SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Virus
2.2. Recombinant Protein Expression and Purification
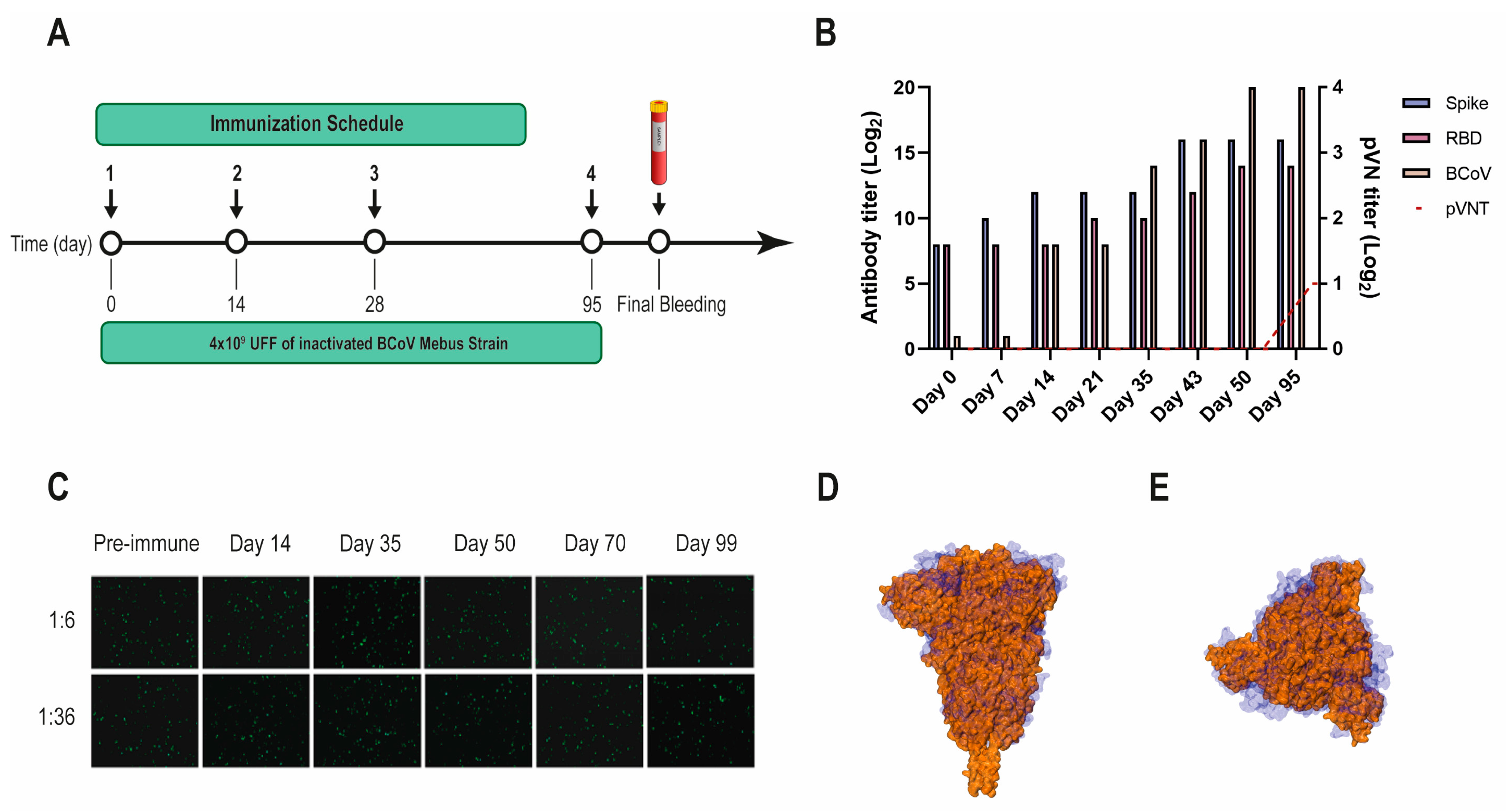
2.3. Llama Immunization and Library Construction
2.4. Isolation of SARS-CoV-2 S-2P- and RBD-Specific Nanobodies
2.5. Periplasmic Extract ELISA (PEE) and Phage ELISA (rPE)
2.6. Phylogenetic Analysis and Germline Origin
2.7. Expression and Purification of Nanobodies
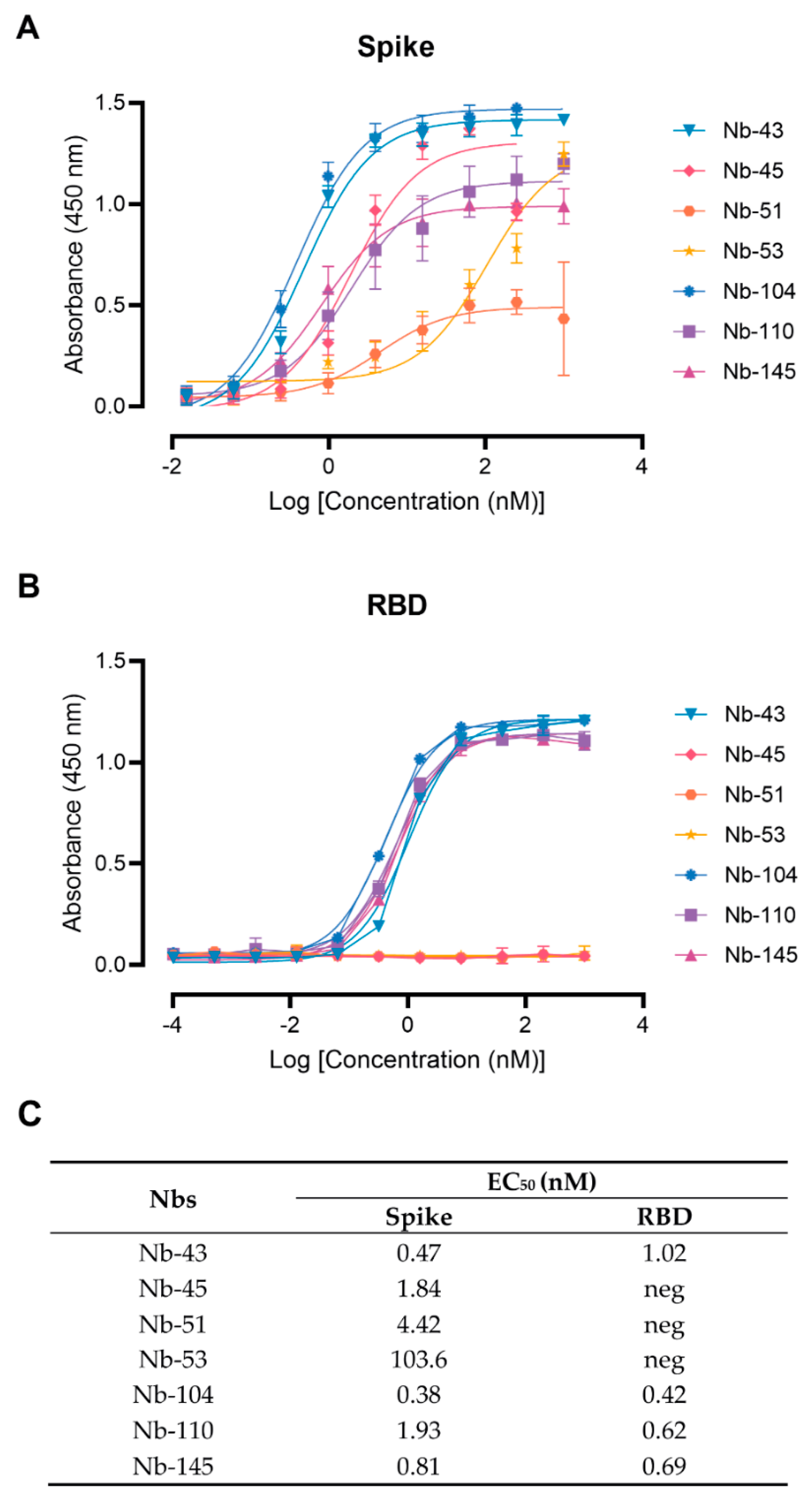
2.8. Binding Affinity to the Target Antigen Estimated by ELISA EC50
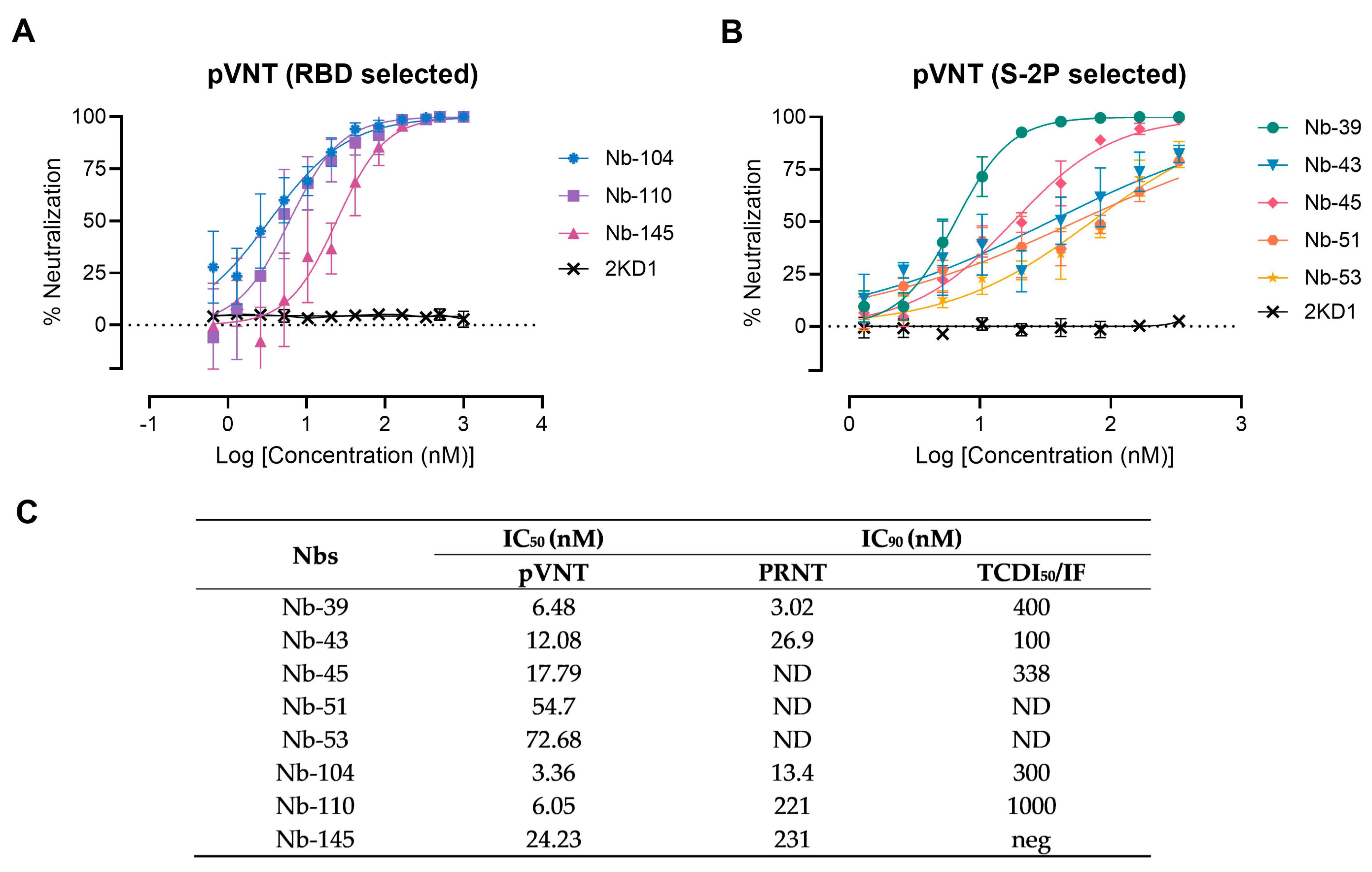
2.9. Pseudovirus Neutralization Test (pVNT)
2.10. Wild-Type Virus Neutralization Assays (VNA)
2.11. Competition of Nanobodies with ACE2
2.12. Interference of Nanobody Binding to Spike by Biliverdin
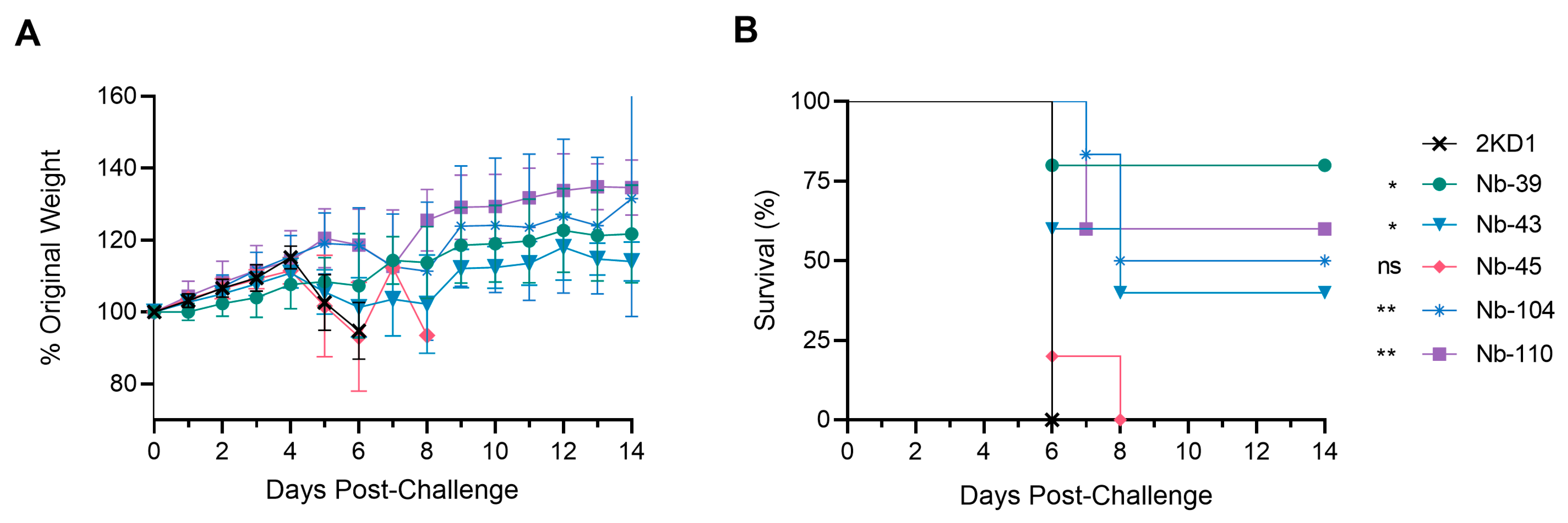
2.13. Efficacy of Nanobodies in a Mouse Model
2.14. In Silico Analysis
3. Results
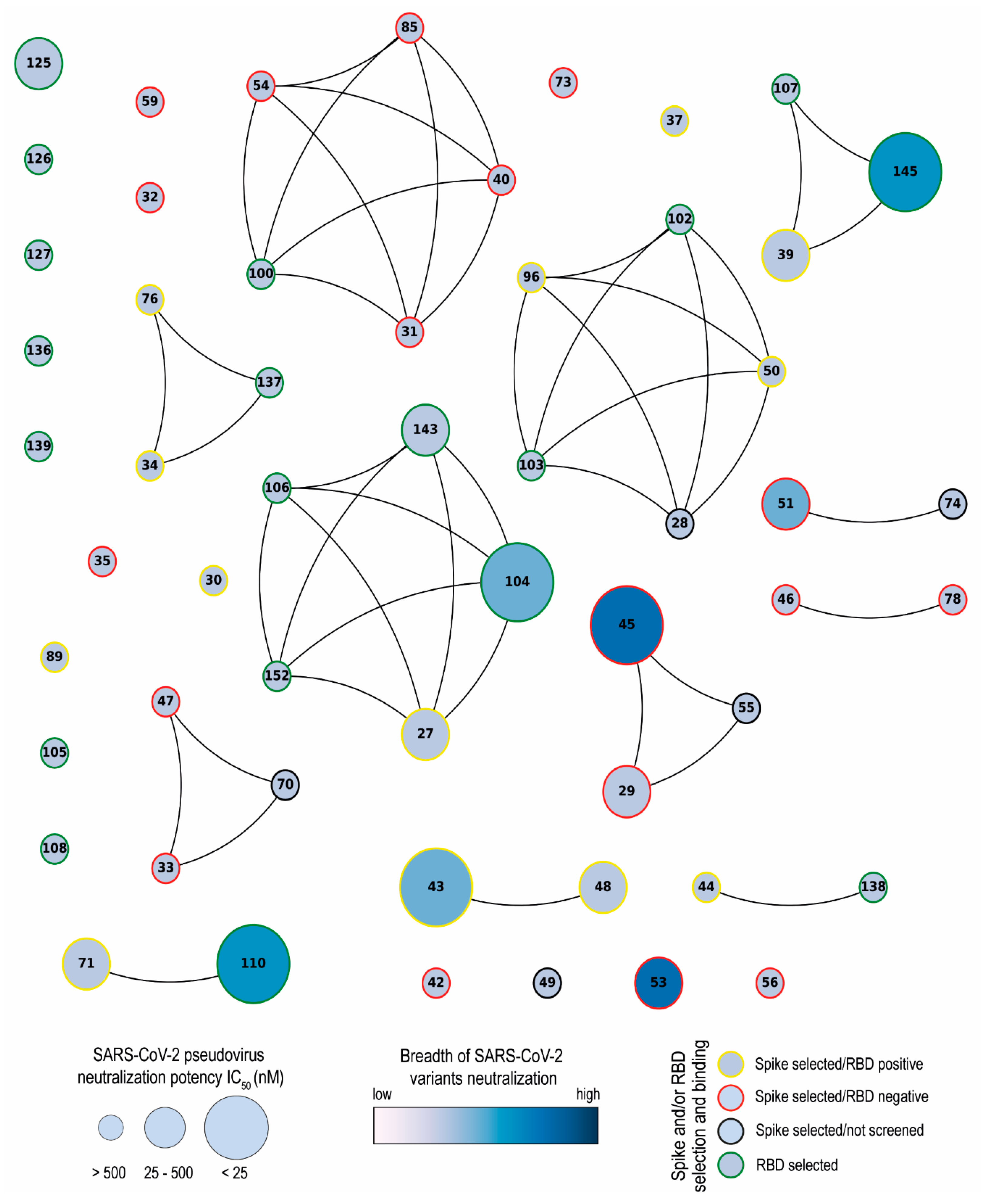
3.1. Selection of SARS-CoV-2 S-2P and RBD-Specific Nanobodies
3.2. SARS-CoV-2 Nanobody Characterization
3.3. Screening of Neutralizing Nanobodies against SARS-CoV-2 WT Strain
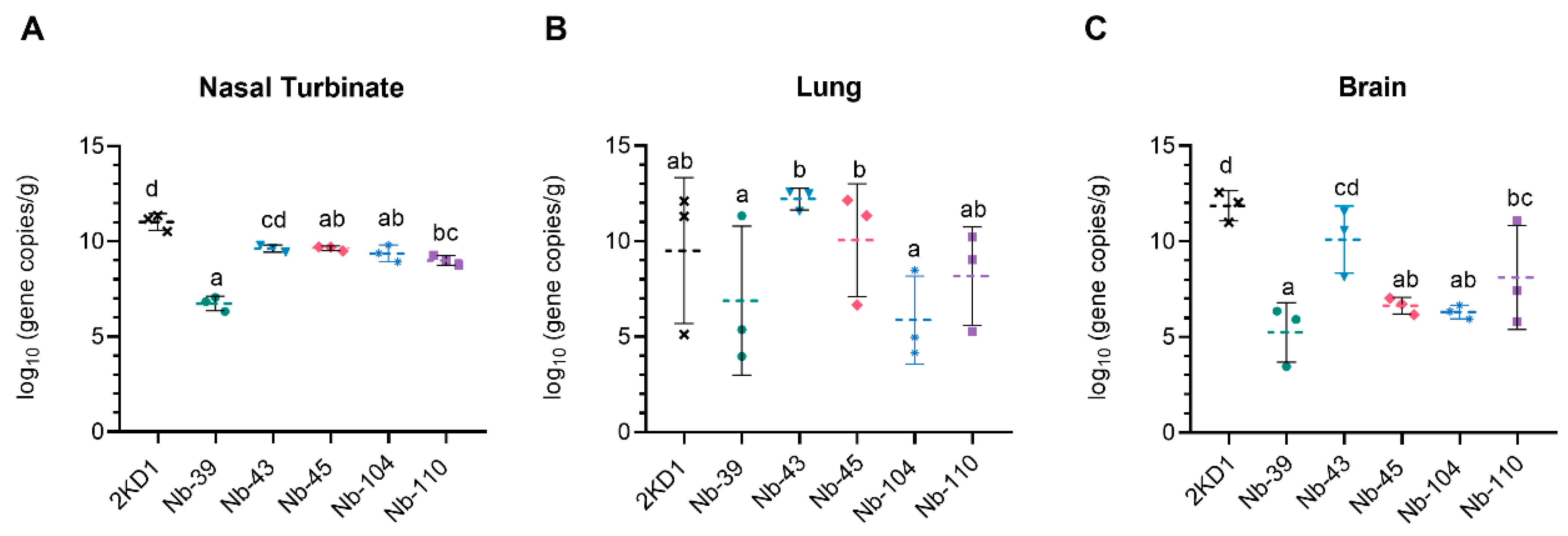
3.4. Protection against SARS-CoV-2 Challenge in k18-hACE2 Mouse Model
3.5. Nanobodies to SARS-CoV-2 WT Strain Neutralize SARS-CoV-2 Variants
3.6. A cocktail of Nanobodies Enhances Neutralization Potencies against Omicron Variants
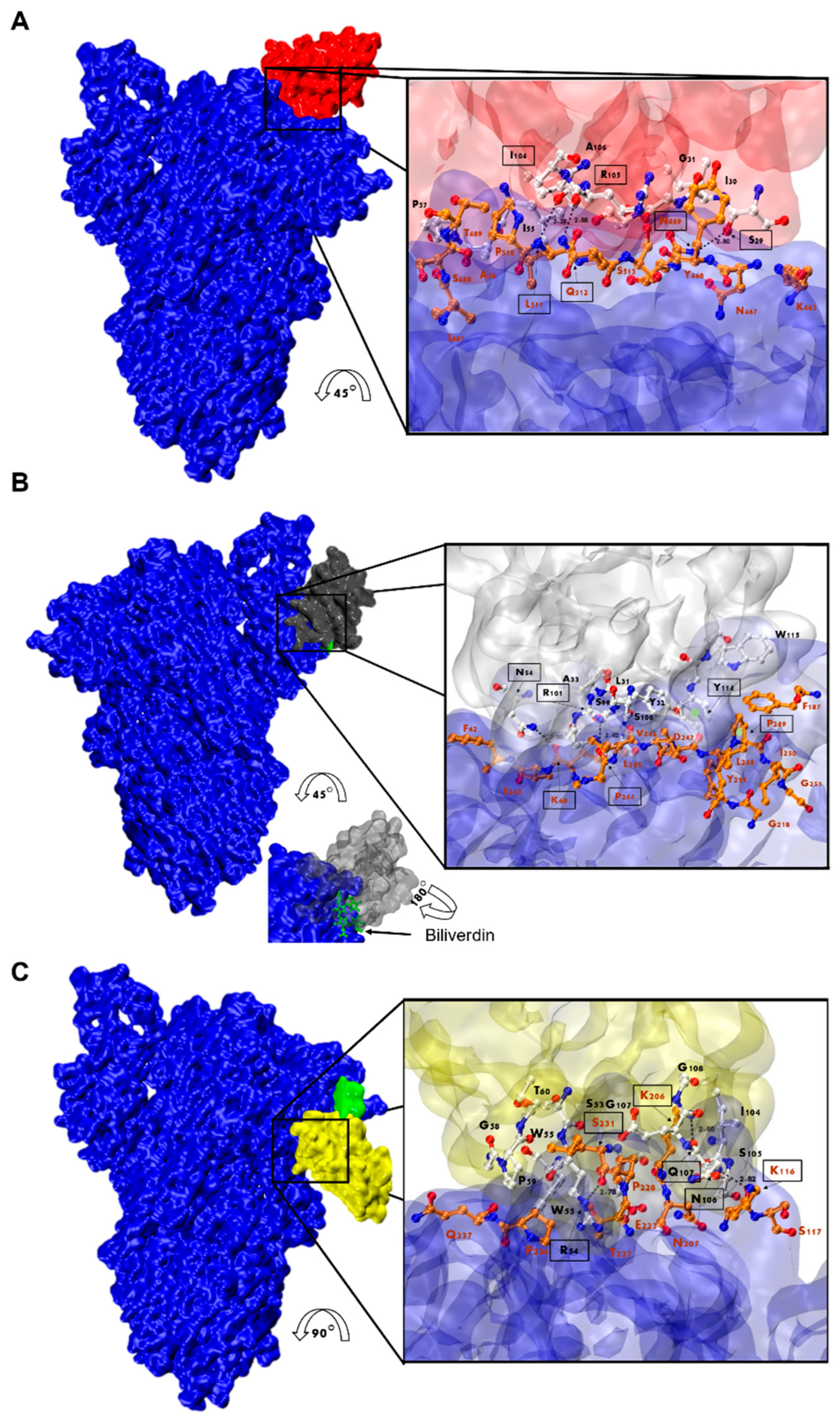
3.7. Nanobody Epitope Mapping and Binding Mode by In Silico Prediction
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Abou Ghayda, R.; Lee, K.H.; Han, Y.J.; Ryu, S.; Hong, S.H.; Yoon, S.; Jeong, G.H.; Yang, J.W.; Lee, H.J.; Lee, J.; et al. The Global Case Fatality Rate of Coronavirus Disease 2019 by Continents and National Income: A Meta-analysis. J. Med. Virol. 2022, 94, 2402–2413. [Google Scholar] [CrossRef]
- Mathieu, E.; Ritchie, H.; Rodés-Guirao, L.; Appel, C.; Gavrilov, D.; Giattino, C.; Hasell, J.; Macdonald, B.; Dattani, S.; Beltekian, D.; et al. Mortality Risk of COVID-19. Available online: https://ourworldindata.org/mortality-risk-covid (accessed on 21 December 2023).
- World Health Organization. WHO Coronavirus (COVID-19). Available online: https://covid19.who.int/ (accessed on 12 December 2023).
- Ministerio de Salud Argentina Información Epidemiológica. Available online: www.argentina.gob.ar/salud/coronavirus-COVID-19/informacion-epidemiologica/febrero-2023 (accessed on 21 December 2023).
- Paules, C.I.; Marston, H.D.; Fauci, A.S. Coronavirus Infections—More Than Just the Common Cold. JAMA 2020, 323, 707. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Kok, K.-H.; Zhu, Z.; Chu, H.; To, K.K.-W.; Yuan, S.; Yuen, K.-Y. Genomic Characterization of the 2019 Novel Human-Pathogenic Coronavirus Isolated from a Patient with Atypical Pneumonia after Visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef]
- Yang, H.; Rao, Z. Structural Biology of SARS-CoV-2 and Implications for Therapeutic Development. Nat. Rev. Microbiol. 2021, 19, 685–700. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell Entry Mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- World Health Organization. COVID-19 Vaccine Tracker. Available online: https://covid19.trackvaccines.org/agency/who/ (accessed on 21 December 2023).
- Data, O.W. Coronavirus (COVID-19). Vaccinations. Available online: https://ourworldindata.org/covid-vaccinations (accessed on 21 December 2023).
- Cromer, D.; Juno, J.A.; Khoury, D.; Reynaldi, A.; Wheatley, A.K.; Kent, S.J.; Davenport, M.P. Prospects for Durable Immune Control of SARS-CoV-2 and Prevention of Reinfection. Nat. Rev. Immunol. 2021, 21, 395–404. [Google Scholar] [CrossRef]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.I.; Correia, I.; Seagal, J.; DeGoey, D.A.; Schrimpf, M.R.; Hardee, D.J.; Noey, E.L.; Kati, W.M. Antiviral Drug Discovery for the Treatment of COVID-19 Infections. Viruses 2022, 14, 961. [Google Scholar] [CrossRef] [PubMed]
- Gudima, G.; Kofiadi, I.; Shilovskiy, I.; Kudlay, D.; Khaitov, M. Antiviral Therapy of COVID-19. Int. J. Mol. Sci. 2023, 24, 8867. [Google Scholar] [CrossRef] [PubMed]
- Indari, O.; Jakhmola, S.; Manivannan, E.; Jha, H.C. An Update on Antiviral Therapy Against SARS-CoV-2: How Far Have We Come? Front. Pharmacol. 2021, 12, 632677. [Google Scholar] [CrossRef] [PubMed]
- Widyasari, K.; Kim, J. A Review of the Currently Available Antibody Therapy for the Treatment of Coronavirus Disease 2019 (COVID-19). Antibodies 2023, 12, 5. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Updates on Bebtelovimab. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-bebtelovimab (accessed on 21 December 2023).
- Liu, M.; Gan, H.; Liang, Z.; Liu, L.; Liu, Q.; Mai, Y.; Chen, H.; Lei, B.; Yu, S.; Chen, H.; et al. Review of Therapeutic Mechanisms and Applications Based on SARS-CoV-2 Neutralizing Antibodies. Front. Microbiol. 2023, 14, 1122868. [Google Scholar] [CrossRef]
- Food and Drug Administration. Coronavirus (COVID-19) Update: FDA Authorizes New Monoclonal Antibody for Treatment of COVID-19 That Retains Activity against Omicron Variant. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-new-long-acting-monoclonal-antibodies-pre-exposure (accessed on 21 December 2023).
- Naidoo, D.B.; Chuturgoon, A.A. The Potential of Nanobodies for COVID-19 Diagnostics and Therapeutics. Mol. Diagn. Ther. 2023, 27, 193–226. [Google Scholar] [CrossRef]
- Hamers-Casterman, C.; Atarhouch, T.; Muyldermans, S.; Robinson, G.; Hamers, C.; Songa, E.B.; Bendahman, N.; Hamers, R. Naturally Occurring Antibodies Devoid of Light Chains. Nature 1993, 363, 446–448. [Google Scholar] [CrossRef]
- Muyldermans, S. Nanobodies: Natural Single-Domain Antibodies. Annu. Rev. Biochem. 2013, 82, 775–797. [Google Scholar] [CrossRef]
- Koromyslova, A.D.; Hansman, G.S. Nanobody Binding to a Conserved Epitope Promotes Norovirus Particle Disassembly. J. Virol. 2015, 89, 2718–2730. [Google Scholar] [CrossRef] [PubMed]
- Salmen, W.; Hu, L.; Bok, M.; Chaimongkol, N.; Ettayebi, K.; Sosnovtsev, S.V.; Soni, K.; Ayyar, B.V.; Shanker, S.; Neill, F.H.; et al. A Single Nanobody Neutralizes Multiple Epochally Evolving Human Noroviruses by Modulating Capsid Plasticity. Nat. Commun. 2023, 14, 6516. [Google Scholar] [CrossRef] [PubMed]
- Muyldermans, S. A Guide to: Generation and Design of Nanobodies. FEBS J. 2021, 288, 2084–2102. [Google Scholar] [CrossRef] [PubMed]
- Rossotti, M.A.; Bélanger, K.; Henry, K.A.; Tanha, J. Immunogenicity and Humanization of Single-domain Antibodies. FEBS J. 2022, 289, 4304–4327. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Poulain, A.; Perret, S.; Malenfant, F.; Mullick, A.; Massie, B.; Durocher, Y. Rapid Protein Production from Stable CHO Cell Pools Using Plasmid Vector and the Cumate Gene-Switch. J. Biotechnol. 2017, 255, 16–27. [Google Scholar] [CrossRef]
- Argentinian AntiCovid Consortium. Structural and Functional Comparison of SARS-CoV-2-Spike Receptor Binding Domain Produced in Pichia Pastoris and Mammalian Cells. Sci. Rep. 2020, 10, 21779. [Google Scholar] [CrossRef] [PubMed]
- Arce, L.P.; Pavan, M.F.; Bok, M.; Gutiérrez, S.E.; Estein, S.M.; Santos, A.T.; Condorí, W.E.; Uhart, M.M.; Parreño, V.; Vizoso-Pinto, M.G.; et al. A Multispecies Competitive Nanobody-Based ELISA for the Detection of Antibodies against Hepatitis E Virus. Sci. Rep. 2023, 13, 15448. [Google Scholar] [CrossRef] [PubMed]
- Garaicoechea, L.; Aguilar, A.; Parra, G.I.; Bok, M.; Sosnovtsev, S.V.; Canziani, G.; Green, K.Y.; Bok, K.; Parreño, V. Llama Nanoantibodies with Therapeutic Potential against Human Norovirus Diarrhea. PLoS ONE 2015, 10, e0133665. [Google Scholar] [CrossRef] [PubMed]
- Pardon, E.; Laeremans, T.; Triest, S.; Rasmussen, S.G.F.; Wohlkönig, A.; Ruf, A.; Muyldermans, S.; Hol, W.G.J.; Kobilka, B.K.; Steyaert, J. A General Protocol for the Generation of Nanobodies for Structural Biology. Nat. Protoc. 2014, 9, 674–693. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.G.; Bok, M.; Vlasova, A.N.; Chattha, K.S.; Gómez-Sebastián, S.; Nuñez, C.; Alvarado, C.; Lasa, R.; Escribano, J.M.; Garaicoechea, L.L.; et al. Recombinant Monovalent Llama-Derived Antibody Fragments (VHH) to Rotavirus VP6 Protect Neonatal Gnotobiotic Piglets against Human Rotavirus-Induced Diarrhea. PLoS Pathog. 2013, 9, e1003334. [Google Scholar] [CrossRef] [PubMed]
- Giudicelli, V.; Brochet, X.; Lefranc, M.-P. IMGT/V-QUEST: IMGT Standardized Analysis of the Immunoglobulin (IG) and T Cell Receptor (TR) Nucleotide Sequences. Cold Spring Harb. Protoc. 2011, 2011, pdb.prot5633. [Google Scholar] [CrossRef] [PubMed]
- Ehrenmann, F.; Kaas, Q.; Lefranc, M.-P. IMGT/3Dstructure-DB and IMGT/DomainGapAlign: A Database and a Tool for Immunoglobulins or Antibodies, T Cell Receptors, MHC, IgSF and MhcSF. Nucleic Acids Res. 2010, 38, D301–D307. [Google Scholar] [CrossRef]
- Crooks, G.E.; Hon, G.; Chandonia, J.-M.; Brenner, S.E. WebLogo: A Sequence Logo Generator: Figure 1. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Vega, C.G.; Garaicoechea, L.L.; Degiuseppe, J.I.; Bok, M.; Rivolta, A.A.; Piantanida, A.P.; Asenzo, G.; Adúriz Guerrero, M.; Wigdorovitz, A.; Stupka, J.A.; et al. ROTADIAL: The First Nanobody-Based Immunoassay to Detect Group A Rotavirus. J. Virol. Methods 2021, 298, 114279. [Google Scholar] [CrossRef]
- Bioquest, A. Quest GraphTM IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 15 December 2023).
- Berguer, P.M.; Blaustein, M.; Bredeston, L.M.; Craig, P.O.; D’Alessio, C.; Elias, F.; Farré, P.C.; Fernández, N.B.; Gentili, H.G.; Gándola, Y.B.; et al. Covalent Coupling of Spike’s Receptor Binding Domain to a Multimeric Carrier Produces a High Immune Response against SARS-CoV-2. Sci. Rep. 2022, 12, 692. [Google Scholar] [CrossRef]
- Ferrara, F.; Temperton, N. Pseudotype Neutralization Assays: From Laboratory Bench to Data Analysis. Methods Protoc. 2018, 1, 8. [Google Scholar] [CrossRef]
- NIAID Plque Reduction Calculator. Available online: https://bioinformatics.niaid.nih.gov/plaquereduction (accessed on 21 December 2023).
- Zhu, Y.; Nikolic, D.; Van Breemen, R.B.; Silverman, R.B. Mechanism of Inactivation of Inducible Nitric Oxide Synthase by Amidines. Irreversible Enzyme Inactivation without Inactivator Modification. J. Am. Chem. Soc. 2005, 127, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Callahan, V.; Hawks, S.; Crawford, M.A.; Lehman, C.W.; Morrison, H.A.; Ivester, H.M.; Akhrymuk, I.; Boghdeh, N.; Flor, R.; Finkielstein, C.V.; et al. The Pro-Inflammatory Chemokines CXCL9, CXCL10 and CXCL11 Are Upregulated Following SARS-CoV-2 Infection in an AKT-Dependent Manner. Viruses 2021, 13, 1062. [Google Scholar] [CrossRef] [PubMed]
- Estadística y Biometría y de Diseño de Experimentos de la Universidad Nacional de Córdoba InfoStat Software Estadístico. Available online: http://www.infostat.com.ar (accessed on 10 December 2023).
- Cohen, T.; Halfon, M.; Schneidman-Duhovny, D. NanoNet: Rapid and Accurate End-to-End Nanobody Modeling by Deep Learning. Front. Immunol. 2022, 13, 958584. [Google Scholar] [CrossRef]
- Dominguez, C.; Boelens, R.; Bonvin, A.M.J.J. HADDOCK: A Protein−Protein Docking Approach Based on Biochemical or Biophysical Information. J. Am. Chem. Soc. 2003, 125, 1731–1737. [Google Scholar] [CrossRef]
- van Zundert, G.C.P.; Rodrigues, J.P.G.L.M.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A.M.J.J. The HADDOCK2.2 Web Server: User-Friendly Integrative Modeling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Xiang, Y.; Huang, W.; Liu, H.; Sang, Z.; Nambulli, S.; Tubiana, J.; Williams, K.L.; Duprex, W.P.; Schneidman-Duhovny, D.; Wilson, I.A.; et al. Superimmunity by Pan-Sarbecovirus Nanobodies. Cell Rep. 2022, 39, 111004. [Google Scholar] [CrossRef]
- Rosa, A.; Pye, V.E.; Graham, C.; Muir, L.; Seow, J.; Ng, K.W.; Cook, N.J.; Rees-Spear, C.; Parker, E.; dos Santos, M.S.; et al. SARS-CoV-2 Can Recruit a Heme Metabolite to Evade Antibody Immunity. Sci. Adv. 2021, 7, eabg7607. [Google Scholar] [CrossRef]
- Valenzuela-Nieto, G.; Miranda-Chacon, Z.; Salinas-Rebolledo, C.; Jara, R.; Cuevas, A.; Berking, A.; Rojas-Fernandez, A. Nanobodies: COVID-19 and Future Perspectives. Front. Drug Discov. 2022, 2, 927164. [Google Scholar] [CrossRef]
- Wrapp, D.; De Vlieger, D.; Corbett, K.S.; Torres, G.M.; Van Breedam, W.; Roose, K.; van Schie, L.; Hoffmann, M.; Pöhlmann, S.; Graham, B.S.; et al. Structural Basis for Potent Neutralization of Betacoronaviruses by Single-Domain Camelid Antibodies. Cell 2020, 181, 1004–1015.e15. [Google Scholar] [CrossRef]
- Rossotti, M.A.; van Faassen, H.; Tran, A.T.; Sheff, J.; Sandhu, J.K.; Duque, D.; Hewitt, M.; Wen, X.; Bavananthasivam, J.; Beitari, S.; et al. Arsenal of Nanobodies Shows Broad-Spectrum Neutralization against SARS-CoV-2 Variants of Concern in Vitro and in Vivo in Hamster Models. Commun. Biol. 2022, 5, 933. [Google Scholar] [CrossRef] [PubMed]
- Hanke, L.; Vidakovics Perez, L.; Sheward, D.J.; Das, H.; Schulte, T.; Moliner-Morro, A.; Corcoran, M.; Achour, A.; Karlsson Hedestam, G.B.; Hällberg, B.M.; et al. An Alpaca Nanobody Neutralizes SARS-CoV-2 by Blocking Receptor Interaction. Nat. Commun. 2020, 11, 4420. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, Y.; Aw, Z.Q.; Chen, B.; Yang, Z.; Lei, Y.; Cheng, L.; Liang, Q.; Hong, J.; Yang, Y.; et al. Broadly Neutralizing and Protective Nanobodies against SARS-CoV-2 Omicron Subvariants BA.1, BA.2, and BA.4/5 and Diverse Sarbecoviruses. Nat. Commun. 2022, 13, 7957. [Google Scholar] [CrossRef]
- Walter, J.D.; Scherer, M.; Hutter, C.A.J.; Garaeva, A.A.; Zimmermann, I.; Wyss, M.; Rheinberger, J.; Ruedin, Y.; Earp, J.C.; Egloff, P.; et al. Biparatopic Sybodies Neutralize SARS-CoV-2 Variants of Concern and Mitigate Drug Resistance. EMBO Rep. 2022, 23, e54199. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; Jin, Y.; Zhu, Y.; Wu, Y.; Li, C.; Kong, Y.; Song, W.; Tian, X.; Zhan, W.; et al. A Non-ACE2 Competing Human Single-Domain Antibody Confers Broad Neutralization against SARS-CoV-2 and Circulating Variants. Signal Transduct. Target. Ther. 2021, 6, 378. [Google Scholar] [CrossRef]
- Weinstein, J.B.; Bates, T.A.; Leier, H.C.; McBride, S.K.; Barklis, E.; Tafesse, F.G. A Potent Alpaca-Derived Nanobody That Neutralizes SARS-CoV-2 Variants. iScience 2022, 25, 103960. [Google Scholar] [CrossRef]
- Favorskaya, I.A.; Shcheblyakov, D.V.; Esmagambetov, I.B.; Dolzhikova, I.V.; Alekseeva, I.A.; Korobkova, A.I.; Voronina, D.V.; Ryabova, E.I.; Derkaev, A.A.; Kovyrshina, A.V.; et al. Single-Domain Antibodies Efficiently Neutralize SARS-CoV-2 Variants of Concern. Front. Immunol. 2022, 13, 822159. [Google Scholar] [CrossRef]
- Panda, M.; Kalita, E.; Singh, S.; Kumar, K.; Prajapati, V.K. Nanobody-Peptide-Conjugate (NPC) for Passive Immunotherapy against SARS-CoV-2 Variants of Concern (VoC): A Prospective Pan-Coronavirus Therapeutics. Mol. Divers. 2022, 27, 1–27. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, X.; Zeng, W.; Zhou, J.; Chi, X.; Chen, S.; Zheng, P.; Wang, M.; Wu, Y.; Zhao, D.; et al. A Bispecific Nanobody Dimer Broadly Neutralizes SARS-CoV-1 & 2 Variants of Concern and Offers Substantial Protection against Omicron via Low-Dose Intranasal Administration. Cell Discov. 2022, 8, 132. [Google Scholar] [CrossRef]
- Shi, Z.; Li, X.; Wang, L.; Sun, Z.; Zhang, H.; Chen, X.; Cui, Q.; Qiao, H.; Lan, Z.; Zhang, X.; et al. Structural Basis of Nanobodies Neutralizing SARS-CoV-2 Variants. Structure 2022, 30, 707–720.e5. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Grant, O.C.; Montgomery, D.; Ito, K.; Woods, R.J. Analysis of the SARS-CoV-2 Spike Protein Glycan Shield Reveals Implications for Immune Recognition. Sci. Rep. 2020, 10, 14991. [Google Scholar] [CrossRef]
- Mast, F.D.; Fridy, P.C.; Ketaren, N.E.; Wang, J.; Jacobs, E.Y.; Olivier, J.P.; Sanyal, T.; Molloy, K.R.; Schmidt, F.; Rutkowska, M.; et al. Highly Synergistic Combinations of Nanobodies That Target SARS-CoV-2 and Are Resistant to Escape. eLife 2021, 10, e73027. [Google Scholar] [CrossRef]
- Covariants. Available online: https://covariants.org/ (accessed on 21 December 2023).
- Kombe Kombe, A.J.; Zahid, A.; Mohammed, A.; Shi, R.; Jin, T. Potent Molecular Feature-Based Neutralizing Monoclonal Antibodies as Promising Therapeutics Against SARS-CoV-2 Infection. Front. Mol. Biosci. 2021, 8, 670815. [Google Scholar] [CrossRef]
- Vincke, C.; Loris, R.; Saerens, D.; Martinez-Rodriguez, S.; Muyldermans, S.; Conrath, K. General Strategy to Humanize a Camelid Single-Domain Antibody and Identification of a Universal Humanized Nanobody Scaffold. J. Biol. Chem. 2009, 284, 3273–3284. [Google Scholar] [CrossRef]
- Ishiwatari-Ogata, C.; Kyuuma, M.; Ogata, H.; Yamakawa, M.; Iwata, K.; Ochi, M.; Hori, M.; Miyata, N.; Fujii, Y. Ozoralizumab, a Humanized Anti-TNFα NANOBODY® Compound, Exhibits Efficacy Not Only at the Onset of Arthritis in a Human TNF Transgenic Mouse but Also During Secondary Failure of Administration of an Anti-TNFα IgG. Front. Immunol. 2022, 13, 853008. [Google Scholar] [CrossRef]
- Ackaert, C.; Smiejkowska, N.; Xavier, C.; Sterckx, Y.G.J.; Denies, S.; Stijlemans, B.; Elkrim, Y.; Devoogdt, N.; Caveliers, V.; Lahoutte, T.; et al. Immunogenicity Risk Profile of Nanobodies. Front. Immunol. 2021, 12, 578. [Google Scholar] [CrossRef]
- Schepens, B.; van Schie, L.; Nerinckx, W.; Roose, K.; Van Breedam, W.; Fijalkowska, D.; Devos, S.; Weyts, W.; De Cae, S.; Vanmarcke, S.; et al. An Affinity-Enhanced, Broadly Neutralizing Heavy Chain–Only Antibody Protects against SARS-CoV-2 Infection in Animal Models. Sci. Transl. Med. 2021, 13, eabi7826. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-López, E.; Schuhmacher, A.J. Transportation of Single-Domain Antibodies through the Blood–Brain Barrier. Biomolecules 2021, 11, 1131. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bourgeois, J.; Celli, S.; Glacial, F.; Le Sourd, A.; Mecheri, S.; Weksler, B.; Romero, I.; Couraud, P.; Rougeon, F.; et al. Cell-penetrating Anti-GFAP VHH and Corresponding Fluorescent Fusion Protein VHH-GFP Spontaneously Cross the Blood-brain Barrier and Specifically Recognize Astrocytes: Application to Brain Imaging. FASEB J. 2012, 26, 3969–3979. [Google Scholar] [CrossRef]
- Zawilska, J.B.; Kuczyńska, K. Psychiatric and Neurological Complications of Long COVID. J. Psychiatr. Res. 2022, 156, 349–360. [Google Scholar] [CrossRef]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef]
- Gomes, J.R.; Cabrito, I.; Soares, H.R.; Costelha, S.; Teixeira, A.; Wittelsberger, A.; Stortelers, C.; Vanlandschoot, P.; Saraiva, M.J. Delivery of an Anti-Transthyretin Nanobody to the Brain through Intranasal Administration Reveals Transthyretin Expression and Secretion by Motor Neurons. J. Neurochem. 2018, 145, 393–408. [Google Scholar] [CrossRef]
- Soleimanizadeh, A.; Dinter, H.; Schindowski, K. Central Nervous System Delivery of Antibodies and Their Single-Domain Antibodies and Variable Fragment Derivatives with Focus on Intranasal Nose to Brain Administration. Antibodies 2021, 10, 47. [Google Scholar] [CrossRef]
- Pothin, E.; Lesuisse, D.; Lafaye, P. Brain Delivery of Single-Domain Antibodies: A Focus on VHH and VNAR. Pharmaceutics 2020, 12, 937. [Google Scholar] [CrossRef]
- Han, Q.; Wang, S.; Wang, Z.; Zhang, C.; Wang, X.; Feng, N.; Wang, T.; Zhao, Y.; Chi, H.; Yan, F.; et al. Nanobodies with Cross-Neutralizing Activity Provide Prominent Therapeutic Efficacy in Mild and Severe COVID-19 Rodent Models. Virol. Sin. 2023, 38, 787–800. [Google Scholar] [CrossRef]
- Wu, X.; Cheng, L.; Fu, M.; Huang, B.; Zhu, L.; Xu, S.; Shi, H.; Zhang, D.; Yuan, H.; Nawaz, W.; et al. A Potent Bispecific Nanobody Protects HACE2 Mice against SARS-CoV-2 Infection via Intranasal Administration. Cell Rep. 2021, 37, 109869. [Google Scholar] [CrossRef]
- Valenzuela Nieto, G.; Jara, R.; Watterson, D.; Modhiran, N.; Amarilla, A.A.; Himelreichs, J.; Khromykh, A.A.; Salinas-Rebolledo, C.; Pinto, T.; Cheuquemilla, Y.; et al. Potent Neutralization of Clinical Isolates of SARS-CoV-2 D614 and G614 Variants by a Monomeric, Sub-Nanomolar Affinity Nanobody. Sci. Rep. 2021, 11, 3318. [Google Scholar] [CrossRef] [PubMed]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An Ultrapotent Synthetic Nanobody Neutralizes SARS-CoV-2 by Stabilizing Inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, C.; Xia, S.; Tian, X.; Kong, Y.; Wang, Z.; Gu, C.; Zhang, R.; Tu, C.; Xie, Y.; et al. Identification of Human Single-Domain Antibodies against SARS-CoV-2. Cell Host Microbe 2020, 27, 891–898.e5. [Google Scholar] [CrossRef]
- Huo, J.; Le Bas, A.; Ruza, R.R.; Duyvesteyn, H.M.E.; Mikolajek, H.; Malinauskas, T.; Tan, T.K.; Rijal, P.; Dumoux, M.; Ward, P.N.; et al. Neutralizing Nanobodies Bind SARS-CoV-2 Spike RBD and Block Interaction with ACE2. Nat. Struct. Mol. Biol. 2020, 27, 846–854. [Google Scholar] [CrossRef]
- Ye, G.; Gallant, J.; Zheng, J.; Massey, C.; Shi, K.; Tai, W.; Odle, A.; Vickers, M.; Shang, J.; Wan, Y.; et al. The Development of Nanosota-1 as Anti-SARS-CoV-2 Nanobody Drug Candidates. eLife 2021, 10, e64815. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Dong, J.; Huang, B.; Wang, B.; Titong, A.; Gallolu Kankanamalage, S.; Jia, Z.; Wright, M.; Parthasarathy, P.; Liu, Y. Development of Humanized Tri-Specific Nanobodies with Potent Neutralization for SARS-CoV-2. Sci. Rep. 2020, 10, 17806. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Nambulli, S.; Xiao, Z.; Liu, H.; Sang, Z.; Duprex, W.P.; Schneidman-Duhovny, D.; Zhang, C.; Shi, Y. Versatile and Multivalent Nanobodies Efficiently Neutralize SARS-CoV-2. Science 2020, 370, 1479–1484. [Google Scholar] [CrossRef]
- Koenig, P.-A.; Das, H.; Liu, H.; Kümmerer, B.M.; Gohr, F.N.; Jenster, L.-M.; Schiffelers, L.D.J.; Tesfamariam, Y.M.; Uchima, M.; Wuerth, J.D.; et al. Structure-Guided Multivalent Nanobodies Block SARS-CoV-2 Infection and Suppress Mutational Escape. Science 2021, 371, eabe6230. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, L.; Liu, X.; Zhang, J.; Yin, Y.; Luan, L.; Jiang, D.; Yang, X.; Li, L.; Xiong, H.; et al. A Potent Synthetic Nanobody with Broad-Spectrum Activity Neutralizes SARS-CoV-2 Virus and the Omicron Variant BA.1 through a Unique Binding Mode. J. Nanobiotechnol. 2022, 20, 411. [Google Scholar] [CrossRef]
- Hopkins, C.W.; Le Grand, S.; Walker, R.C.; Roitberg, A.E. Long-Time-Step Molecular Dynamics through Hydrogen Mass Repartitioning. J. Chem. Theory Comput. 2015, 11, 1864–1874. [Google Scholar] [CrossRef]
- Tian, C.; Kasavajhala, K.; Belfon, K.A.A.; Raguette, L.; Huang, H.; Migues, A.N.; Bickel, J.; Wang, Y.; Pincay, J.; Wu, Q.; et al. Ff19SB: Amino-Acid-Specific Protein Backbone Parameters Trained against Quantum Mechanics Energy Surfaces in Solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef]



| Nbs | pVNT IC50 (nM) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alpha | Beta | Delta | Omicron B.1.1.529 | Omicron BA.2 | Omicron XBB | Omicron XBB.1.5 | ||
| RBD binders | Nb-43 | - | - | - | 394.7 | 204.8 | - | - |
| Nb-104 | 18.29 | - | 121.8 | - | - | - | - | |
| Nb-110 | 133.9 | 324.8 | 121.8 | - | - | - | - | |
| Nb-145 | 86.65 | 451.6 | 384.4 | - | - | - | - | |
| Non-RBD binders | Nb-45 | 83.66 | 237.5 | 248.0 | 214.6 | 112.0 | 30.73 | 42.17 |
| Nb-51 | 215.3 | 616.3 | - | - | - | 285.6 | 316.9 | |
| Nb-53 | 91.2 | 112.4 | 701.8 | 322.6 | 135.3 | 145.2 | 136.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavan, M.F.; Bok, M.; Betanzos San Juan, R.; Malito, J.P.; Marcoppido, G.A.; Franco, D.R.; Militello, D.A.; Schammas, J.M.; Bari, S.E.; Stone, W.; et al. SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice. Viruses 2024, 16, 185. https://doi.org/10.3390/v16020185
Pavan MF, Bok M, Betanzos San Juan R, Malito JP, Marcoppido GA, Franco DR, Militello DA, Schammas JM, Bari SE, Stone W, et al. SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice. Viruses. 2024; 16(2):185. https://doi.org/10.3390/v16020185
Chicago/Turabian StylePavan, María Florencia, Marina Bok, Rafael Betanzos San Juan, Juan Pablo Malito, Gisela Ariana Marcoppido, Diego Rafael Franco, Daniela Ayelen Militello, Juan Manuel Schammas, Sara Elizabeth Bari, William Stone, and et al. 2024. "SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice" Viruses 16, no. 2: 185. https://doi.org/10.3390/v16020185
APA StylePavan, M. F., Bok, M., Betanzos San Juan, R., Malito, J. P., Marcoppido, G. A., Franco, D. R., Militello, D. A., Schammas, J. M., Bari, S. E., Stone, W., López, K., Porier, D. L., Muller, J. A., Auguste, A. J., Yuan, L., Wigdorovitz, A., Parreño, V. G., & Ibañez, L. I. (2024). SARS-CoV-2 Specific Nanobodies Neutralize Different Variants of Concern and Reduce Virus Load in the Brain of h-ACE2 Transgenic Mice. Viruses, 16(2), 185. https://doi.org/10.3390/v16020185







