Factors Associated with SARS-CoV-2 Infection in Fully Vaccinated Nursing Home Residents and Workers
Abstract
1. Introduction
2. Methods
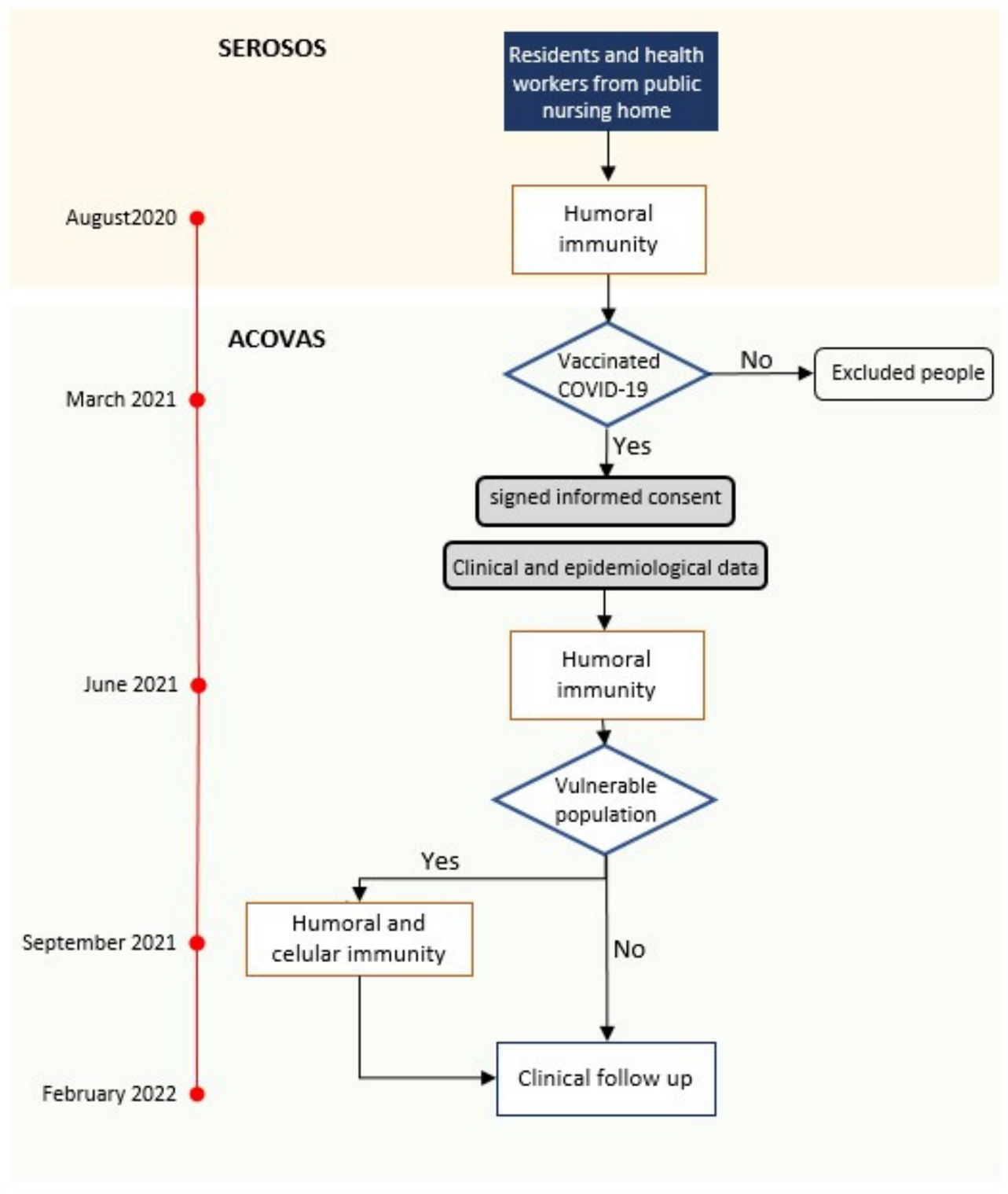
2.1. Study Design
2.2. Population
2.3. Laboratory Analysis
2.4. Statistical Analysis
3. Results
3.1. Clinical Parameters
3.2. Immunological Parameters
| Residents (267) | Staff (302) | p | |||
| August 2020 | Concentration of anti-spike antibodies (mean [SD] AU/µL) | 1.5 [3.2] | 0.4 [1.8] | <0.01 | |
| Proportion of positive serologies (%) | 203 (76.0) | 106 (35.1 | <0.01 | ||
| June 2021 | Concentration of anti-spike IgG antibodies (mean [SD] AU/µL) | 15.7 [17.9] | 7.7 [12.7] | <0.01 | |
| Positive serologies (%) | 267 (100) | 301 (99.7) | 0.4 | ||
| September 2021 | Concentration of anti-spike IgG antibodies (mean [SD] AU/µL) | 9.4 [12.7] | 2.3 [9.4] | <0.01 | |
| Positive serologies (%) | 189 (100) | 58 (100) | 1 | ||
| Proportion of neutralizing antibodies (mean % [SD]) | 63.3 [34.1] | 17.7 [17.2] | <0.01 | ||
| Celullar immunity (IGRA positivity) | CD4+ (%) | 117 (62.2) | 24 (39.3) | <0.01 | |
| CD8+ (%) | 71 (37.8) | 7 (11.5) | <0.01 | ||
| CD4+/CD8+ (%) | 123 (65.4) | 24 (39.3) | <0.01 | ||
| History of COVID-19 | |||||||
|---|---|---|---|---|---|---|---|
| Residents | Staff | ||||||
| Yes | No | p | Yes | No | p | ||
| No. of participants | 206 | 59 | 122 | 164 | |||
| August 2020 | Concentration of anti-spike IgG antibodies (mean [SD] AU/µL) | 1.76 [3.48] | 0.69 [1.30] | <0.01 | 0.94 [2.68] | 0.07 [0.36] | <0.01 |
| Proportion of positive serologies (%) | 83.5 | 52.5 | <0.01 | 63.9 | 14.0 | <0.01 | |
| June 2021 | Concentration of anti-spike IgG antibodies (mean [SD] AU/µL) | 20.65 [25.23] | 8.83 [12.57] | <0.01 | 16.63 [22.32] | 3.13 [6.81] | <0.01 |
| Proportion of positive serologies (%) | 100 | 100 | 1 | 100 | 100 | 1 | |
| Elevation in the concentration of anti-spike IgG antibodies from August 2020 | 18.89 [23.79] | 8.14 [12.07] | <0.01 | 15.67 [21.21] | 3.06 [6.75] | <0.01 | |
3.3. The Omicron Epidemic
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Candel, F.J.; Barreiro, P.; San Román, J.; Carretero, M.d.M.; Sanz, J.C.; Pérez-Abeledo, M.; Ramos, B.; Viñuela-Prieto, J.M.; Canora, J.; Martínez-Peromingo, F.J.; et al. The demography and characteristics of SARS-CoV-2 seropositive residents and staff of nursing homes for older adults in the Community of Madrid: The SeroSOS study. Age Ageing 2021, 50, 1038–1047. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; Fernández de Larrea, N.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; de la Fuente, S.; Mills, P.; Muñoz, A.; Muñez, E.; Ramos, A.; Fernández-Cruz, A.; Arias, A.; Pintos, I.; Vargas, J.A.; et al. Major determinants of death in patients hospitalized with COVID-19 during the first epidemic wave in Madrid, Spain. Medicine 2021, 100, e25634. [Google Scholar] [CrossRef]
- San Román, J.; Candel, F.J.; Carretero, M.d.M.; Sanz, J.C.; Pérez-Abeledo, M.; Barreiro, P.; Viñuela-Prieto, J.M.; Ramos, B.; Canora, J.; Barba, R.; et al. Cross-sectional analysis of risk factors for outbreak of COVID-19 in nursing homes for older adults in the Community of Madrid. Gerontology 2023, 69, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Menéndez Colino, R.; Merello de Miguel, A.; Argentina, F.; Barcons Marques, M.; Chaparro Jiménez, B.; López Hernández, P.; Jiménez Bueno, S.; Montero Vega, M.D.; García Rodríguez, J.; Ferrer Simo, B.; et al. Evolución de la COVID-19 en las residencias de personas mayores desde la según ola hasta la vacunación. Descripción de un programa de coordinación entre atención primaria, geriatría y salud pública. Rev. Esp. Salud Pública 2021, 95, e1–e11. [Google Scholar]
- Mateos-Nozal, J.; Galán Montemayor, J.C.; Lores Torres, S.; Barreiro, P.; Paños Zamora, A.; Martín Martínez, F.; García Castelo, D.; Rodríguez-Domínguez, M.; Martínez Peromingo, F.J.; Cruz-Jentoft, A.J. SARS-CoV-2 B1.1.7 Variant outbreak in a fully vaccinated nursing home—Madrid, June 2021. J. Am. Med. Dir. Assoc. 2021, 22, 2266–2268. [Google Scholar] [CrossRef] [PubMed]
- Dagan, N.; Barda, N.; Kepten, E.; Miron, O.; Perchik, S.; Katz, M.A.; Hernán, M.A.; Lipsitch, M.; Reis, B.; Balicer, R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021, 384, 1412–1423. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.; Haas, E.J.; Milo, R.; Alroy-Preis, S.; Ash, N.; Huppert, A. Waning Immunity after the BNT162b2 Vaccine in Israel. N. Engl. J. Med. 2021, 385, e85. [Google Scholar] [CrossRef] [PubMed]
- Mlcochova, P.; Kemp, S.A.; Dhar, M.S.; Papa, G.; Meng, B.; Ferreira, I.A.T.M.; Datir, R.; Collier, D.A.; Albecka, A.; Singh, S.; et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature 2021, 599, 114–119. [Google Scholar] [CrossRef]
- Tian, D.; Sun, Y.; Xu, H.; Ye, Q. The emergence and epidemic characteristics of the highly mutated SARS-CoV-2 Omicron variant. J. Med. Virol. 2022, 94, 2376–2383. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. SARS-CoV-2 Variants of Concern as of 27 July 2023. Available online: https://www.ecdc.europa.eu/en/covid-19/variants-concern (accessed on 26 July 2023).
- Nemet, I.; Kliker, L.; Lustig, Y.; Zuckerman, N.; Erster, O.; Cohen, C.; Kreiss, Y.; Alroy-Preis, S.; Regev-Yochay, G.; Mendelson, E.; et al. Third BNT162b2 Vaccination Neutralization of SARS-CoV-2 Omicron Infection. N. Engl. J. Med. 2022, 386, 492–494. [Google Scholar] [CrossRef]
- Regev-Yochay, G.; Gonen, T.; Gilboa, M.; Mandelboim, M.; Indenbaum, V.; Amit, S.; Meltzer, L.; Asraf, K.; Cohen, C.; Fluss, R.; et al. Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. N. Engl. J. Med. 2022, 386, 1377–1380. [Google Scholar] [CrossRef]
- Suzuki, R.; Yamasoba, D.; Kimura, I.; Wang, L.; Kishimoto, M.; Ito, J.; Morioka, Y.; Nao, N.; Nasser, H.; Uriu, K.; et al. Attenuated fusogenicity and pathogenicity of SARS-CoV-2 Omicron variant. Nature 2022, 603, 700–705. [Google Scholar] [CrossRef]
- Shuai, H.; Chan, J.F.; Hu, B.; Chai, Y.; Yuen, T.T.; Yin, F.; Huang, X.; Yoon, C.; Hu, J.C.; Liu, H.; et al. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature 2022, 603, 693–699. [Google Scholar] [CrossRef]
- Shao, J.; Fan, R.; Hu, J.; Zhang, T.; Lee, C.; Huang, X.; Wang, F.; Liang, H.; Jin, Y.; Jiang, Y.; et al. Clinical Progression and Outcome of Hospitalized Patients Infected with SARS-CoV-2 Omicron Variant in Shanghai, China. Vaccines 2022, 10, 1409. [Google Scholar] [CrossRef]
- Maslo, C.; Friedland, R.; Toubkin, M.; Laubscher, A.; Akaloo, T.; Kama, B. Characteristics and Outcomes of Hospitalized Patients in South Africa During the COVID-19 Omicron Wave Compared with Previous Waves. JAMA 2022, 327, 583–584. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirseborn, F.; Toffa, S.; Rickeard, T.; Gallagher, E. COVID-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N. Engl. J. Med. 2022, 386, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Pajon, R.; Doria-Rose, N.A.; Shen, X.; Schmidt, S.D.; O’Dell, S.; McDanal, C.; Feng, W.; Tong, J.; Eaton, A.; Maglinao, M.; et al. SARS-CoV-2 Omicron Variant Neutralization after mRNA-1273 Booster Vaccination. N. Engl. J. Med. 2022, 386, 1088–1091. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Beltran, W.F.; St Denis, K.J.; Hoelzemer, A.; Lam, E.C.; Nitido, A.D.; Sheehan, M.L.; Berrios, C.; Ofoman, O.; Chang, C.C.; Hauser, B.M.; et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 Omicron variant. Cell 2022, 185, 457–466. [Google Scholar] [CrossRef] [PubMed]
- UK Health Security Agency. SARS-CoV-2 Variants of Concern and Variants under Investigation in England Technical Briefing: Update on Hospitalization and Vaccine Effectiveness for Omicron VOC-21 NOV01 (B.1.1.529). Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1044481/Technical-Briefing-31-Dec-2021-Omicron_severity_update.pdf (accessed on 30 July 2023).
- Barda, N.; Dagan, N.; Cohen, C.; Hernán, M.A.; Lipsitch, M.; Kohane, I.S.; Reis, B.Y.; Balicer, R.D. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: An observational study. Lancet 2021, 398, 2093–2100. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Nozal, J.; Pérez Panizo, N.; Zárate-Sáez, C.M.; Vaquero-Pinto, M.N.; Roldán-Plaza, C.; Mejía Ramírez-Arellano, M.V.; Sánchez García, E.; Garza-Martínez, A.J.; Cruz-Jentoft, A.J. Proactive geriatric comanagement of nursing home patients by a new hospital-based Liaison Geriatric Unit: A new model for the future. J. Am. Med. Dir. Assoc. 2022, 23, 308–310. [Google Scholar] [CrossRef]
- Wang, E.; Liu, A.; Wang, Z.; Shang, X.; Zhang, L.; Jin, Y.; Ma, Y.; Zhang, L.; Bai, T.; Song, J.; et al. The prognostic value of the Barthel Index for mortality in patients with COVID-19: A cross-sectional study. Front. Public Health 2023, 10, 978237. [Google Scholar] [CrossRef] [PubMed]
- Vinyoles, E.; Vila, J.; Argimon, J.M.; Espinàs, J.; Abos, T.; Limón, E. Concordance among Mini-Examen Cognoscitivo and Mini-Mental State Examination in cognitive impairment screening. Aten Primaria 2002, 30, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Shin, S.; Nam, M.; Hong, Y.J.; Roh, E.Y.; Park, K.U.; Song, E.Y. Performance evaluation of three automated quantitative immunoassays and their correlation with a surrogate virus neutralization test in coronavirus disease 19 patients and pre-pandemic controls. J. Clin. Lab. Anal. 2021, 35, e23921. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, P.; Sanz, J.C.; San Román, J.; Pérez-Abeledo, M.; Carretero, M.; Megías, G.; Viñuela-Prieto, J.M.; Ramos, B.; Canora, J.; Martínez-Peromingo, F.J.; et al. A Pilot Study for the Evaluation of an Interferon Gamma Release Assay (IGRA) To Measure T-Cell Immune Responses after SARS-CoV-2 Infection or Vaccination in a Unique Cloistered Cohort. J. Clin. Microbiol. 2022, 60, e0219921. [Google Scholar] [CrossRef]
- Red de Vigilancia Epidemiológica. Informe Epidemiológico Semanal Comunidad de Madrid. Semana 32. 16 August 2023. Available online: https://www.comunidad.madrid/sites/default/files/doc/sanidad/epid/informe_epidemiologico_semanal.pdf (accessed on 18 August 2023).
- Kaufman, H.W.; Letovsky, S.; Meyer, W.A., 3rd; Gillim, L.; Assimon, M.M.; Kabelac, C.A.; Kroner, J.W.; Reynolds, S.L.; Eisenberg, M. SARS-CoV-2 spike-protein targeted serology test results and their association with subsequent COVID-19-related outcomes. Front. Public Health 2023, 11, 1193246. [Google Scholar] [CrossRef] [PubMed]
- Aalto, U.L.; Pitkälä, K.H.; Andersen-Ranberg, K.; Bonin-Guillaume, S.; Cruz-Jentoft, A.J.; Eriksdotter, M.; Gordon, A.L.; Gosch, M.; Holmerova, I.; Kautiainen, H.; et al. COVID-19 pandemic and mortality in nursing homes across USA and Europe up to October 2021. Eur. Geriatr. Med. 2022, 13, 705–709. [Google Scholar] [CrossRef]
- Hernandez-Suarez, C.; Murillo-Zamora, E. Waning immunity to SARS-CoV-2 following vaccination or infection. Front. Med. 2022, 9, 972083. [Google Scholar] [CrossRef]
- Blain, H.; Tuaillon, E.; Gamon, L.; Pisoni, A.; Miot, S.; Rolland, Y.; Picot, M.; Bousquet, J. Antibody response after one and two jabs of the BNT162b2 vaccine in nursing home residents: The CONsort-19 study. Allergy 2021, 77, 271–281. [Google Scholar] [CrossRef]
- Primorac, D.; Brlek, P.; Matišić, V.; Molnar, V.; Vrdoljak, K.; Zadro, R.; Parčina, M. Cellular Immunity-The Key to Long-Term Protection in Individuals Recovered from SARS-CoV-2 and after Vaccination. Vaccines 2022, 10, 442. [Google Scholar] [CrossRef]
- Van Praet, J.T.; Vandecasteele, S.; De Roo, A.; Vynck, M.; De Vriese, A.S.; Reynders, M. Dynamics of the cellular and humoral immune response after BNT162b2 messenger ribonucleic acid coronavirus disease2019 (COVID-19) vaccination in COVID-19 naïve nursing home residents. J. Infect. Dis. 2021, 224, 1690–1693. [Google Scholar]
- Achiron, A.; Gurevich, M.; Falb, R.; Dreyer-Alster, S.; Sonis, P.; Mandel, M. SARS-CoV-2 antibody dynamics and B-cell memory response over time in COVID-19 convalescent subjects. Clin. Microbiol. Infect. 2021, 27, 1349.e1–1349.e6. [Google Scholar] [CrossRef]
- IMSERSO. Actualización nº 101. Enfermedad por Coronavirus (COVID-19) en Centros Residenciales. 29 January 2023. Available online: https://imserso.es/documents/20123/117116/Inf_resid_20230129.pdf/a7dfa113-f148-a028-88ad-23fa85009e60 (accessed on 1 October 2023).
- Pierobon, S.; Braggion, M.; Fedeli, U.; Nordio, M.; Basso, C.; Zorzi, M. Impact of vaccination on the spread of SARS-CoV-2 infection in north-east Italy nursing homes. A propensity score and risk analysis. Age Ageing 2022, 51, afab224. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; King, T.; Zheng, Y.M.; Oltz, E.M.; Whelan, S.P.J.; Saif, L.J.; Peeples, M.E.; Liu, S.L. SARS-CoV-2 spreads through cell-to-cell transmission. Proc. Natl. Acad. Sci. USA 2022, 119, e2111400119. [Google Scholar] [CrossRef]
- Sekine, T.; Perez-Potti, A.; Rivera-Ballesteros, O.; Strålin, K.; Gorin, J.B.; Olsson, A.; Llewellyn-Lacey, S.; Kamal, H.; Bogdanovic, G.; Muschiol, S.; et al. Robust T Cell Immunity in Convalescent Individuals with Asymptomatic or Mild COVID-19. Cell 2020, 183, 158–168. [Google Scholar] [CrossRef]
- Koyama, T.; Miyakawa, K.; Tokumasu, R.; Jeremiah, S.S.; Kudo, M.; Ryo, A. Evasion of vaccine-induced humoral immunity by emerging sub-variants of SARS-CoV-2. Future Microbiol. 2022, 17, 417–424. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Adrielle Dos Santos, L.; Filho, P.G.G.; Silva, A.M.F.; Santos, J.V.G.; Santos, D.S.; Aquino, M.M.; de Jesus, R.M.; Almeida, M.L.D.; da Silva, J.S.; et al. Recurrent COVID-19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J. Infect. 2021, 82, 399–406. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Q.; Gu, X.; Ren, L.; Huang, T.; Li, Y.; Zhang, H.; Liu, Y.; Zhong, J.; Wang, X.; et al. Durability and cross-reactive immune memory to SARS-CoV-2 in individuals 2 years after recovery from COVID-19: A longitudinal cohort study. Lancet Microbe 2023, 5, e24–e33. [Google Scholar] [CrossRef] [PubMed]
- Candel, F.J.; Barreiro, P.; San Román, J.; Abanades, J.C.; Barba, R.; Barberán, J.; Bibiano, C.; Canora, J.; Cantón, R.; Calvo, C.; et al. Recommendations for use of antigenic tests in the diagnosis of acute SARS-CoV-2 infection in the second pandemic wave: Attitude in different clinical settings. Rev. Esp. Quimioter. 2020, 33, 466–484. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, A.C.; Vrancken, B.; Haralambous, C.; Alexandrou, M.; Gregoriou, I.; Ioannides, M.; Ioannou, C.; Kalakouta, O.; Karagiannis, C.; Marcou, M.; et al. Unraveling the dynamics of Omicron (BA.1, BA.2, and BA.5) waves and emergence of the Deltacton variant: Genomic epidemiology of the SARS-CoV-2 epidemic in Cyprus (Oct 2021–Oct 2022). Viruses 2023, 15, 1933. [Google Scholar] [CrossRef] [PubMed]
| Residents | Staff | p | |
|---|---|---|---|
| No | 267 | 302 | |
| Age (mean [SD] years) | 87.6 [7.7] | 50.7 [10.3] | <0.01 |
| Females (%) | 217 (81.3) | 248 (82.1) | 0.6 |
| Low weight (%) | 65 (24.5) | 4 (1.4) | <0.01 |
| Obesity (%) | 68 (25.7) | 39 (13.9) | <0.01 |
| Hypertension (%) | 180 (67.7) | 55 (19.2) | <0.01 |
| Diabetes mellitus (%) | 64 (24.0) | 8 (2.8) | <0.01 |
| Dyslipidemia (%) | 69 (25.9) | 54 (18.9) | <0.05 |
| COPD (%) | 26 (9.8) | 10 (3.5) | <0.05 |
| Heart disease (%) | 121 (45.7) | 10 (3.5) | <0.01 |
| Active malignancy (%) | 18 (6.8) | 2 (0.7) | <0.01 |
| Immunodepression (%) | 8 (3.0) | 9 (3.1) | 0.9 |
| Barthel score (mean [SD] points) | 39.0 [40.0] | -- | -- |
| Lobo-MMT score (mean [SD] points) | 12.6 [10.9] | -- | -- |
| No of comorbidities (mean [SD]) | 3.0 [1.3] | 0.7 [0.9] | <0.01 |
| COVID-19 before June 2021 (%) | 206 (77.7) | 122 (42.7) | <0.01 |
| COVID-19 before September 2021 (%) | 206 (77.7) | 125 (43.0) | <0.01 |
| OR (95% CI), p | |
|---|---|
| Age (per year) | 1.00 (0.98–1.03), 0.7 |
| Sex (male vs. female) | 0.53 (0.25–1.12), 0.1 |
| History of COVID-19 | 1.35 (0.70–2.61), 0.4 |
| Concentration of anti-spike antibodies (per AU/mL) | 1.02 (0.99–1.06), 0.2 |
| Proportion of neutralizing anti-spike antibodies (per point) | 1.02 (1.01–1.03), <0.001 |
| All (%) | Residents (%) | Staff (%) | p | Vulnerable (%) | Not-Vulnerable (%) | p | |
|---|---|---|---|---|---|---|---|
| SARS-CoV-2 infection | 82 (14.4) | 32 (11.9) | 50 (16.6) | 0.1 | 32 (13.0) | 50 (15.5) | 0.4 |
| Any cause death | 37 (6.5) | 37 (13.9) | 0 (0) | <0.001 | 26 (10.5) | 11 (3.4) | 0.001 |
| SARS-CoV2 Infection | Any Cause Death | |||||
|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | |
| No. (%) | 32 (11.9) | 235 (88.1) | - | 37 (13.9) | 229 (86.1) | - |
| Females (%) | 28 (87.5) | 189 (80.4) | 0.3 | 26 (70.3) | 191 (83.0) | 0.06 |
| Low weight (%) | 6 (18.8) | 59 (25.3) | 0.4 | 16 (44.4) | 49 (21.4) | 0.003 |
| Obesity (%) | 6 (18.8) | 62 (26.6) | 0.3 | 6 (16.7) | 62 (27.1) | 0.2 |
| Hypertension (%) | 21 (65.6) | 159 (67.9) | 0.8 | 24 (64.9) | 156 (68.1) | 0.7 |
| Diabetes mellitus (%) | 12 (37.5) | 52 (22.1) | 0.06 | 7 (18.9) | 57 (24.8) | 0.4 |
| Dyslipidemia (%) | 12 (37.5) | 57 (24.4) | 0.1 | 6 (16.2) | 63 (27.5) | 0.1 |
| COPD (%) | 0 (0) | 26 (11.1) | 0.05 | 2 (5.4) | 24 (10.5) | 0.3 |
| Heart disease (%) | 7 (21.9) | 114 (48.9) | 0.004 | 19 (52.8) | 102 (44.5) | 0.4 |
| Active malignancy (%) | 2 (6.3) | 16 (6.9) | 0.9 | 6 (16.7) | 12 (5.2) | 0.01 |
| Immunodepression (%) | 0 (0) | 8 (3.4) | 0.3 | 0 (0.0) | 8 (3.5) | 0.3 |
| Barthel score (mean [SD] points) | 38.9 (32.1) | 39.0 (30.7) | 0.9 | 25.6 (27.6) | 41.1 (30.7) | 0.1 |
| Dementia (%) | 25 (78.1) | 162 (69.2) | 0.3 | 29 (80.6) | 158 (68.7) | 0.1 |
| Lobo-MMT score (mean [SD] points) | 9.2 (11.2) | 13.1 (10.8) | 0.1 | 9.8 (9.8) | 13.1 (11.0) | 0.2 |
| No. of comorbidities (mean [SD]) | 2.8 (1.2) | 3.1 (1.3) | 0.4 | 3.1 (1.3) | 3.0 (1.3) | 0.9 |
| COVID-19 before June 2021 (%) | 24 (75.0) | 182 (78.1) | 0.7 | 27 (75.0) | 179 (78.2) | 0.7 |
| COVID-19 before September 2021 (%) | 24 (75.0) | 182 (78.1) | 0.7 | 27 (75.0) | 179 (78.2) | 0. 7 |
| SARS-CoV2 Infection | Any Cause Death | |||||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | |||
| No (%) | 32 (12) | 235 (88) | - | 37 (13.9) | 230 (86.1) | - | ||
| August 2020 | IgG anti-spike antibodies (mean [SD] AU/µL) | 1.9 [4.6] | 1.4 [2.9] | 0.4 | 1.5 [4.0] | 1.5 [3.0] | 0.5 | |
| Positive serologies (%) | 26 (81.3) | 177 (75.3) | 0.5 | 28 (75.7) | 175 (76.1) | 1 | ||
| June 2021 | IgG anti-spike antibodies (mean [SD] AU/µL) | 13.7 [16.7] | 16.0 [18.0] | 0.5 | 14.2 [19.2] | 18.5 [24.1] | 0.5 | |
| Positive serologies (%) | 32 (100) | 235 (100) | 1 | 37 (100) | 230 (100) | 1 | ||
| September 2021 | IgG anti-spike antibodies (mean [SD] AU/µL) | 8.4 [3.4] | 9.5 [12.7] | 0.7 | 7.1 [7.0] | 10.3 [16.2] | 0.5 | |
| Positive serologies (%) | 21 (100) | 168 (100) | 1 | 20 (100) | 169 (100) | 1 | ||
| Proportion ofneutralizing antibodies (mean % [SD]) | 62.4 [33.0] | 63.4 [34.3] | 0.9 | 63.7 [38.3] | 63.2 [33.7] | 0.9 | ||
| Cellular immunity (positivity): | CD4+ (%) | 14 (66.7) | 103 (61.7) | 0.7 | 13 (65.0) | 104 (61.9) | 0.8 | |
| CD8+ (%) | 9 (42.9) | 62 (37.1) | 0.6 | 3 (15.0) | 68 (40.5) | 0.03 | ||
| CD4+/CD8+ (%) | 14 (66.7) | 109 (65.3) | 0.9 | 13 (65.0) | 123 (65.4) | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos-Nozal, J.; Rodríguez-Domínguez, M.; San Román, J.; Candel, F.J.; Villarrubia, N.; Pérez-Panizo, N.; Segura, E.; Cuñarro, J.M.; Ramírez-Arellano, M.V.M.; Rodríguez-Ramos, R.; et al. Factors Associated with SARS-CoV-2 Infection in Fully Vaccinated Nursing Home Residents and Workers. Viruses 2024, 16, 186. https://doi.org/10.3390/v16020186
Mateos-Nozal J, Rodríguez-Domínguez M, San Román J, Candel FJ, Villarrubia N, Pérez-Panizo N, Segura E, Cuñarro JM, Ramírez-Arellano MVM, Rodríguez-Ramos R, et al. Factors Associated with SARS-CoV-2 Infection in Fully Vaccinated Nursing Home Residents and Workers. Viruses. 2024; 16(2):186. https://doi.org/10.3390/v16020186
Chicago/Turabian StyleMateos-Nozal, Jesús, Mario Rodríguez-Domínguez, Jesús San Román, Francisco Javier Candel, Noelia Villarrubia, Nuria Pérez-Panizo, Esther Segura, Juan Manuel Cuñarro, Manuel V. Mejía Ramírez-Arellano, Rafael Rodríguez-Ramos, and et al. 2024. "Factors Associated with SARS-CoV-2 Infection in Fully Vaccinated Nursing Home Residents and Workers" Viruses 16, no. 2: 186. https://doi.org/10.3390/v16020186
APA StyleMateos-Nozal, J., Rodríguez-Domínguez, M., San Román, J., Candel, F. J., Villarrubia, N., Pérez-Panizo, N., Segura, E., Cuñarro, J. M., Ramírez-Arellano, M. V. M., Rodríguez-Ramos, R., Pariente-Rodríguez, R., Villar, L. M., Ramos, P., Cantón, R., Cruz-Jentoft, A. J., & Galán, J. C. (2024). Factors Associated with SARS-CoV-2 Infection in Fully Vaccinated Nursing Home Residents and Workers. Viruses, 16(2), 186. https://doi.org/10.3390/v16020186






