18 | 26 | 0.00279 | 0 | −0.00279 | No | 38 | No |
| 19 | 26 | 0.00312 | 0 | −0.00312 | No | 39 | No |
| 20 | 28 | 0.00025 | 0 | −0.00025 | No | 39 | Yes |
| 21 | 33 | 0.00011 | 0.0011 | −0.00099 | No | 40 | Missing |
| 22 | 30 | 0.16459 | 0.29646 | 0.13187 | No | 39 | No |
| 23 | 35 | 0.00036 | 0.00036 | 0 | No | 41 | No |
| 24 | 33 | 0.04063 | 0.00375 | −0.03688 | No | 39 | No |
| 25 | 32 | 0.00134 | 5.06819 | 5.06686 | No | 39 | No |
| 26 | 28 | 0.00004 | 0.0004 | 0.00036 | No | 39 | No |
| 27 | 33 | 0.05176 | 1.00443 | 0.95267 | No | 40 | No |
| 28 | 38 | 0.00015 | 0 | −0.00015 | No | 38 | Missing |
| 29 | 33 | 0.00486 | 0.62714 | 0.62228 | No | 40 | No |
| 30 | 26 | 0.02287 | 0.01027 | −0.0126 | No | 40 | No |
| 31 | 26 | 2.46677 | 0.08264 | −2.38413 | No | 39 | Missing |
| 32 | 24 | 1.14868 | 0.10666 | −1.04202 | No | 40 | No |
| 33 | 47 | 0.00065 | 0 | −0.00065 | No | 39 | No |
| 34 | 31 | 0.00327 | 0.00133 | −0.00194 | No | 40 | No |
| 35 | 31 | 0.00108 | 0.01091 | 0.00983 | No | 41 | Yes |
| 36 | 32 | 0.12291 | 0.04096 | −0.08195 | No | 39 | No |
| 37 | 30 | 0.00473 | 0.01793 | 0.0132 | No | 37 | No |
| 38 | 31 | 2.07335 | 0.38704 | −1.6863 | No | 40 | Missing |
| 39 | 33 | 0.00063 | 0 | −0.00063 | No | 40 | No |
| 40 | 29 | 0.01605 | 0.12466 | 0.10861 | No | 40 | No |
| 41 | 29 | 0.00227 | 0 | −0.00227 | No | 39 | No |
| 42 | 32 | 0.39554 | 0.00759 | −0.38795 | No | 39 | No |
| 43 | 38 | 0.45647 | 0.03041 | −0.42606 | No | 41 | Yes |
| 44 | 35 | 0.00308 | 0.00004 | −0.00304 | No | 39 | No |
| 45 | 36 | 0.0001 | 0 | −0.0001 | No | 40 | No |
| 46 | 36 | 0.01013 | 0 | −0.01013 | No | 41 | No |
| 47 | 35 | 0.00072 | 0.00524 | 0.00451 | No | 41 | Yes |
| 48 | 26 | 0.00307 | 0.00062 | −0.00244 | No | 39 | No |
| HPV16 Viral Load (Number of Copies/Cell) | Odds Ratio (95% CI) | ||
|---|---|---|---|
| Number of Preterm Births/Total Women | Crude | Adjusted c | |
| Viral load in the first trimester (continuous) | 5/48 a | 1.15 (1.01–1.31) | 1.13 (1.03–1.25) |
| Viral load in the third trimester (continuous) | 5/45 b | 1.75 (0.90–3.41) | 1.84 (0.80–4.23) |
| First trimester | |||
| Low viral load (≤0.5 copies/cell) | 2/39 | Referent | Referent |
| High viral load (>0.5 copies/cell) | 3/9 | 9.25 (1.27–67.42) | 13.04 (1.58–107.57) |
| Third trimester | |||
| Low viral load (≤0.5 copies/cell) | 3/39 | Referent | Referent |
| High viral load (>0.5 copies/cell) | 2/6 | 6.00 (0.76–47.36) | 6.75 (0.76–59.67) |
| First trimester | |||
| Low viral load (≤1 copy/cell) | 2/40 | Referent | Referent |
| High viral load (>1 copy/cell) | 3/8 | 11.40 (1.52–85.73) | 15.03 (1.75–129.26) |
| Third trimester | |||
| Low viral load (≤1 copy/cell) | 3/41 | Referent | Referent |
| High viral load (>1 copy/cell) | 2/4 | 12.67 (1.29–124.51) | 14.02 (1.28–153.48) |
| First trimester | |||
| Low viral load (≤2 copies/cell) | 3/42 | Referent | Referent |
| High viral load (>2 copies/cell) | 2/6 | 6.50 (0.83–51.20) | 6.24 (0.66–59.06) |
| Third trimester | |||
| Low viral load (≤2 copies/cell) | 4/43 | Referent | Referent |
| High viral load (>2 copies/cell) | 1/2 | 9.75 (0.51–187.53) | 14.67 (0.72–300.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khayargoli, P.; Mayrand, M.-H.; Niyibizi, J.; Audibert, F.; Laporte, L.; Lacaille, J.; Carceller, A.M.; Lacroix, J.; Comète, É.; Coutlée, F.; et al. Association between Human Papillomavirus 16 Viral Load in Pregnancy and Preterm Birth. Viruses 2024, 16, 298. https://doi.org/10.3390/v16020298
Khayargoli P, Mayrand M-H, Niyibizi J, Audibert F, Laporte L, Lacaille J, Carceller AM, Lacroix J, Comète É, Coutlée F, et al. Association between Human Papillomavirus 16 Viral Load in Pregnancy and Preterm Birth. Viruses. 2024; 16(2):298. https://doi.org/10.3390/v16020298
Chicago/Turabian StyleKhayargoli, Pranamika, Marie-Hélène Mayrand, Joseph Niyibizi, François Audibert, Louise Laporte, Julie Lacaille, Ana Maria Carceller, Jacques Lacroix, Émilie Comète, François Coutlée, and et al. 2024. "Association between Human Papillomavirus 16 Viral Load in Pregnancy and Preterm Birth" Viruses 16, no. 2: 298. https://doi.org/10.3390/v16020298
APA StyleKhayargoli, P., Mayrand, M.-H., Niyibizi, J., Audibert, F., Laporte, L., Lacaille, J., Carceller, A. M., Lacroix, J., Comète, É., Coutlée, F., & Trottier, H., on behalf of the HERITAGE Study Group. (2024). Association between Human Papillomavirus 16 Viral Load in Pregnancy and Preterm Birth. Viruses, 16(2), 298. https://doi.org/10.3390/v16020298






