Postural Abnormalities in Children with Congenital Zika Syndrome-Related Neurological and Visual Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Sociodemographic and Clinical Questionnaire
2.4. Measures
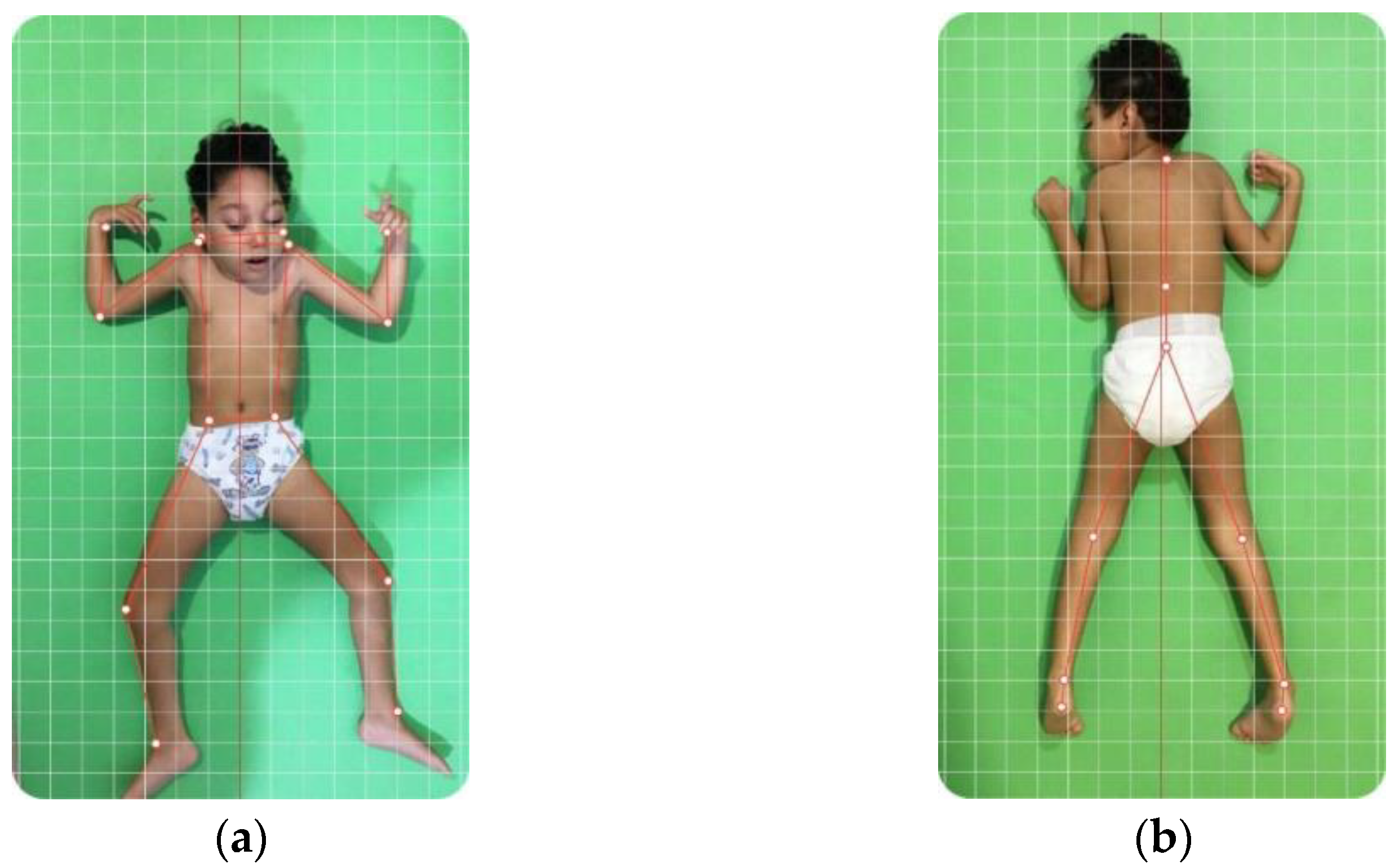
2.4.1. Posture Assessment
2.4.2. Visual Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan American Health Organization; World Health Organization. Epidemiological Alert: Neurological Syndrome, Congenital Malformations, and Zika Virus Infection: Implications for Public Health in the Americas. PAHO/WHO 2015. Available online: https://www.paho.org (accessed on 5 November 2023).
- Zanluca, C.; Melo, V.C.; Mosimann, A.L.; Santos, G.I.; Santos, C.N.; Luz, K. First report of autochthonous transmission of Zika virus in Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.; de Sequeira, P.C.; de Mendonça, M.C.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Motta, I.J.; Spencer, B.R.; Cordeiro da Silva, S.G.; Arruda, M.B.; Dobbin, J.A.; Gonzaga, Y.B.; Arcuri, I.P.; Tavares, R.C.; Atta, E.H.; Fernandes, R.F.; et al. Evidence for transmission of Zika virus by platelet transfusion. N. Engl. J. Med. 2016, 375, 1101–1103. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.A.; Staples, J.E.; Dobyns, W.B.; Pessoa, A.; Ventura, C.V.; Da Fonseca, E.B.; Ribeiro, E.M.; Ventura, L.O.; Neto, N.N.; Arena, J.F.; et al. Characterizing the pattern of anomalies in Congenital Zika Syndrome for pediatric clinicians. JAMA Pediatr. 2017, 171, 288–295. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Del Campo, M.; García-Alix, A.; Ventura, L.O.; Boquett, J.A.; van der Linden, V.; Pessoa, A.; van der Linden Júnior, H.; Ventura, C.V.; Leal, M.C.; et al. Neurodevelopment in Children Exposed to Zika in utero: Clinical and Molecular Aspects. Front. Genet. 2022, 13, 758715. [Google Scholar] [CrossRef]
- Melo, A.; Gama, G.L.; Da Silva Júnior, R.A.; De Assunção, P.L.; Tavares, J.S.; Da Silva, M.B.; Costa, K.N.; Vânia, M.L.; Evangelista, M.A.; De Amorim, M.M. Motor function in children with congenital Zika syndrome. Dev. Med. Child Neurol. 2020, 2, 221–226. [Google Scholar] [CrossRef]
- Ventura, P.A.; Lage, M.L.C.; de Carvalho, A.L.; Fernandes, A.S.; Taguchi, T.B.; Nascimento-Carvalho, C.M. Early Gross Motor Development Among Brazilian Children with Microcephaly Born Right After Zika Virus Infection Outbreak. J. Dev. Behav. Pediatr. 2020, 41, 134–140. [Google Scholar] [CrossRef]
- Teixeira, G.A.; Dantas, D.N.A.; de Lima Carvalho, G.A.F.; da Silva, A.N.; de Carvalho Lira, A.L.B.; Enders, B.C. Analysis of the concept of the Zika Virus congenital syndrome. Ciênc. Saúde Coletiva 2020, 25, 567–574. [Google Scholar] [CrossRef]
- Van der Linden, V.; Rolim Filho, E.L.; Lins, O.G.; van der Linden, A.; de Fátima Viana Vasco Aragão, M.; Brainer-Lima, A.M.; Di Cavalcanti Sousa Cruz, D.; Rocha, M.A.W.; da Silva, P.F.S.; Durce Costa Gomes Carvalho, M.; et al. Congenital Zika syndrome with arthrogryposis: Retrospective case series study. BMJ 2016, 354, i3899. [Google Scholar] [CrossRef]
- Harsanyi, S.; Zamborsky, R.; Krajciova, L.; Kokavec, M.; Danisovic, L. Developmental dysplasia of the hip: A review of etiopathogenesis, risk factors, and genetic aspects. Medicina 2020, 56, 153. [Google Scholar] [CrossRef]
- Van der Linden, H.; Silveira-Moriyama, L.; van der Linden, V.; Pessoa, A.; Valente, K.; Mink, J.; Paciorkowski, A. Movement disorders in children with congenital Zika virus syndrome. Brain Dev. 2020, 42, 720–729. [Google Scholar] [CrossRef] [PubMed]
- Ventura, L.O.; Ventura, C.V.; Lawrence, L.; van der Linden, V.; van der Linden, A.; Gois, A.L.; Cavalcanti, M.M.; Barros, E.A.; Dias, N.C.; Berrocal, A.M.; et al. Visual impairment in children with congenital Zika syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2017, 21, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.F.; Kattah, M.; Ventura, L.O.; Gois, A.L.; Rocha, C.; Andrade, C.G.; Mendonza-Santiesteban, C.; Ventura, C.V. Pattern-Reversal Visual Evoked Potential in Children With Congenital Zika Syndrome. J. Pediatr. Ophthalmol. Strabismus 2021, 58, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Ventura, C.V.; Zin, A.; de Paula Freitas, B.; Ventura, L.O.; Rocha, C.; Costa, F.; Nery, N., Jr.; De Senna, T.C.; Moreira, M.E.L.; Maia, M.; et al. Ophthalmological Manifestations in Congenital Zika Syndrome in 469 Brazilian Children. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2021, 25, 158.e1–158.e8. [Google Scholar] [CrossRef]
- De Pádua, M.; Sauer, J.F.; João, S.M.A. Quantitative Postural Analysis of Children With Congenital Visual Impairment. J. Manipulative Physiol. Ther. 2018, 41, 62–70. [Google Scholar] [CrossRef]
- Henderson, A.D.; Ventura, C.V.; Huisman, T.A.G.M.; Meoded, A.; Hazin, A.N.; van der Linden, V. Characterization of Visual Pathway Abnormalities in Infants With Congenital Zika Syndrome Using Computed Tomography and Magnetic Resonance Imaging. J. Neuroophthalmol. 2021, 41, e598–e605. [Google Scholar] [CrossRef]
- Kendall, F.P.; McCreary, E.K.; Provance, P.G.; Rodgers, M.M.; Romani, W.A. Muscles: Testing and Function with Posture and Pain, 5th ed.; Lippincott Williams & Wilkins: Baltimore, MD, USA, 2005; 451p. [Google Scholar]
- Lanners, J.; Piccioni, A.; Fea, F.; Goergen, E. Early intervention for children with cerebral visual impairment: Preliminary results. J. Intellect. Disabil. Res. 1999, 43, 1–12. [Google Scholar] [CrossRef]
- Moreira, R.; Teles, A.; Fialho, R.; Baluz, R.; Santos, T.C.; Goulart-Filho, R.; Rocha, L.; Silva, F.J.; Gupta, N.; Bastos, V.H.; et al. Mobile Applications for Assessing Human Posture: A Systematic Literature Review. Electronics 2020, 9, 1196. [Google Scholar] [CrossRef]
- Fazzi, E.; Signorini, S.G.; Bova, S.M.; La Piana, R.; Ondei, P.; Bertone, C.; Misefari, W.; Bianchi, P.E. Spectrum of visual disorders in children with cerebral visual impairment. J. Child Neurol. 2007, 22, 294–301. [Google Scholar] [CrossRef]
- Ventura, L.O.; Lawrence, L.; Ventura, C.V.; Dutton, G.N.; Marinho, P.; Ferro, P.F.; Gois, A.L.; Dias, N.C.; Ventura, L.; Moore, C.A.; et al. Response to Correction of Refractive Errors and Hypoaccommodation in Children with Congenital Zika Syndrome. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2017, 21, 480–484. [Google Scholar] [CrossRef]
- Lidbeck, C.; Bartonek, Å.; Yadav, P.; Tedroff, K.; Åstrand, P.; Hellgren, K.; Gutierrez-Farewik, E.M. The role of visual stimuli on standing posture in children with bilateral cerebral palsy. BMC Neurol. 2016, 16, 151. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, E.B.; Hadders-Algra, M. Postural dysfunction in children with cerebral palsy: Some implications for therapeutic guidance. Neural. Plast. 2005, 12, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Cunha, A.B.; Polido, G.J.; Bella, G.P.; Garbellini, D.; Fornasari, C.A. Relação entre alinhamento postural e desempenho motor em crianças com paralisia cerebral. Fisioter. Pesqui. 2009, 16, 22–27. [Google Scholar] [CrossRef]
- Pone, M.V.D.S.; Gomes da Silva, T.O.; Ribeiro, C.T.M.; Aguiar, E.B.D.; Mendes, P.H.B.; Gomes Junior, S.C.D.S.; Hamanaka, T.; Zin, A.A.; Pereira Junior, J.P.; Moreira, M.E.L.; et al. Acquired Hip Dysplasia in Children with Congenital Zika Virus Infection in the First Four Years of Life. Viruses 2022, 14, 2643. [Google Scholar] [CrossRef]
- Almeida, K.J.; Martins, A.C.B.; Almendra, I.C.C.G.E.; Meneses, G.M.S.D.; Sampaio, T.D.D.O.; Campêlo, J.D.C.M.; Bor-Seng-Shu, E. Clinical aspects of congenital microcephaly syndrome by Zika virus in a rehabilitation center for patients with microcephaly. Rev. Assoc. Med. Bras. 2019, 65, 1249–1253. [Google Scholar] [CrossRef]
- Matos, M.A.; Nascimento, M.A.S.T.; Merriman, J.W. Orthopaedic approach to the congenital Zika syndrome. Int. Orthop. 2021, 45, 559–564. [Google Scholar] [CrossRef]
- Alghadir, A.H.; Alotaibi, A.Z.; Iqbal, Z.A. Postural stability in people with visual impairment. Brain Behav. 2019, 9, e01436. [Google Scholar] [CrossRef]
- Alotaibi, A.Z.; Alghadir, A.; Iqbal, Z.A.; Anwer, S. Effect of absence of vision on posture. J. Phys. Ther. Sci. 2016, 28, 1374–1377. [Google Scholar] [CrossRef]
- Bertolini, S.M.M.G.; Polyana, M.; Paula, K.P. Postura corporal: Aspectos estruturais funcionais para promoção da saúde. Saúde E Pesqui. 2015, 8, 125–130. [Google Scholar] [CrossRef][Green Version]

| Variables | n (%) |
|---|---|
| Age in months (mean ± SD) | 6.8 ± 0.7 |
| Sex | |
| Male | 12 (50.0) |
| Female | 12 (50.0) |
| Microcephaly | |
| Yes | 19 (79.2) |
| No | 5 (20.8) |
| Severity of microcephaly | |
| ≥3 SD and ≤2 SD | 5 (26.3) |
| ≤3 SD | 14 (73.7) |
| Hypertonia | |
| Yes | 24 (100) |
| No | 0 (0) |
| Anticonvulsant medication | |
| Yes | 24 (100) |
| No | 0 (0) |
| Hydrocephalus | |
| Yes | 6 (25.0) |
| Patient | Zika Serology | Brain Imaging | Reduced Brain Volume | Lateral Ventricular Enlargement | Fourth Ventricular Enlargement | Third Ventricular Enlargement | Fissure Enlargement | Sulci Reduction | Lissencephaly | Agyria | Pachygyria | Calcifications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | CT | No | Yes | No | No | No | No | No | No | No | Yes |
| 2 | Yes | CT | Yes | Yes | No | No | No | Yes | Yes | No | No | Yes |
| 3 | Yes | MRI | Yes | Yes | No | Yes | No | Yes | Yes | Yes | No | Yes |
| 4 | Yes | CT | Yes | Yes | No | No | No | No | No | No | No | Yes |
| 5 | Yes | CT | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | Yes |
| 6 | No | CT | No | Yes | No | Yes | No | No | No | No | No | Yes |
| 7 | Yes | CT | Yes | Yes | Yes | Yes | Yes | No | Yes | No | Yes | Yes |
| 8 | Yes | CT | Yes | Yes | No | Yes | Yes | No | Yes | No | Yes | Yes |
| 9 | Yes | CT | Yes | Yes | No | No | No | Yes | Yes | No | No | Yes |
| 10 | Yes | CT | Yes | Yes | No | Yes | No | Yes | Yes | No | No | Yes |
| 11 | Yes | MRI | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| 12 | Yes | CT | Yes | Yes | No | No | No | Yes | Yes | Yes | Yes | Yes |
| 13 | Yes | CT | Yes | Yes | Yes | Yes | No | No | Yes | No | No | Yes |
| 14 | Yes | CT | Yes | Yes | No | Yes | No | No | No | No | No | Yes |
| 15 | Yes | CT | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| 16 | Yes | CT | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| 17 | No | CT | Yes | Yes | Yes | Yes | No | No | No | No | No | Yes |
| 18 | Yes | CT | Yes | Yes | No | Yes | No | Yes | Yes | No | Yes | Yes |
| 19 | Yes | MRI | Yes | Yes | No | No | No | Yes | Yes | No | No | No |
| 20 | No | CT | No | Yes | No | No | No | No | No | No | No | Yes |
| 21 | No | CT | No | Yes | No | No | Yes | No | No | No | No | Yes |
| 22 | No | CT | No | Yes | Yes | Yes | Yes | No | No | No | No | Yes |
| 23 | Yes | MRI | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| 24 | No | MRI | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Posture Abnormalities | n (%) |
|---|---|
| Head | |
| Left tilt | 11 (45.8) |
| Right tilt | 7 (29.2) |
| None | 6 (25) |
| Shoulder | |
| Left elevation | 13 (54.2) |
| Right elevation | 10 (41.7) |
| None | 1 (4.2) |
| Spine | |
| Left deviation | 5 (20.8) |
| Right deviation | 5 (20.8) |
| None | 14 (58.3) |
| Hip | |
| Left elevation | 10 (41.7) |
| Right elevation | 8 (33.4) |
| None | 6 (25) |
| Knee | |
| Varus | 22 (84.6) |
| Valgus | 10 (38.4) |
| None | 2 (7.7) |
| Ankle | |
| Eversion | 19 (73.1) |
| Inversion | 14 (53.9) |
| None | 0 |
| Ophthalmological Evaluation | n (%) |
|---|---|
| Visual impairment | |
| Normal | 1 (4.8) |
| Mild | 3 (14.3) |
| Moderate | 6 (28.6) |
| Severe | 6 (28.6) |
| Blindness | 5 (23.8) |
| Structural findings | 8 (38.1) |
| Retina | |
| Yes | 7 (33.3) |
| No | 1 (4.8) |
| Optic nerve | |
| Yes | 6 (28.6) |
| No | 2 (9.5) |
| Strabismus | |
| Yes | 15 (71.4) |
| No | 6 (28.6) |
| Nystagmus | |
| Yes | 4 (19.0) |
| No | 17 (81.0) |
| Refractive errors | |
| Myopia | 8 (38.1) |
| Hyperopia | 13 (61.9) |
| Astigmatism | 21 (100) |
| Body Segments | Visual Impairment Classification | ||||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | Blindness | p-Value * | |
| Head | 3/3 (100.0%) | 5/6 (83.3%) | 4/6 (66.7%) | 3/5 (60.0%) | 0.759 |
| Shoulder | 3/3 (100.0%) | 5/6 (83.3%) | 5/6 (83.3%) | 5/5 (100.0%) | 1 |
| Hip | 3/3 (100.0%) | 5/6 (83.3%) | 4/6 (66.7%) | 3/5 (60.0%) | 0.759 |
| Right knee | 3/3 (100.0%) | 5/6 (83.3%) | 4/6 (66.7%) | 4/5 (80.0%) | 0.910 |
| Left knee | 3/3 (100.0%) | 6/6 (100.0%) | 5/6 (83.3%) | 4/5 (80.0%) | 0.829 |
| Spine | 1/3 (33.3%) | 4/6 (66.7%) | 2/6 (33.3%) | 2/5 (40.0%) | 0.694 |
| Left ankle | 1/3 (33.3%) | 6/6 (100.0%) | 4/6 (66.7%) | 4/5 (80.0%) | 0.254 |
| Right ankle | 3/3 (100.0%) | 5/6 (83.3%) | 6/6 (100%) | 5/5 (100.0%) | 0.110 |
| Body Segments | Visual Impairment Classification | ||||
|---|---|---|---|---|---|
| Mild Median (Range) | Moderate Median (Range) | Severe Median (Range) | Blindness Median (Range) | p-Value * | |
| Head | 34.7 (14.3−36.7) | 17.5 (1.0−176.9) | 4.25 (0.3−19.1) | 4.3 (0.8−30.8) | 0.243 |
| Shoulder | 6.5 (1.9−18.4) | 6.3 (1.1−173.3) | 4.45 (1.6−17.4) | 8.8 (4.0−12.2) | 0.804 |
| Hip | 9.1 (7.1−17.7) | 3.3 (0.3−175.2) | 4.9 (0.0−10.5) | 2.4 (0.2−8.9) | 0.438 |
| Right knee | 40.9 (10.4−109.1) | 39.25 (1.7−75.9) | 49.65 (6.3−97.6) | 25.6 (6.5−113.4) | 0.971 |
| Left knee | 64.3 (60.5−66) | 35.8 (21.2−117.1) | 43.35 (6.5−135.4) | 22.7 (7.1−107.5) | 0.526 |
| Spine | 2.9 (0.2−12.9) | 8.15 (0.1−15.4) | 0.8 (0.7−17.7) | 2.7 (0.5−37.2) | 0.908 |
| Left ankle | 2.4 (2.1−13.6) | 17.7 (15.1−21.2) | 13.9 (0.7−54.2) | 15.0 (2.5−25.9) | 0.293 |
| Right ankle | 15.7 (8.6−19.1) | 12.5 (2.8−30.8) | 15.85 (7.5−28.8) | 12.4 (9.8−47.1) | 0.641 |
| Body Segments | Severity Microcephaly | p-Value * | |
|---|---|---|---|
| Less Severe Median (Range) | Severe Median (Range) | ||
| Head | 3.7 (0.3–17.9) | 10.80 (0.8–176.9) | 0.212 |
| Shoulders | 6.50 (1.7–10.5) | 8.80 (1.6–173.3) | 0.308 |
| Hip | 4.70 (1.7–9.1) | 2.40 (0–175.2) | 0.496 |
| Right knee | 10.40 (1.7–49.6) | 28.90 (6.5–113.4) | 0.145 |
| Left knee | 30.70 (6.5–64.3) | 37.30 (7.1–107.5) | 0.583 |
| Spine | 5.30 (0.2–15.4) | 2.90 (0.5–37.2) | 0.733 |
| Left ankle | 11.10 (0.7–21.2) | 15.10 (1.9–54.2) | 0.411 |
| Right ankle | 15.80 (9.2–19.9) | 12.40 (2.8–47.1) | 0.910 |
| Microcephaly Classification | Postural Deviation | Visual Impairment Classification [n/total (%)] | p-Value | |||
|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Blindness | |||
| Mild | Head | 1/1(100) | 1/2(50) | 1/2(50) | 0/0(0) | 1.00 |
| Severe | 1/1(100) | 2/2(100) | 2/3(66.7) | 3/5(60) | 1.00 | |
| Mild | Shoulder | 1/1(100) | 2/2(100) | 2/2(100) | 0/0(0) | 0.545 |
| Severe | 1/1(100) | 2/2(100) | 2/3(66.7) | 5/5(100) | 1.00 | |
| Mild | Hip | 1/1(100) | 2/2(100) | 2/2(100) | 0/0(0) | 1.00 |
| Severe | 1/1(100) | 1/2(50) | 1/3(33.3) | 3/5(60) | 1.00 | |
| Mild | Right knee | 1/1(100) | 1/2(50) | 1/2(50) | 0/0(0) | 1.00 |
| Severe | 1/1(100) | 2/2(100) | 2/3(66.7) | 4/5(80) | 1.00 | |
| Mild | Left knee | 1/1(100) | 2/2(100) | 1/2(50) | 0/0(0) | 1.00 |
| Severe | 1/1(100) | 2/2(100) | 3/3(100) | 4/5(80) | 1.00 | |
| Mild | Spine | 0/1(0) | 2/2(100) | 1/2(50) | 0/0(0) | 0.600 |
| Severe | 0/1(0) | 2/2(100) | 1/3(33.3) | 2/5(40.0) | 0.610 | |
| Mild | Right ankle | 0/1(0) | 2/2(100) | 1/2(50) | 0/0(0) | 0.600 |
| Severe | 0/1(0) | 2/2(100) | 2/3(66.7) | 4/5(80) | 0.515 | |
| Mild | Left ankle | 1/1(100) | 2/2(100) | 2/2(100) | 0/0(0) | 1.00 |
| Severe | 1/1(100) | 1/2(50) | 3/3(100) | 5/5(100) | 0.273 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borba, R.; Rodrigues, A.; Ventura, C.V.; Marques, C.; Nóbrega, L.; Higino, T.; Santos, D.; Sallum, J.; Ventura, L.O. Postural Abnormalities in Children with Congenital Zika Syndrome-Related Neurological and Visual Impairment. Viruses 2024, 16, 1959. https://doi.org/10.3390/v16121959
Borba R, Rodrigues A, Ventura CV, Marques C, Nóbrega L, Higino T, Santos D, Sallum J, Ventura LO. Postural Abnormalities in Children with Congenital Zika Syndrome-Related Neurological and Visual Impairment. Viruses. 2024; 16(12):1959. https://doi.org/10.3390/v16121959
Chicago/Turabian StyleBorba, Raíne, Amanda Rodrigues, Camila V. Ventura, Cláudia Marques, Lucélia Nóbrega, Taciana Higino, Dalmir Santos, Juliana Sallum, and Liana O. Ventura. 2024. "Postural Abnormalities in Children with Congenital Zika Syndrome-Related Neurological and Visual Impairment" Viruses 16, no. 12: 1959. https://doi.org/10.3390/v16121959
APA StyleBorba, R., Rodrigues, A., Ventura, C. V., Marques, C., Nóbrega, L., Higino, T., Santos, D., Sallum, J., & Ventura, L. O. (2024). Postural Abnormalities in Children with Congenital Zika Syndrome-Related Neurological and Visual Impairment. Viruses, 16(12), 1959. https://doi.org/10.3390/v16121959






