Correction: Stante et al. Four Novel Caudoviricetes Bacteriophages Isolated from Baltic Sea Water Infect Colonizers of Aurelia aurita. Viruses 2023, 15, 1525
Figure/Table Legend
- Figure 1. Plaque and virion morphology of isolated bacteriophages KMM1-KMM4. (A) Plaque morphologies were detected on MB double-agar layer plates after 16 h of incubation at 30 °C. Plaques formed on a lawn of Staphylococcus sp., PQ151711 (KMM1), Citrobacter freundii, OQ398153 (KMM2), and Citrobacter sp., OQ398154 (KMM3, KMM4). Scale bars represent 1 mm. (B) Transmission electron micrographs of phage lysates KMM1–KMM4, scale bars represent 50 nm.
- Figure 2. Infection cycles of isolated phages. One-step growth curves over 120 min were performed to calculate the latent period (green arrow) and burst size (orange arrow). (A) KMM1-infected Staphylococcus sp. (PQ151711) after 27 min with the release of 55 pfu/cell, (B) KMM2-infected Citrobacter freundii (OQ398153) after 20 min with the release of 280 pfu/cell, (C) KMM3- and (D) KMM4-infected Citrobacter sp. (OQ398154) after 45 and 30 min, respectively, with the release of 60 and 120 pfu/cell, respectively. Values represent the mean of three biological replicates.
- Table 4. Adsorption dynamics of bacteriophages KMM1–KMM4. Adsorption rates were determined 5 min after phage addition to the primary hosts (Staphylococcus sp., Citrobacter freundii, and Citrobacter sp.). The number of phages adsorbed to the cells generated a decrease in phage titer. The percentage of adsorbed phages and the adsorption constant (k) were calculated. Values are the mean of three biological replicates with corresponding standard deviations.
Error in Figure/Table
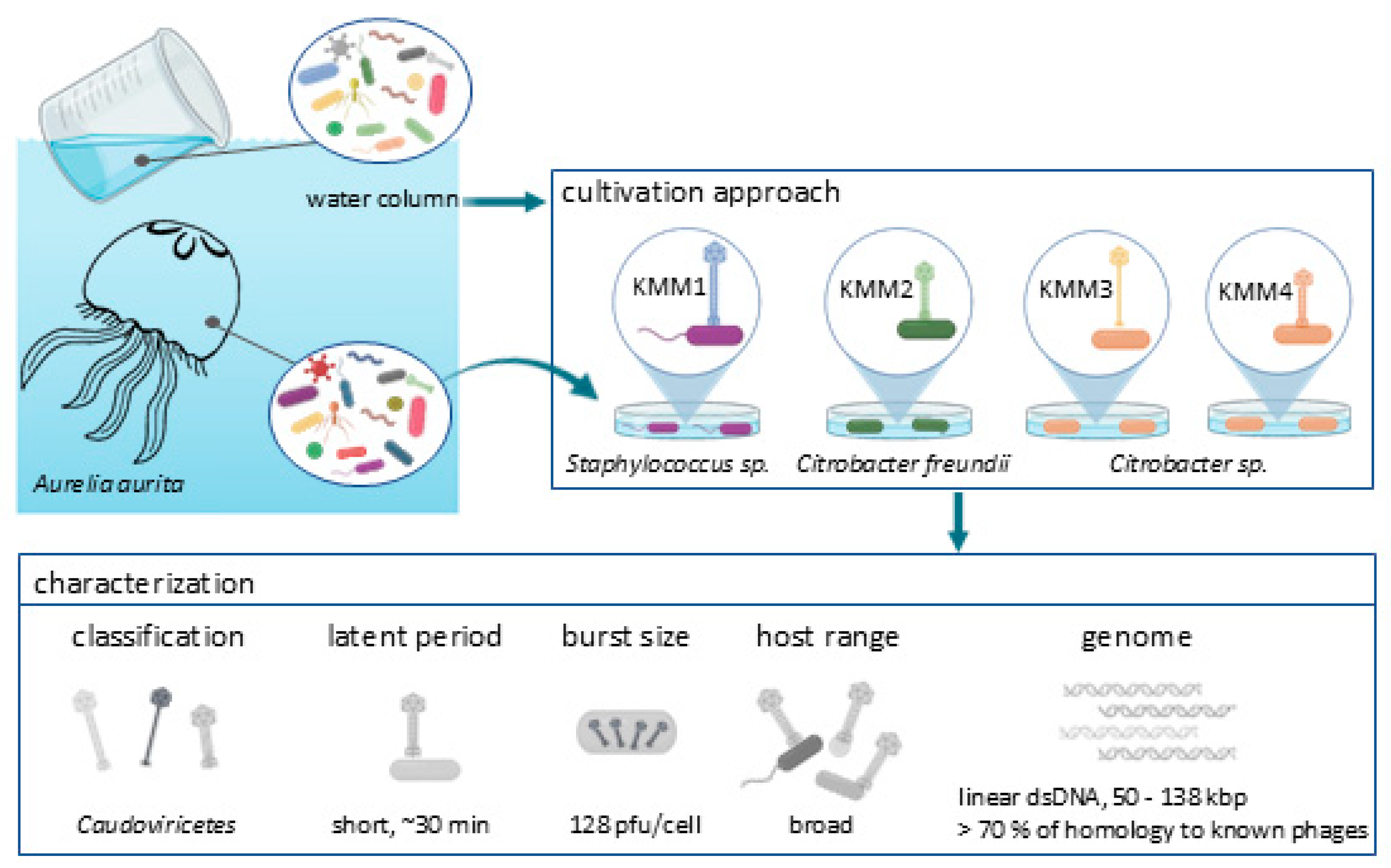
- Graphical abstract
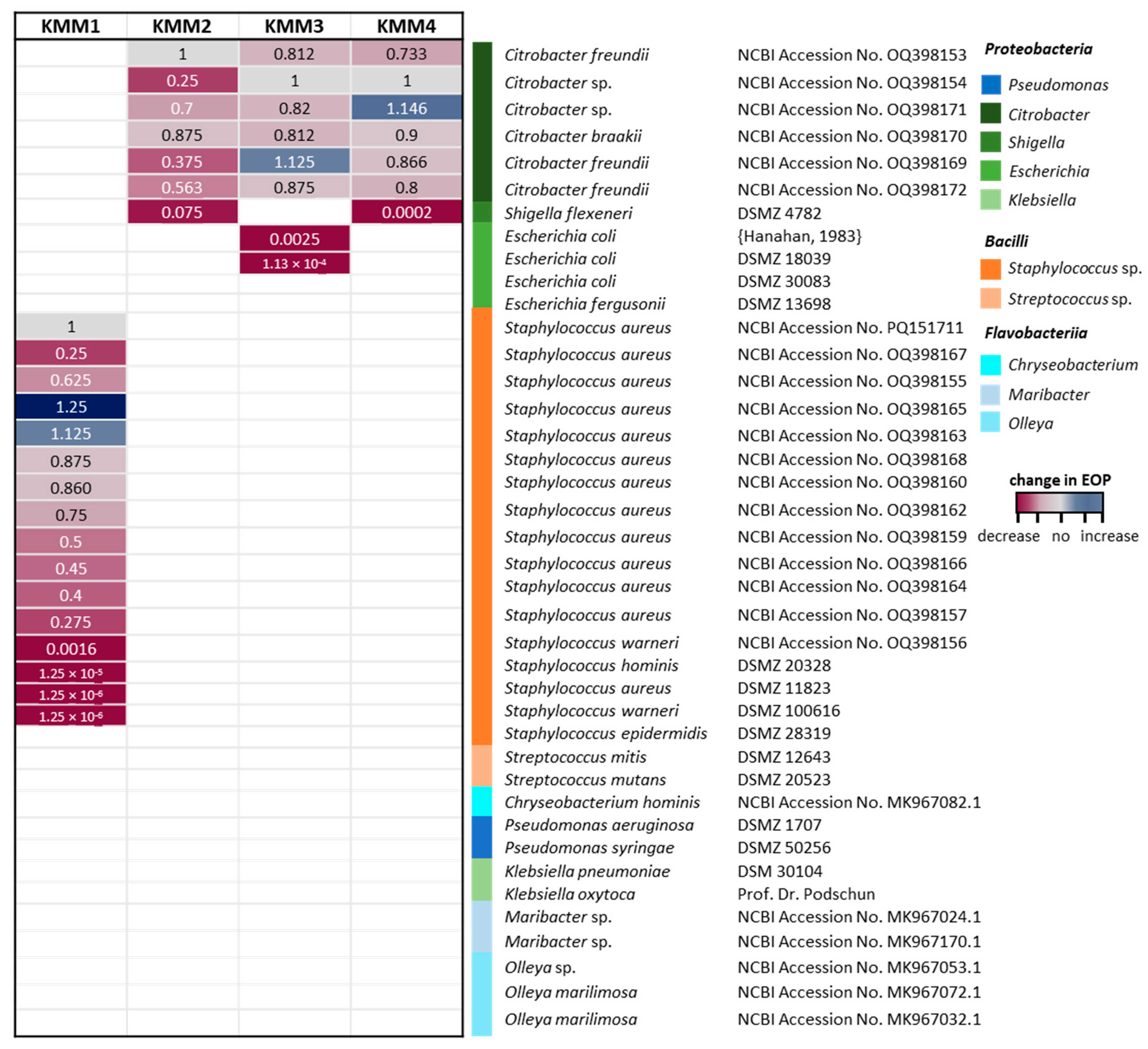
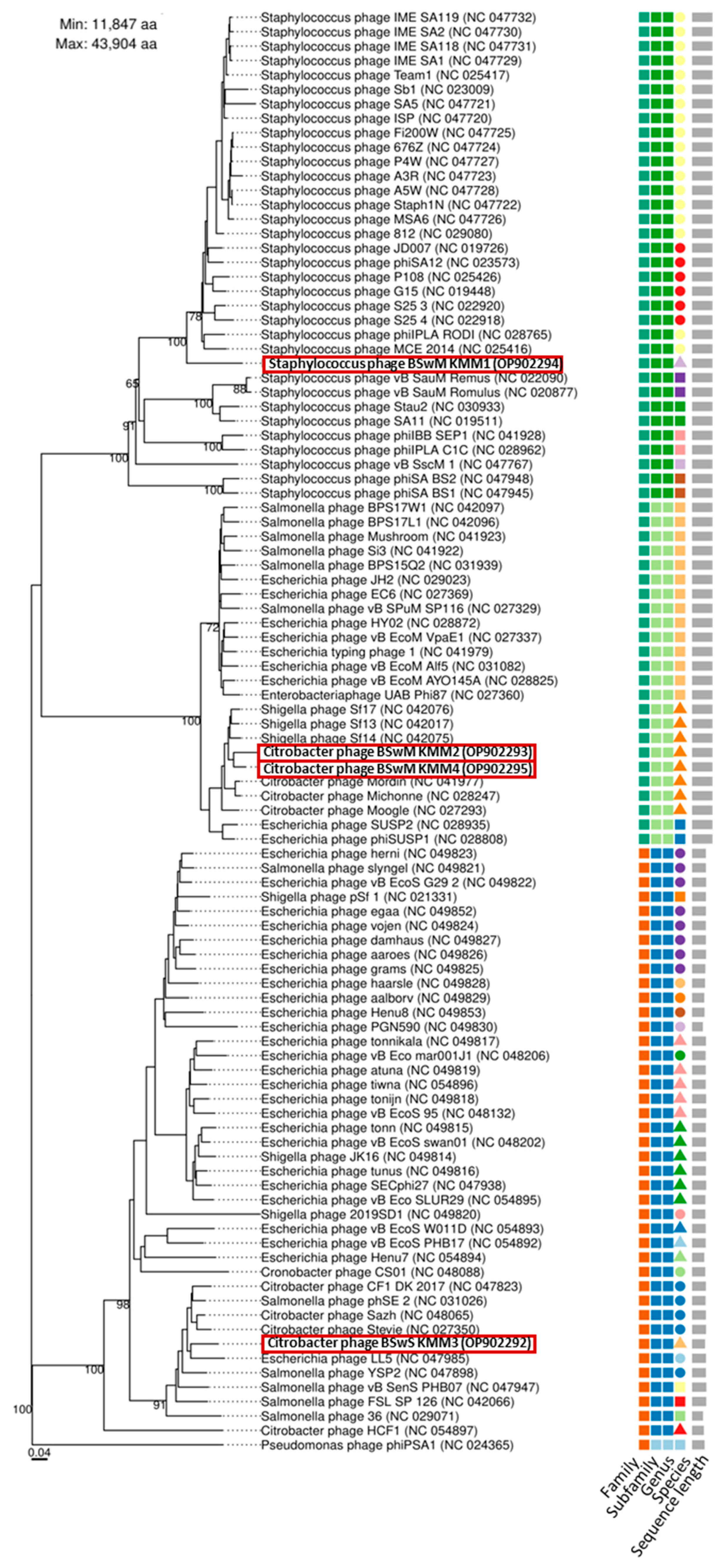
3.4. All Isolated Phages Are Highly Specific and Effective
- The corrected paragraph 2–5 of Section 4 was as follows:
References
- 114.
- Shineh, G.; Mobaraki, M.; Perves Bappy, M.J.; Mills, D.K. Biofilm formation, and related impacts on healthcare, food processing and packaging, industrial manufacturing, marine industries, and sanitation—A review. Appl. Microbiol. 2023, 3, 629–665.
- 115.
- Zammuto, V.; Rizzo, M.G.; Spano, A.; Spagnuolo, D.; Di Martino, A.; Morabito, M.; Manghisi, A.; Genovese, G.; Guglielmino, S.; Calabrese, G. Effects of crude polysaccharides from marine macroalgae on the adhesion and biofilm formation of Pseudomonas aeruginosa and Staphylococcus aureus. Algal Res. 2022, 63, 102646.
- 116.
- Sentenac, H.; Loyau, A.; Leflaive, J.; Schmeller, D.S. The significance of biofilms to human, animal, plant and ecosystem health. Funct. Ecol. 2022, 36, 294–313.
- 117.
- Singh, A.K.; Kaur, R.; Verma, S.; Singh, S. Antimicrobials and antibiotic resistance genes in water bodies: Pollution, risk, and control. Front. Environ. Sci. 2022, 10, 830861.
- 118.
- Jampani, M.; Mateo-Sagasta, J.; Chandrasekar, A.; Fatta-Kassinos, D.; Graham, D.W.; Gothwal, R.; Moodley, A.; Chadag, V.M.; Wiberg, D.; Langan, S. Fate and transport modelling for evaluating antibiotic resistance in aquatic environments: Current knowledge and research priorities. J. Hazard. Mater. 2023, 461, 132527.
- 119.
- Lajqi Berisha, N.; Poceva Panovska, A.; Hajrulai-Musliu, Z. Antibiotic Resistance and Aquatic Systems: Importance in Public Health. Water 2024, 16, 2362.
- 120.
- Sarkar, S.; Kamle, M.; Bharti, A.; Kumar, P. Antibiotic-resistant bacteria risks and challenges for human health and environment: An overview. World J. Environ. Biosci. 2023, 12, 26–34.
- 121.
- Ferheen, I.; Spurio, R.; Marcheggiani, S. Vehicle transmission of antibiotic-resistant pathogens mediated by plastic debris in aquatic ecosystems. iScience 2024, 27, 110026.
- 123.
- Schilcher, K.; Horswill, A.R. Staphylococcal biofilm development: Structure, regulation, and treatment strategies. Microbiol. Mol. Biol. Rev. 2020, 84, 10–1128.
- 124.
- Nogales, B.; Lanfranconi, M.P.; Piña-Villalonga, J.M.; Bosch, R. Anthropogenic perturbations in marine microbial communities. FEMS Microbiol. Rev. 2011, 35, 275–298.
- 125.
- Gambino, D.; Vicari, D.; Vitale, M.; Schirò, G.; Mira, F.; Giglia, M.L.; Riccardi, A.; Gentile, A.; Giardina, S.; Carrozzo, A. Study on bacteria isolates and antimicrobial resistance in wildlife in Sicily, southern Italy. Microorganisms 2021, 9, 203.
- 128.
- Royam, M.M.; Nachimuthu, R. Isolation, characterization, and efficacy of bacteriophages isolated against Citrobacter spp. an in vivo approach in a zebrafish model (Danio rerio). Res. Microbiol. 2020, 171, 341–350.
- 129.
- Castledine, M.; Buckling, A. Critically evaluating the relative importance of phage in shaping microbial community composition. Trends Microbiol. 2024, 32, 957–969.
- 131.
- Santos, J.D.; Vitorino, I.; Reyes, F.; Vicente, F.; Lage, O.M. From ocean to medicine: Pharmaceutical applications of metabolites from marine bacteria. Antibiotics 2020, 9, 455.
- 132.
- García, P.; Madera, C.; Martinez, B.; Rodríguez, A.; Suárez, J.E. Prevalence of bacteriophages infecting Staphylococcus aureus in dairy samples and their potential as biocontrol agents. J. Dairy Sci. 2009, 92, 3019–3026.
- 133.
- Sala-Comorera, L.; Nolan, T.M.; Reynolds, L.J.; Venkatesh, A.; Cheung, L.; Martin, N.A.; Stephens, J.H.; Gitto, A.; O’Hare, G.M.; O’Sullivan, J.J. Bacterial and bacteriophage antibiotic resistance in marine bathing waters in relation to rivers and urban streams. Front. Microbiol. 2021, 12, 718234.
- 134.
- Blanco-Picazo, P.; Roscales, G.; Toribio-Avedillo, D.; Gómez-Gómez, C.; Avila, C.; Ballesté, E.; Muniesa, M.; Rodríguez-Rubio, L. Antibiotic resistance genes in phage particles from Antarctic and Mediterranean seawater ecosystems. Microorganisms 2020, 8, 1293.
- 135.
- Liu, K.; Wang, C.; Zhou, X.; Guo, X.; Yang, Y.; Liu, W.; Zhao, R.; Song, H. Bacteriophage therapy for drug-resistant Staphylococcus aureus infections. Front. Cell. Infect. Microbiol. 2024, 14, 1336821.
- 136.
- Petrovic Fabijan, A.; Lin, R.C.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R. Safety of bacteriophage therapy in severe Staphylococcus aureus infection. Nat. Microbiol. 2020, 5, 465–472.
- 147.
- Rai, S.; Kaur, B.; Singh, P.; Singh, A.; Benjakul, S.; Vijay Kumar Reddy, S.; Nagar, V.; Tyagi, A. Perspectives on phage therapy for health management in aquaculture. Aquac. Int. 2024, 32, 1349–1393.
- 148.
- Lomelí-Ortega, C.O.; Balcázar, J.L.; Quiroz-Guzmán, E. Phage therapy and aquaculture: Progress and challenges. Int. Microbiol. 2023, 26, 439–441.
- 149.
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31.
- 150.
- Schackart, K.E., III; Graham, J.B.; Ponsero, A.J.; Hurwitz, B.L. Evaluation of computational phage detection tools for metagenomic datasets. Front. Microbiol. 2023, 14, 1078760.
Reference
- Stante, M.; Weiland-Bräuer, N.; Repnik, U.; Werner, A.; Bramkamp, M.; Chibani, C.M.; Schmitz, R.A. Four Novel Caudoviricetes Bacteriophages Isolated from Baltic Sea Water Infect Colonizers of Aurelia aurita. Viruses 2023, 15, 1525. [Google Scholar] [CrossRef] [PubMed]
| Strain No. | Strain | Reference | Phylum | Class | Order | Family | Source | Growth Medium | Growth Temp. | Use in This Study |
|---|---|---|---|---|---|---|---|---|---|---|
| 74 | Micrococcus luteus | MK967048.1 | Actinomycetota | Actinomycetia | Micrococcales | Micrococcaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 75 | Arthrobacter sp. | MK967049.1 | Actinomycetota | Actinomycetia | Micrococcales | Micrococcaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 83 | Gordonia terrae | MK967057.1 | Actinomycetota | Actinomycetia | Mycobacteriales | Gordoniaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 15 | Sulfitobacter sp. | MK967015.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 20 | Sulfitobacter pontiacus | MK967020.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 23 | Sulfitobacter sp. | MK967023.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 69 | Rhodobacter sp. | MK967043.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | M. leidyi Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 78 | Sulfitobacter sp. | MK967052.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 86 | Ruegeria sp. | MK967060.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 89 | Ruegeria sp. | MK967063.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 100 | Sulfitobacter sp. | MK967074.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 117 | Ruegeria mobilis | MK967091.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | A. aurita polyp North Atlantic husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 147 | Phaeobacter gallaeciensis | MK967120.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 188 | Sulfitobacter pseudonitzschiae | MK967160.1 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | Artificial Seawater 30 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 13 | Bacillus cereus | MK967013.1 | Bacillota | Bacilli | Bacillales | Bacillaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 16 | Bacillus sp. | MK967016.1 | Bacillota | Bacilli | Bacillales | Bacillaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 17 | Bacillus cereus | MK967017.1 | Bacillota | Bacilli | Bacillales | Bacillaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 19 | Bacillus sp. | MK967019.1 | Bacillota | Bacilli | Bacillales | Bacillaceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 76 | Bacillus weihenstephanensis | MK967050.1 | Bacillota | Bacilli | Bacillales | Bacillaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 85 | Staphylococcus warneri | MK967059.1 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 88 | Staphylococcus sp. | MK967062.1 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 73 | Enterococcus casseliflavus | MK967047.1 | Bacillota | Bacilli | Lactobacillales | Enterococcaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 24 | Maribacter sp. | MK967024.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 57 | Olleya marilimosa | MK967032.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 79 | Olleya sp. | MK967053.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 181 | Chryseobacterium sp. | MK967154.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Weeksellaceae | Artificial Seawater 30 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 257 | Chryseobacterium sp. | MK967218.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Weeksellaceae | M. leidyi Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 22 | Pseudolateromonas sp. | MK967022.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 91 | Pseudoalteromonas prydzensis | MK967065.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 101 | Pseudoalteromonas issachenkonii | MK967075.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 167 | Pseudoalteromonas sp. | MK967140.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 203 | Pseudoalteromonas espejiana | MK967174.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | Artificial Seawater 30 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 219 | Pseudoalteromonas tunicata | MK967188.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 224 | Pseudoalteromonas lipolytica | MK967191.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Pseudoalteromonadaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 105 | Shewanella basaltis | MK967079.1 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Shewanellaceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 21 | Cobetia amphilecti | MK967021.1 | Pseudomonadota | Gammaproteobacteria | Oceanospirillales | Halomonadaceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 55 | Marinomonas hwangdonensis | MK967030.1 | Pseudomonadota | Gammaproteobacteria | Oceanospirillales | Oceanospirillaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 222 | Marinomonas pontica | MK967189.1 | Pseudomonadota | Gammaproteobacteria | Oceanospirillales | Oceanospirillaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 262 | Oceanospirillaceae bacterium | MK967222.1 | Pseudomonadota | Gammaproteobacteria | Oceanospirillales | Oceanospirillaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 11 | Pseudomonas sp. | MK967012.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 90 | Pseudomonas putida | MK967064.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 92 | Pseudomonas putida | MK967066.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 93 | Pseudomonas sp. | MK967067.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 94 | Pseudomonas sp. | MK967068.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 132 | Pseudomonas fluorescens | MK967106.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 196 | Pseudomonas syringae | MK967168.1 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Artificial Seawater 30 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 77 | Vibrio anguillarum | MK967051.1 | Pseudomonadota | Gammaproteobacteria | Vibrionales | Vibrionaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 80 | Vibrio anguillarum | MK967054.1 | Pseudomonadota | Gammaproteobacteria | Vibrionales | Vibrionaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 18 | Staphylococcus aureus | OQ398157 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 134 | Staphylococcus aureus | OQ398164 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | enrichment/ first screening |
| 87 | Staphylococcus aureus | OQ398160 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | enrichment/ first screening |
| 14 | Staphylococcus warneri | OQ398156 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening |
| 6 | Citrobacter freundii | OQ398153 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening/ host range |
| 7 | Citrobacter sp. | OQ398154 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening/ host range |
| 8 | Staphylococcus aureus | PQ151711 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | enrichment/ first screening/ host range |
| 62 | Sulfitobacter pontiacus | OQ398158 | Pseudomonadota | Alphaproteobacteria | Rhodobacterales | Rhodobacteraceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | host range |
| 97 | Shewanella sp. | OQ398161 | Pseudomonadota | Gammaproteobacteria | Alteromonadales | Shewanellaceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | host range |
| 199 | Staphylococcus aureus | OQ398168 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | Artificial Seawater 30 PSU | Marine Bouillon | 30 °C | host range |
| DSMZ 11823 | Staphylococcus aureus | DSMZ 11823 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | clinical material | Trypticase Soy Yeast Broth | 37 °C | host range |
| 67 | Staphylococcus aureus | OQ398159 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | M. leidyi Baltic Sea husbandry | Marine Bouillon | 30 °C | host range |
| 102 | Staphylococcus aureus | OQ398162 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | host range |
| 158 | Staphylococcus aureus | OQ398165 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | host range |
| 161 | Staphylococcus aureus | OQ398166 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | host range |
| 127 | Staphylococcus aureus | OQ398163 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita polyp North Atlantic husbandry | Marine Bouillon | 30 °C | host range |
| DSMZ 28319 | Staphylococcus epidermidis | DSMZ 28319 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | catheter sepsis | Trypticase Soy Yeast Broth | 37 °C | host range |
| DSMZ 20328 | Staphylococcus hominis | DSMZ 20328 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | human skin | Trypticase Soy Yeast Broth | 37 °C | host range |
| DSMZ 100616 | Staphylococcus warneri | DSMZ 100616 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | cleanroom facility, TAS | Trypticase Soy Yeast Broth | 30 °C | host range |
| DSMZ 12643 | Streptococcus mitis | DSMZ 12643 | Bacillota | Bacilli | Lactobacillales | Streptococcaceae | oral cavity, human | Trypticase Soy Yeast Broth | 37 °C | host range |
| DSMZ 20523 | Streptococcus mutans | DSMZ 20523 | Bacillota | Bacilli | Lactobacillales | Streptococcaceae | carious dentine | Trypticase Soy Yeast Broth | 37 °C | host range |
| 296 | Citrobacter braakii | OQ398170 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | A. aurita polyp North Atlantic husbandry | Marine Bouillon | 30 °C | host range |
| 283 | Citrobacter freundii | OQ398169 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | host range |
| 321 | Citrobacter freundii | OQ398172 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | Artifical Seawater 30 PSU | Marine Bouillon | 30 °C | host range |
| 313 | Citrobacter sp. | OQ398171 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | Artifical Seawater 18 PSU | Marine Bouillon | 30 °C | host range |
| DSMZ 18039 | Escherichia coli | DSMZ 18039 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | unknown source | Luria-Bertani Bouillon | 37 °C | host range |
| strain 8 | Escherichia coli | [47] | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | unknown source | Luria-Bertani Bouillon | 37 °C | host range |
| DSMZ 30083 | Escherichia coli | DSMZ 30083 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | urine | Luria-Bertani Bouillon | 37 °C | host range |
| DSMZ 13698 | Escherichia fergusonii | DSMZ 13698 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | faeces of 1-year-old boy | Luria-Bertani Bouillon | 37 °C | host range |
| strain 27 | Klebsiella oxytoca | Prof. Dr. Podschun, (National Reference Laboratory for Klebsiella species, Kiel University) | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | unknown source | Nutrient Broth | 30 °C | host range |
| DSMZ 30104 | Klebsiella pneumoniae | DSMZ 30104 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | unknown source | Nutrient Broth | 30 °C | host range |
| DSMZ 4782 | Shigella flexeneri | DSMZ 4782 | Pseudomonadota | Gammaproteobacteria | Enterobacterales | Enterobacteriaceae | unknown source | Caso Bouillon | 37 °C | host range |
| DSMZ 1707 | Pseudomonas aeruginosa | DSMZ 1707 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | unknown source | Caso Bouillon | 30 °C | host range |
| 9 | Staphylococcus aureus | OQ398155 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | A. aurita medusa Baltic Sea | Marine Bouillon | 30 °C | host range |
| 170 | Staphylococcus aureus | OQ398167 | Bacillota | Bacilli | Bacillales | Staphylococcaceae | Artificial Seawater 18 PSU | Marine Bouillon | 30 °C | host range |
| DSMZ 50256 | Pseudomonas syringae | DSMZ 50256 | Pseudomonadota | Gammaproteobacteria | Pseudomonadales | Pseudomonadaceae | Triticum aestivum, glume rot of wheat | Caso Bouillon | 30 °C | host range |
| 24 | Maribacter sp. | MK967024.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | A. aurita medusa Baltic Sea husbandry | Marine Bouillon | 30 °C | host range |
| 79 | Olleya sp. | MK967053.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | A. aurita polyp Baltic Sea husbandry | Marine Bouillon | 30 °C | host range |
| 98 | Olleya marilimosa | MK967072.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | host range |
| 108 | Chryseobacterium hominis | MK967082.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | A. aurita polyp North Sea husbandry | Marine Bouillon | 30 °C | host range |
| 57 | Olleya marilimosa | MK967032.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | M. leidyi Baltic Sea | Marine Bouillon | 30 °C | host range |
| 199 | Maribacter sp. | MK967170.1 | Bacteroidota | Flavobacteriia | Flavobacteriales | Flavobacteriaceae | Artificial Seawater 30 PSU | Marine Bouillon | 30 °C | host range |
| Phage | NCBI Accession No. | No. of Reads | No. of Filtered Reads | Sequence Coverage | N50 | Genome Length (bps) | GC Content (%) | Predicted ORFs | Unknown Proteins |
|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus phage BSwM KMM1 | OP902294 | 4.085 | 3.214 | 247.488 | 17.553 | 137.386 | 31.77 | 259 | 200 |
| Citrobacter phage BSwM KMM2 | OP902295 | 810 | 595 | 74.163 | 22.118 | 88.537 | 39.55 | 137 | 94 |
| Citrobacter phage BSwS KMM3 | OP902292 | 837 | 598 | 130.676 | 20.517 | 49.164 | 43.17 | 92 | 58 |
| Citrobacter phage BSwM KMM4 | OP902293 | 6.433 | 5.371 | 544.327 | 23.894 | 86.911 | 39.02 | 138 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stante, M.; Weiland-Bräuer, N.; Repnik, U.; Werner, A.; Bramkamp, M.; Chibani, C.M.; Schmitz, R.A. Correction: Stante et al. Four Novel Caudoviricetes Bacteriophages Isolated from Baltic Sea Water Infect Colonizers of Aurelia aurita. Viruses 2023, 15, 1525. Viruses 2024, 16, 1880. https://doi.org/10.3390/v16121880
Stante M, Weiland-Bräuer N, Repnik U, Werner A, Bramkamp M, Chibani CM, Schmitz RA. Correction: Stante et al. Four Novel Caudoviricetes Bacteriophages Isolated from Baltic Sea Water Infect Colonizers of Aurelia aurita. Viruses 2023, 15, 1525. Viruses. 2024; 16(12):1880. https://doi.org/10.3390/v16121880
Chicago/Turabian StyleStante, Melissa, Nancy Weiland-Bräuer, Urska Repnik, Almut Werner, Marc Bramkamp, Cynthia M. Chibani, and Ruth A. Schmitz. 2024. "Correction: Stante et al. Four Novel Caudoviricetes Bacteriophages Isolated from Baltic Sea Water Infect Colonizers of Aurelia aurita. Viruses 2023, 15, 1525" Viruses 16, no. 12: 1880. https://doi.org/10.3390/v16121880
APA StyleStante, M., Weiland-Bräuer, N., Repnik, U., Werner, A., Bramkamp, M., Chibani, C. M., & Schmitz, R. A. (2024). Correction: Stante et al. Four Novel Caudoviricetes Bacteriophages Isolated from Baltic Sea Water Infect Colonizers of Aurelia aurita. Viruses 2023, 15, 1525. Viruses, 16(12), 1880. https://doi.org/10.3390/v16121880






