DENV-1 Titer Impacts Viral Blocking in wMel Aedes aegypti with Brazilian Genetic Background
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas
2.2. Wolbachia DNA Detection and Quantification in Ae. aegypti
2.3. Viral Strain and Oral Infections
2.4. Mosquito Saliva Collection and Intrathoracic Microinjection
2.5. DENV-1 RNA Detection and Quantification in Mosquitoes
2.6. Statistical Analysis
3. Results
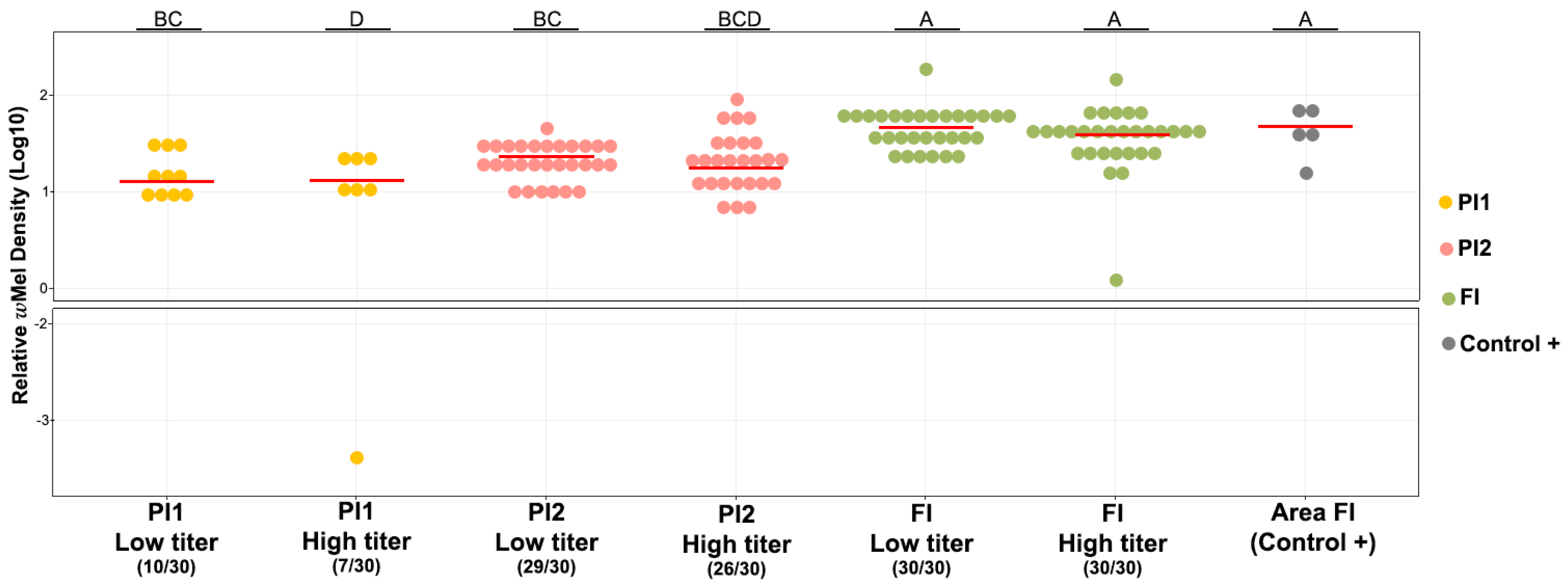
3.1. Prevalence and Density of wMel in Field and Lab Ae. aegypti Mosquitoes
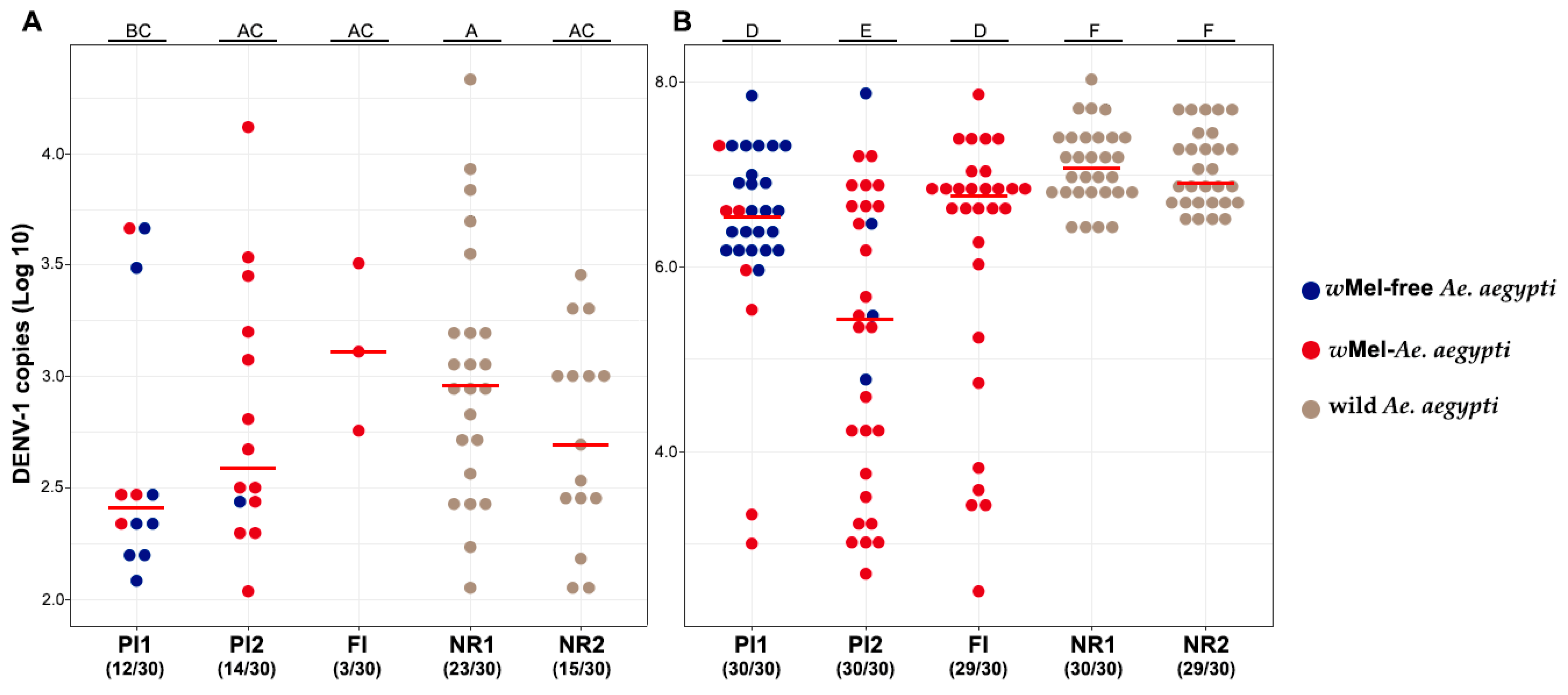
3.2. wMel Detection in Ae. aegypti Mosquitoes Orally Exposed to Two DENV-1 Titers
3.3. Vector Competence Assays
3.4. Saliva Infectivity for DENV-1
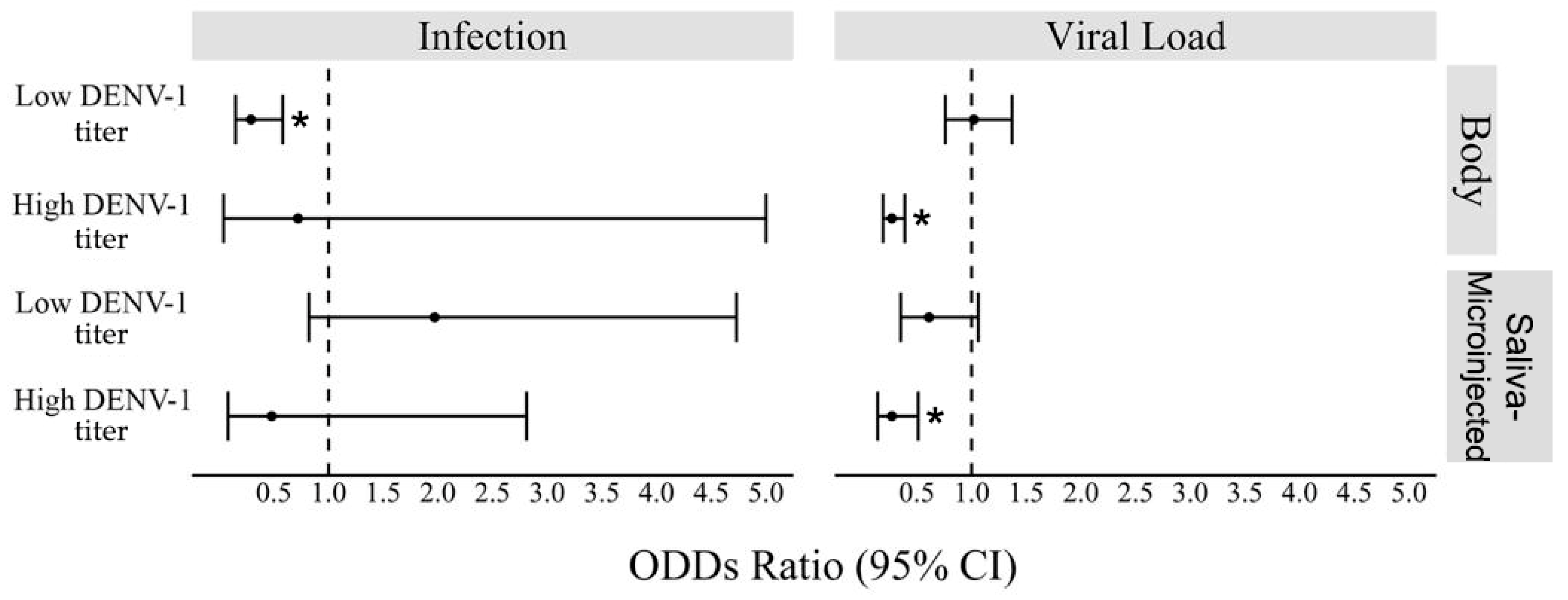
3.5. Interactions of wMel in DENV-1 Exposed Ae. aegypti
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- WHO Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue#:~:text=Dengue%20is%20a%20viral%20infection,million%20infections%20occurring%20each%20year (accessed on 7 August 2023).
- PAHO Epidemiological Update for Dengue, Chikungunya and Zika in 2022. Available online: https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/annual-arbovirus-bulletin-2022.html (accessed on 7 August 2023).
- Maciel-de-Freitas, R.; Marques, W.A.; Peres, R.C.; Cunha, S.P.; Lourenço-de-Oliveira, R. Variation in Aedes aegypti (Diptera: Culicidae) Container Productivity in a Slum and a Suburban District of Rio de Janeiro during Dry and Wet Seasons. Mem. Inst. Oswaldo Cruz 2007, 102, 489–496. [Google Scholar] [CrossRef]
- David, M.R.; Dantas, E.S.; Maciel-de-Freitas, R.; Codeço, C.T.; Prast, A.E.; Lourenço-de-Oliveira, R. Influence of Larval Habitat Environmental Characteristics on Culicidae Immature Abundance and Body Size of Adult Aedes aegypti. Front. Ecol. Evol. 2021, 9, 626757. [Google Scholar] [CrossRef]
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A Global Public Health Threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef]
- Zug, R.; Hammerstein, P. Still a Host of Hosts for Wolbachia: Analysis of Recent Data Suggests That 40% of Terrestrial Arthropod Species Are Infected. PLoS ONE 2012, 7, e38544. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Bian, G.; Xu, Y.; Lu, P.; Xie, Y.; Xi, Z. The Endosymbiotic Bacterium Wolbachia Induces Resistance to Dengue Virus in Aedes aegypti. PLoS Pathog. 2010, 6, e1000833. [Google Scholar] [CrossRef] [PubMed]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia Strain Blocks Dengue and Invades Caged Aedes aegypti Populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef] [PubMed]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master Manipulators of Invertebrate Biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Turelli, M. Evolution of incompatibility-inducing microbes and their hosts. Evolution 1994, 48, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Turelli, M.; Hoffmann, A.A. Evolutionary Ecology of Wolbachia Releases for Disease Control. Annu. Rev. Genet. 2019, 53, 93–116. [Google Scholar] [CrossRef]
- Garcia, G.d.A.; Sylvestre, G.; Aguiar, R.; da Costa, G.B.; Martins, A.J.; Lima, J.B.P.; Petersen, M.T.; Lourenço-de-Oliveira, R.; Shadbolt, M.F.; Rašić, G.; et al. Matching the Genetics of Released and Local Aedes aegypti Populations Is Critical to Assure Wolbachia Invasion. PLoS Negl. Trop. Dis. 2019, 13, e0007023. [Google Scholar] [CrossRef]
- Lau, M.-J.; Ross, P.A.; Hoffmann, A.A. Infertility and Fecundity Loss of Wolbachia-Infected Aedes aegypti Hatched from Quiescent Eggs Is Expected to Alter Invasion Dynamics. PLoS Negl. Trop. Dis. 2021, 15, e0009179. [Google Scholar] [CrossRef]
- Petersen, M.T.; Couto-Lima, D.; Garcia, G.A.; Pavan, M.G.; David, M.R.; Maciel-de-Freitas, R. Dengue Exposure and Wolbachia wMel Strain Affects the Fertility of Quiescent Eggs of Aedes aegypti. Viruses 2023, 15, 952. [Google Scholar] [CrossRef]
- Rocha, M.N.; Duarte, M.M.; Mansur, S.B.; e Silva, B.D.M.; Pereira, T.N.; Adelino, T.É.R.; Giovanetti, M.; Alcantara, L.C.J.; Santos, F.M.; Costa, V.R.d.M.; et al. Pluripotency of Wolbachia against Arboviruses: The Case of Yellow Fever. Gates Open Res. 2019, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.N.; Rocha, M.N.; Sucupira, P.H.F.; Carvalho, F.D.; Moreira, L.A. Wolbachia Significantly Impacts the Vector Competence of Aedes aegypti for Mayaro Virus. Sci. Rep. 2018, 8, 6889. [Google Scholar] [CrossRef]
- Ferguson, N.M.; Kien, D.T.H.; Clapham, H.; Aguas, R.; Trung, V.T.; Chau, T.N.B.; Popovici, J.; Ryan, P.A.; O’Neill, S.L.; McGraw, E.A.; et al. Modeling the Impact on Virus Transmission of Wolbachia-Mediated Blocking of Dengue Virus Infection of Aedes aegypti. Sci. Transl. Med. 2015, 7, 279ra37. [Google Scholar] [CrossRef]
- Carrington, L.B.; Tran, B.C.N.; Le, N.T.H.; Luong, T.T.H.; Nguyen, T.T.; Nguyen, P.T.; Nguyen, C.V.V.; Nguyen, H.T.C.; Vu, T.T.; Vo, L.T.; et al. Field- and Clinically Derived Estimates of Wolbachia-Mediated Blocking of Dengue Virus Transmission Potential in Aedes aegypti Mosquitoes. Proc. Natl. Acad. Sci. USA 2018, 115, 361–366. [Google Scholar] [CrossRef]
- Flores, H.A.; Taneja de Bruyne, J.; O’Donnell, T.B.; Tuyet Nhu, V.; Thi Giang, N.; Thi Xuan Trang, H.; Thi Thuy Van, H.; Thi Long, V.; Thi Dui, L.; Le Anh Huy, H.; et al. Multiple Wolbachia Strains Provide Comparative Levels of Protection against Dengue Virus Infection in Aedes aegypti. PLoS Pathog. 2020, 16, e1008433. [Google Scholar] [CrossRef]
- Caragata, E.P.; Rocha, M.N.; Pereira, T.N.; Mansur, S.B.; Dutra, H.L.C.; Moreira, L.A. Pathogen Blocking in Wolbachia-Infected Aedes aegypti Is Not Affected by Zika and Dengue Virus Co-Infection. PLoS Negl. Trop. Dis. 2019, 13, e0007443. [Google Scholar] [CrossRef]
- Ant, T.H.; Mancini, M.V.; McNamara, C.J.; Rainey, S.M.; Sinkins, S.P. Wolbachia-Virus Interactions and Arbovirus Control through Population Replacement in Mosquitoes. Pathog. Glob. Health 2023, 117, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Boehm, E.C.; Jaeger, A.S.; Ries, H.J.; Castañeda, D.; Weiler, A.M.; Valencia, C.C.; Weger-Lucarelli, J.; Ebel, G.D.; O’Connor, S.L.; Friedrich, T.C.; et al. Wolbachia-Mediated Resistance to Zika Virus Infection in Aedes aegypti Is Dominated by Diverse Transcriptional Regulation and Weak Evolutionary Pressures. PLoS Negl. Trop. Dis. 2023, 17, e0011674. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Longdon, B.; Bauer, S.; Chan, Y.-S.; Miller, W.J.; Bourtzis, K.; Teixeira, L.; Jiggins, F.M. Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Wolbachia Strains. PLoS Pathog. 2014, 10, e1004369. [Google Scholar] [CrossRef] [PubMed]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Ogunlade, S.T.; Adekunle, A.I.; Meehan, M.T.; McBryde, E.S. Quantifying the Impact of Wolbachia Releases on Dengue Infection in Townsville, Australia. Sci. Rep. 2023, 13, 14932. [Google Scholar] [CrossRef]
- Lenharo, M. Dengue Rates Drop after Release of Modified Mosquitoes in Colombia. Nature 2023, 623, 235–236. [Google Scholar] [CrossRef]
- Velez, I.D.; Tanamas, S.K.; Arbelaez, M.P.; Kutcher, S.C.; Duque, S.L.; Uribe, A.; Zuluaga, L.; Martínez, L.; Patiño, A.C.; Barajas, J.; et al. Reduced Dengue Incidence Following City-Wide WMel Wolbachia Mosquito Releases throughout Three Colombian Cities: Interrupted Time Series Analysis and a Prospective Case-Control Study. PLoS Negl. Trop. Dis. 2023, 17, e0011713. [Google Scholar] [CrossRef]
- Ribeiro Dos Santos, G.; Durovni, B.; Saraceni, V.; Souza Riback, T.I.; Pinto, S.B.; Anders, K.L.; Moreira, L.A.; Salje, H. Estimating the Effect of the WMel Release Programme on the Incidence of Dengue and Chikungunya in Rio de Janeiro, Brazil: A Spatiotemporal Modelling Study. Lancet Infect. Dis. 2022, 22, 1587–1595. [Google Scholar] [CrossRef]
- Pinto, S.B.; Riback, T.I.S.; Sylvestre, G.; Costa, G.; Peixoto, J.; Dias, F.B.S.; Tanamas, S.K.; Simmons, C.P.; Dufault, S.M.; Ryan, P.A.; et al. Effectiveness of Wolbachia-Infected Mosquito Deployments in Reducing the Incidence of Dengue and Other Aedes-Borne Diseases in Niterói, Brazil: A Quasi-Experimental Study. PLoS Negl. Trop. Dis. 2021, 15, e0009556. [Google Scholar] [CrossRef]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced Competence to Arboviruses Following the Sustainable Invasion of Wolbachia into Native Aedes aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 10039. [Google Scholar] [CrossRef]
- Calvez, E.; Guillaumot, L.; Girault, D.; Richard, V.; O’Connor, O.; Paoaafaite, T.; Teurlai, M.; Pocquet, N.; Cao-Lormeau, V.-M.; Dupont-Rouzeyrol, M. Dengue-1 Virus and Vector Competence of Aedes aegypti (Diptera: Culicidae) Populations from New Caledonia. Parasit. Vectors 2017, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Bugallo, G.; Boullis, A.; Martinez, Y.; Hery, L.; Rodríguez, M.; Bisset, J.A.; Vega-Rúa, A. Vector Competence of Aedes aegypti from Havana, Cuba, for Dengue Virus Type 1, Chikungunya, and Zika Viruses. PLoS Negl. Trop. Dis. 2020, 14, e0008941. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, O.; Calvez, E.; Inizan, C.; Pocquet, N.; Richard, V.; Dupont-Rouzeyrol, M. Vector Competence of Aedes aegypti from New Caledonia for the Four Recent Circulating Dengue Virus Serotypes. PLoS Negl. Trop. Dis. 2020, 14, e0008303. [Google Scholar] [CrossRef]
- Amoa-Bosompem, M.; Kobayashi, D.; Itokawa, K.; Murota, K.; Faizah, A.N.; Azerigyik, F.A.; Hayashi, T.; Ohashi, M.; Bonney, J.H.K.; Dadzie, S.; et al. Determining Vector Competence of Aedes aegypti from Ghana in Transmitting Dengue Virus Serotypes 1 and 2. Parasit. Vectors 2021, 14, 228. [Google Scholar] [CrossRef]
- Duong, V.; Lambrechts, L.; Paul, R.E.; Ly, S.; Lay, R.S.; Long, K.C.; Huy, R.; Tarantola, A.; Scott, T.W.; Sakuntabhai, A.; et al. Asymptomatic Humans Transmit Dengue Virus to Mosquitoes. Proc. Natl. Acad. Sci. USA 2015, 112, 14688–14693. [Google Scholar] [CrossRef]
- IBGE-Brazilian Institute of Geography and Statistics. Cities and States—Rio de Janeiro. Available online: https://www.ibge.gov.br/cidades-e-estados/rj/rio-de-janeiro.html (accessed on 8 November 2023).
- WMP—World Mosquito Program. Global Progress—Brazil—Rio de Janeiro. Available online: www.worldmosquitoprogram.org/en/global-progress/brazil/niteroi (accessed on 8 November 2023).
- IBGE—Brazilian Institute of Geography and Statistics. Cities and States—Niterói. Available online: https://www.ibge.gov.br/cidades-e-estados/rj/niteroi.html (accessed on 8 November 2023).
- WMP—World Mosquito Program. Global Progress—Brazil—Rio de Janeiro. Available online: https://www.worldmosquitoprogram.org/en/global-progress/brazil/rio-de-janeiro (accessed on 8 November 2023).
- Gesto, J.S.M.; Pinto, S.B.; Dias, F.B.S.; Peixoto, J.; Costa, G.; Kutcher, S.; Montgomery, J.; Green, B.R.; Anders, K.L.; Ryan, P.A.; et al. Large-Scale Deployment and Establishment of Wolbachia Into the Aedes aegypti Population in Rio de Janeiro, Brazil. Front. Microbiol. 2021, 12, 711107. [Google Scholar] [CrossRef]
- Consoli, R.A.G.B.; Lourenço-de-Oliveira, R. Principais Mosquitos de Importância Sanitária No Brasil; Editora FIOCRUZ: Rio de Janeiro, Brazil, 1994. [Google Scholar]
- Dutra, H.L.C.; Rocha, M.N.; Dias, F.B.S.; Mansur, S.B.; Caragata, E.P.; Moreira, L.A. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 2016, 19, 771–774. [Google Scholar] [CrossRef]
- Garcia, G.A.; Lord, A.R.; Santos, L.M.B.; Kariyawasam, T.N.; David, M.R.; Couto-Lima, D.; Tátila-Ferreira, A.; Pavan, M.G.; Sikulu-Lord, M.T.; Maciel-de-Freitas, R. Rapid and Non-Invasive Detection of Aedes aegypti Co-Infected with Zika and Dengue Viruses Using Near Infrared Spectroscopy. Viruses 2022, 15, 11. [Google Scholar] [CrossRef]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-Specific Detection of Dengue Viruses in a Fourplex Real-Time Reverse Transcriptase PCR Assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2021. [Google Scholar]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; van den Hurk, A.; McGraw, E.A.; O’Neill, S.L. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLoS Negl. Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef]
- Ye, Y.H.; Carrasco, A.M.; Frentiu, F.D.; Chenoweth, S.F.; Beebe, N.W.; van den Hurk, A.F.; Simmons, C.P.; O’Neill, S.L.; McGraw, E.A. Wolbachia Reduces the Transmission Potential of Dengue-Infected Aedes aegypti. PLoS Negl. Trop. Dis. 2015, 9, e0003894. [Google Scholar] [CrossRef] [PubMed]
- Terradas, G.; Allen, S.L.; Chenoweth, S.F.; McGraw, E.A. Family Level Variation in Wolbachia-Mediated Dengue Virus Blocking in Aedes aegypti. Parasit. Vectors 2017, 10, 622. [Google Scholar] [CrossRef] [PubMed]
- Novelo, M.; Audsley, M.D.; McGraw, E.A. The Effects of DENV Serotype Competition and Co-Infection on Viral Kinetics in Wolbachia-Infected and Uninfected Aedes aegypti Mosquitoes. Parasit. Vectors 2021, 14, 314. [Google Scholar] [CrossRef] [PubMed]
- Souto-Maior, C.; Sylvestre, G.; Braga Stehling Dias, F.; Gomes, M.G.M.; Maciel-de-Freitas, R. Model-Based Inference from Multiple Dose, Time Course Data Reveals Wolbachia Effects on Infection Profiles of Type 1 Dengue Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2018, 12, e0006339. [Google Scholar] [CrossRef]
- Thi Hue Kien, D.; Edenborough, K.; da Silva Goncalves, D.; Thuy Vi, T.; Casagrande, E.; Thi Le Duyen, H.; Thi Long, V.; Thi Dui, L.; Thi Tuyet Nhu, V.; Thi Giang, N.; et al. Genome Evolution of Dengue Virus Serotype 1 under Selection by Wolbachia Pipientis in Aedes aegypti Mosquitoes. Virus Evol. 2023, 9, vead016. [Google Scholar] [CrossRef]
- Ye, Y.H.; Woolfit, M.; Rancès, E.; O’Neill, S.L.; McGraw, E.A. Wolbachia-Associated Bacterial Protection in the Mosquito Aedes aegypti. PLoS Negl. Trop. Dis. 2013, 7, e2362. [Google Scholar] [CrossRef]
- Frentiu, F.D.; Robinson, J.; Young, P.R.; McGraw, E.A.; O’Neill, S.L. Wolbachia-Mediated Resistance to Dengue Virus Infection and Death at the Cellular Level. PLoS ONE 2010, 5, e13398. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bian, G.; Pan, X.; Xi, Z. Wolbachia Induces Density-Dependent Inhibition to Dengue Virus in Mosquito Cells. PLoS Negl. Trop. Dis. 2012, 6, e1754. [Google Scholar] [CrossRef]
- Osborne, S.E.; Iturbe-Ormaetxe, I.; Brownlie, J.C.; O’Neill, S.L.; Johnson, K.N. Antiviral Protection and the Importance of Wolbachia Density and Tissue Tropism in Drosophila simulans. Appl. Environ. Microbiol. 2012, 78, 6922–6929. [Google Scholar] [CrossRef]
- Ulrich, J.N.; Beier, J.C.; Devine, G.J.; Hugo, L.E. Heat Sensitivity of WMel Wolbachia during Aedes aegypti Development. PLoS Negl. Trop. Dis. 2016, 10, e0004873. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Axford, J.K.; Yang, Q.; Staunton, K.M.; Ritchie, S.A.; Richardson, K.M.; Hoffmann, A.A. Heatwaves Cause Fluctuations in WMel Wolbachia Densities and Frequencies in Aedes aegypti. PLoS Negl. Trop. Dis. 2020, 14, e0007958. [Google Scholar] [CrossRef] [PubMed]
- Axford, J.K.; Ross, P.A.; Yeap, H.L.; Callahan, A.G.; Hoffmann, A.A. Fitness of WAlbB Wolbachia Infection in Aedes aegypti: Parameter Estimates in an Outcrossed Background and Potential for Population Invasion. Am. J. Trop. Med. Hyg. 2016, 94, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.E.; De Bruyne, J.T.; Iturbe-Ormaetxe, I.; Stepnell, J.; Burns, R.L.; Flores, H.A.; O’Neill, S.L. Novel Wolbachia-Transinfected Aedes aegypti Mosquitoes Possess Diverse Fitness and Vector Competence Phenotypes. PLoS Pathog. 2017, 13, e1006751. [Google Scholar] [CrossRef] [PubMed]
- Ant, T.H.; Herd, C.S.; Geoghegan, V.; Hoffmann, A.A.; Sinkins, S.P. The Wolbachia Strain WAu Provides Highly Efficient Virus Transmission Blocking in Aedes aegypti. PLoS Pathog. 2018, 14, e1006815. [Google Scholar] [CrossRef] [PubMed]
- Caragata, E.P.; Rancès, E.; O’Neill, S.L.; McGraw, E.A. Competition for Amino Acids between Wolbachia and the Mosquito Host, Aedes aegypti. Microb. Ecol. 2014, 67, 205–218. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Iturbe-Ormaetxe, I.; Callahan, A.G.; Phillips, B.L.; Billington, K.; Axford, J.K.; Montgomery, B.; Turley, A.P.; O’Neill, S.L. Stability of the WMel Wolbachia Infection Following Invasion into Aedes aegypti Populations. PLoS Negl. Trop. Dis. 2014, 8, e3115. [Google Scholar] [CrossRef]
- Ross, P.A.; Robinson, K.L.; Yang, Q.; Callahan, A.G.; Schmidt, T.L.; Axford, J.K.; Coquilleau, M.P.; Staunton, K.M.; Townsend, M.; Ritchie, S.A.; et al. A Decade of Stability for WMel Wolbachia in Natural Aedes aegypti Populations. PLoS Pathog. 2022, 18, e1010256. [Google Scholar] [CrossRef]
- Dutra, H.L.C.; Ford, S.A.; Allen, S.L.; Bordenstein, S.R.; Chenoweth, S.F.; Bordenstein, S.R.; McGraw, E.A. The Impact of Artificial Selection for Wolbachia-Mediated Dengue Virus Blocking on Phage WO. PLoS Negl. Trop. Dis. 2021, 15, e0009637. [Google Scholar] [CrossRef]
- Amuzu, H.E.; McGraw, E.A. Wolbachia-Based Dengue Virus Inhibition Is Not Tissue-Specific in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0005145. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of WMel Wolbachia in Aedes aegypti Mosquitoes and Reduction of Local Dengue Transmission in Cairns and Surrounding Locations in Northern Queensland, Australia. Gates Open Res. 2019, 3, 1547. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Nguyen, H.L.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S.; et al. Field Evaluation of the Establishment Potential of WMelPop Wolbachia in Australia and Vietnam for Dengue Control. Parasit. Vectors 2015, 8, 563. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.A.; Hoffmann, A.A.; Maciel-de-Freitas, R.; Villela, D.A.M. Aedes aegypti Insecticide Resistance Underlies the Success (and Failure) of Wolbachia Population Replacement. Sci. Rep. 2020, 10, 63. [Google Scholar] [CrossRef]
- Pavan, M.G.; Garcia, G.A.; David, M.R.; Maciel-de-Freitas, R. The Double-Edged Sword Effect of Expanding Wolbachia Deployment in Dengue Endemic Settings. Lancet Reg. Health Am. 2023, 27, 100610. [Google Scholar] [CrossRef]
- ECDC Dengue Worldwide Overview. Available online: https://www.ecdc.europa.eu/en/dengue-monthly (accessed on 28 September 2023).
- United Nations. Department of Economic and Social Affairs Population Division. World Population Prospects: The 2022 Revision. Available online: https://population.un.org/wpp/ (accessed on 28 September 2023).


| DENV-1 Infection Rate | ||
|---|---|---|
| Areas | Low Titer | High Titer |
| NR1 | 80% (8/10) | 77.77% (7/9) |
| PI1 | 50% (2/4) | 50% (3/6) |
| PI2 | 75% (6/8) | 50% (4/8) |
| FI | 66.66% (2/3) | 80% (8/10) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Antônio, J.; David, M.R.; Couto-Lima, D.; Garcia, G.A.; Keirsebelik, M.S.G.; Maciel-de-Freitas, R.; Pavan, M.G. DENV-1 Titer Impacts Viral Blocking in wMel Aedes aegypti with Brazilian Genetic Background. Viruses 2024, 16, 214. https://doi.org/10.3390/v16020214
Corrêa-Antônio J, David MR, Couto-Lima D, Garcia GA, Keirsebelik MSG, Maciel-de-Freitas R, Pavan MG. DENV-1 Titer Impacts Viral Blocking in wMel Aedes aegypti with Brazilian Genetic Background. Viruses. 2024; 16(2):214. https://doi.org/10.3390/v16020214
Chicago/Turabian StyleCorrêa-Antônio, Jessica, Mariana R. David, Dinair Couto-Lima, Gabriela Azambuja Garcia, Milan S. G. Keirsebelik, Rafael Maciel-de-Freitas, and Márcio Galvão Pavan. 2024. "DENV-1 Titer Impacts Viral Blocking in wMel Aedes aegypti with Brazilian Genetic Background" Viruses 16, no. 2: 214. https://doi.org/10.3390/v16020214
APA StyleCorrêa-Antônio, J., David, M. R., Couto-Lima, D., Garcia, G. A., Keirsebelik, M. S. G., Maciel-de-Freitas, R., & Pavan, M. G. (2024). DENV-1 Titer Impacts Viral Blocking in wMel Aedes aegypti with Brazilian Genetic Background. Viruses, 16(2), 214. https://doi.org/10.3390/v16020214







