PLNC8 αβ Potently Inhibits the Flavivirus Kunjin and Modulates Inflammatory and Intracellular Signaling Responses of Alveolar Epithelial Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Virus Strains and Propagation
2.3. Peptides
2.4. Viral Infection and Peptide Treatment of A549 Cells
2.5. RT-PCR
2.6. Plaque Assay
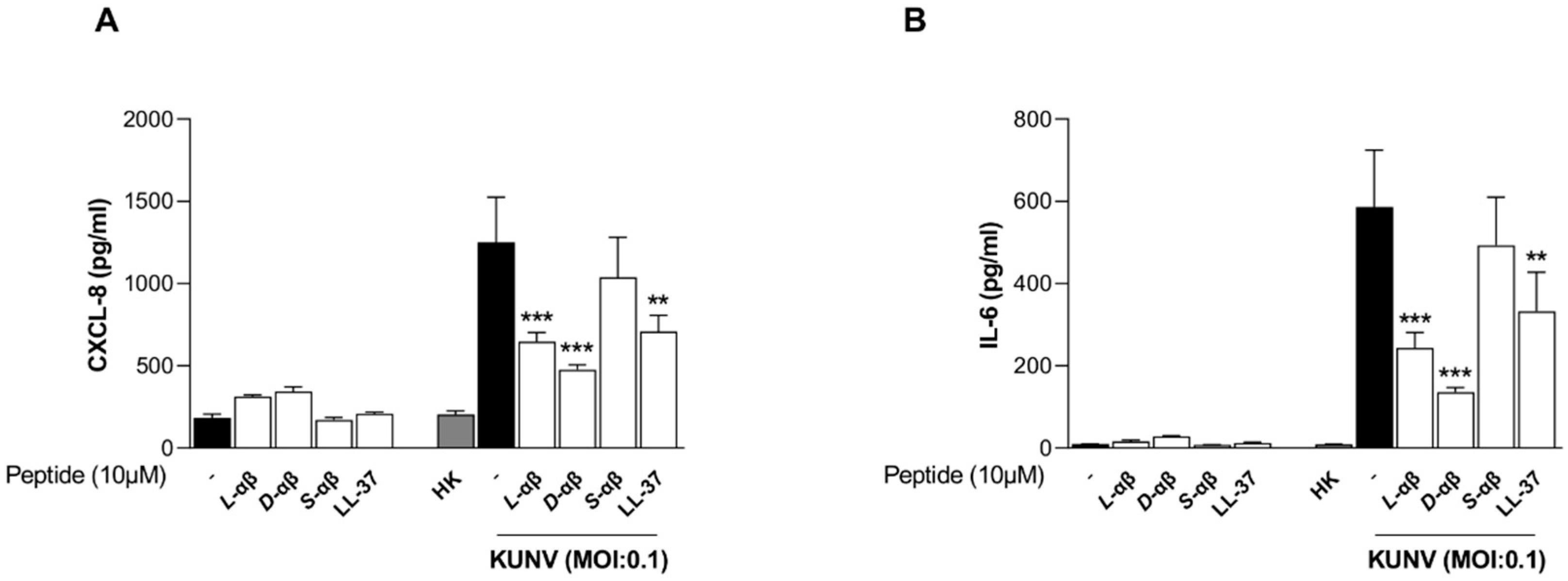
2.7. ELISA
2.8. Cytotoxicity Assay
2.9. Western Blot
2.10. Statistics
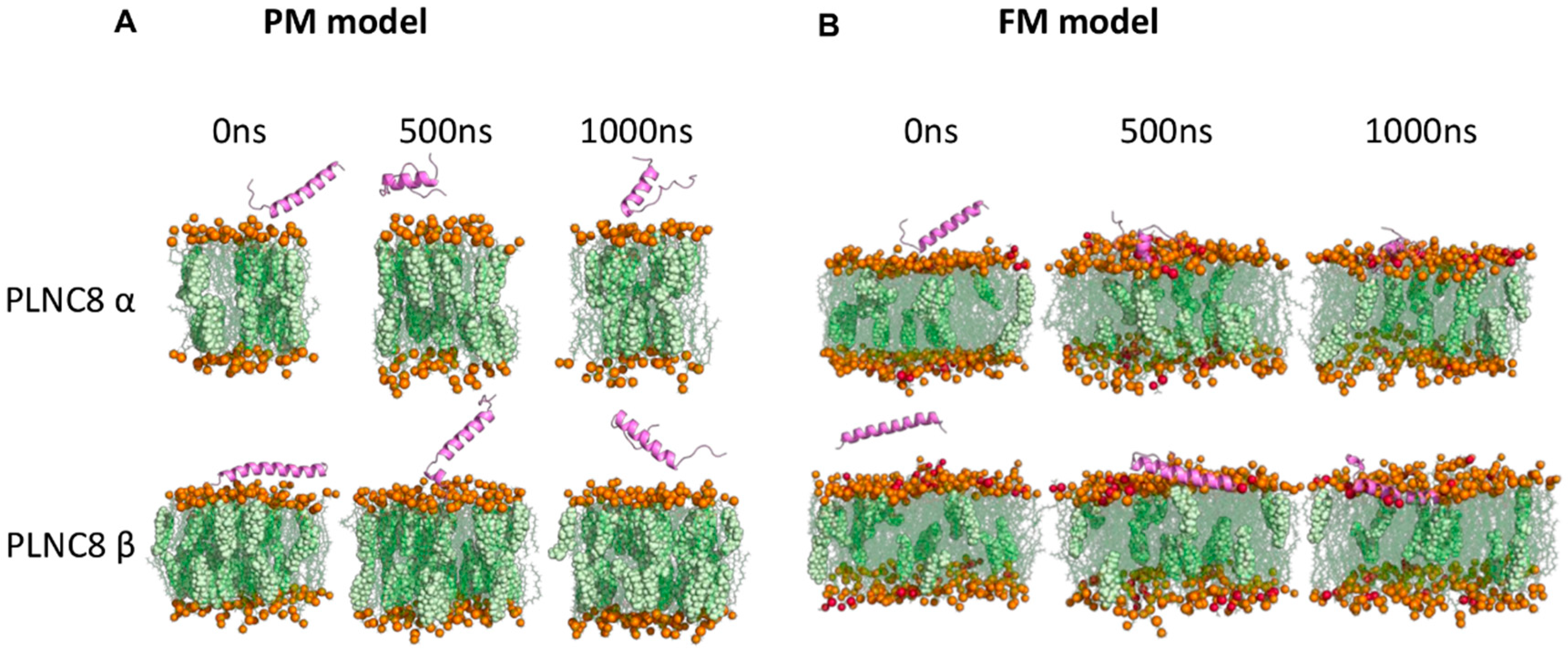
2.11. In Silico Methods
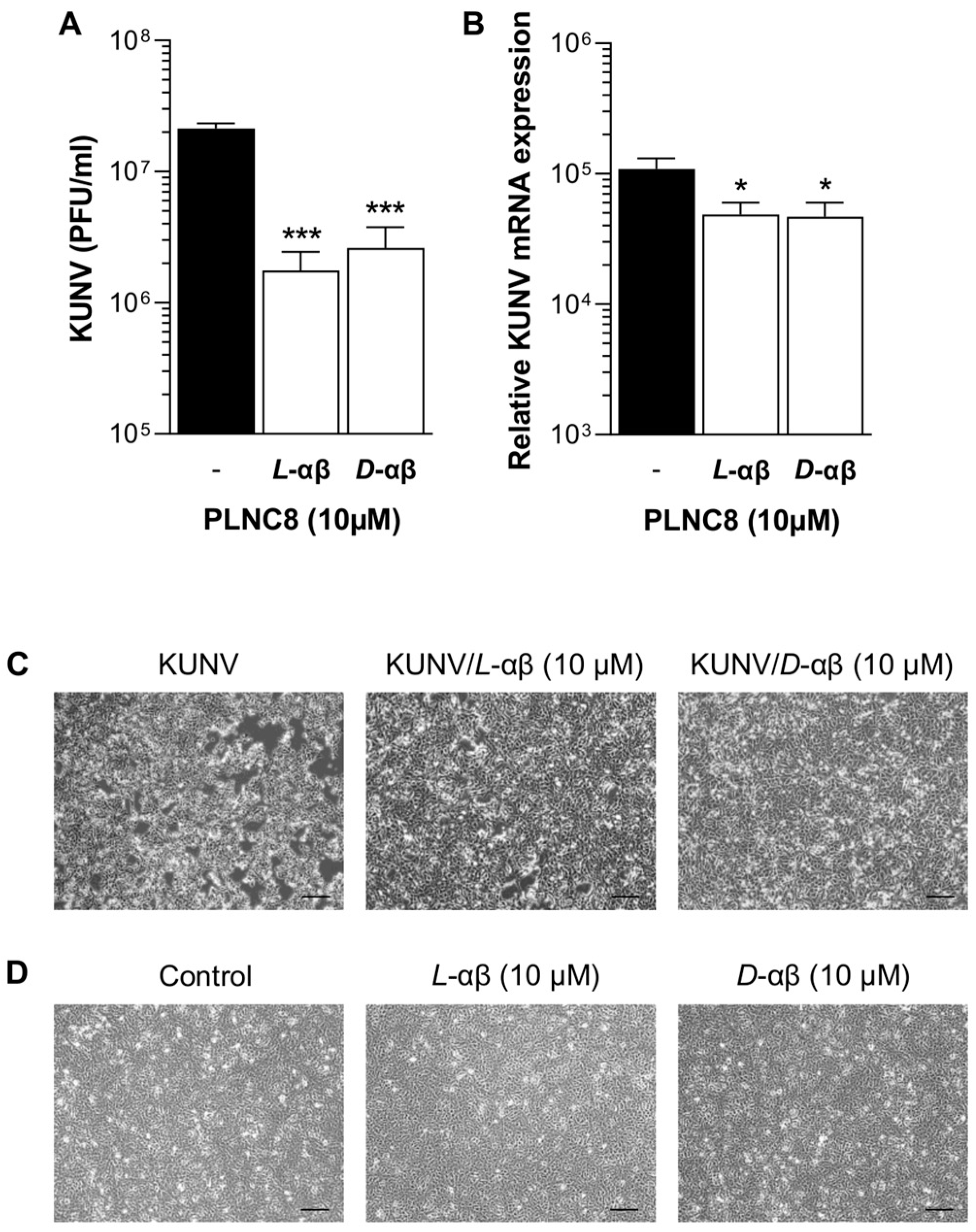
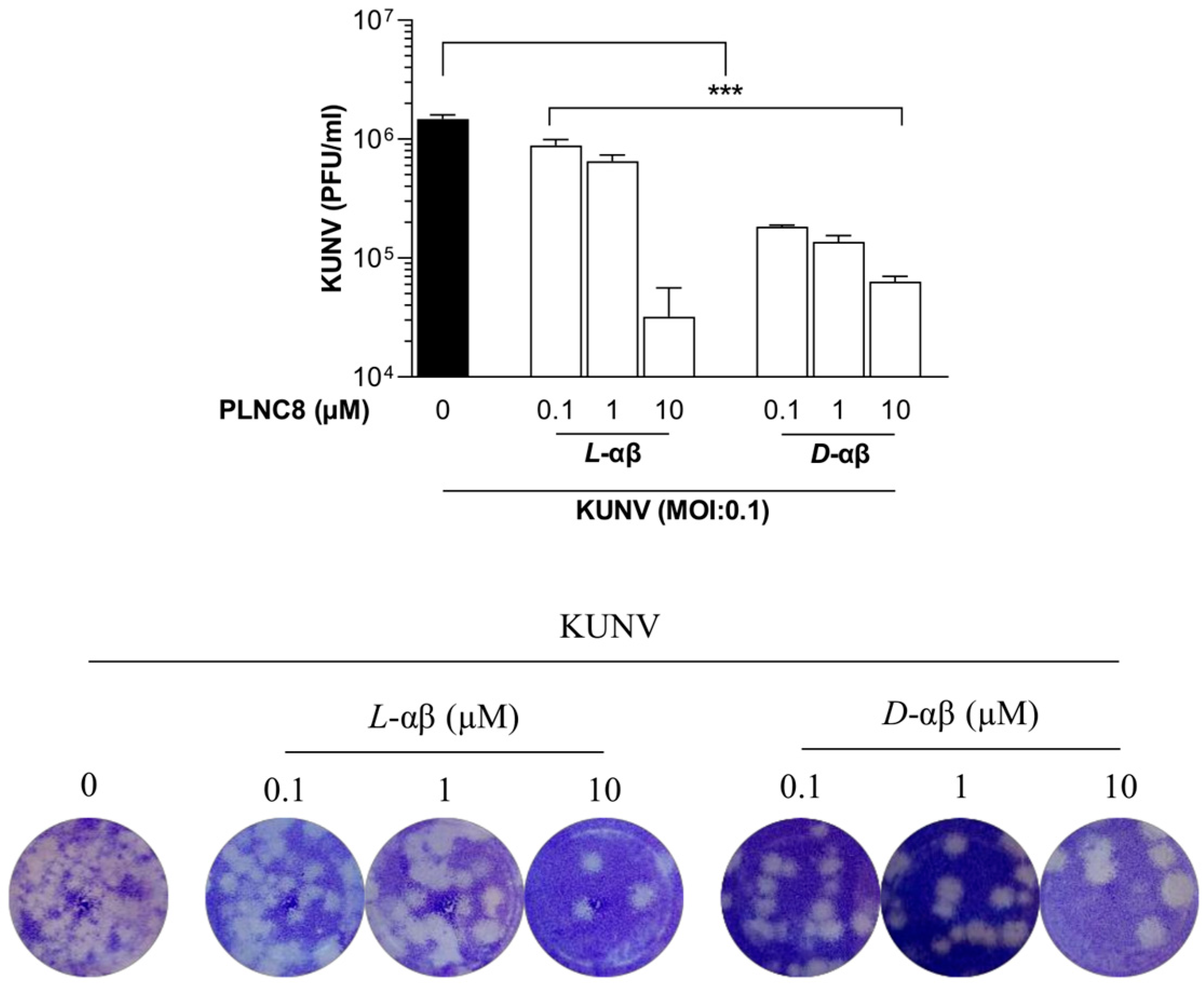
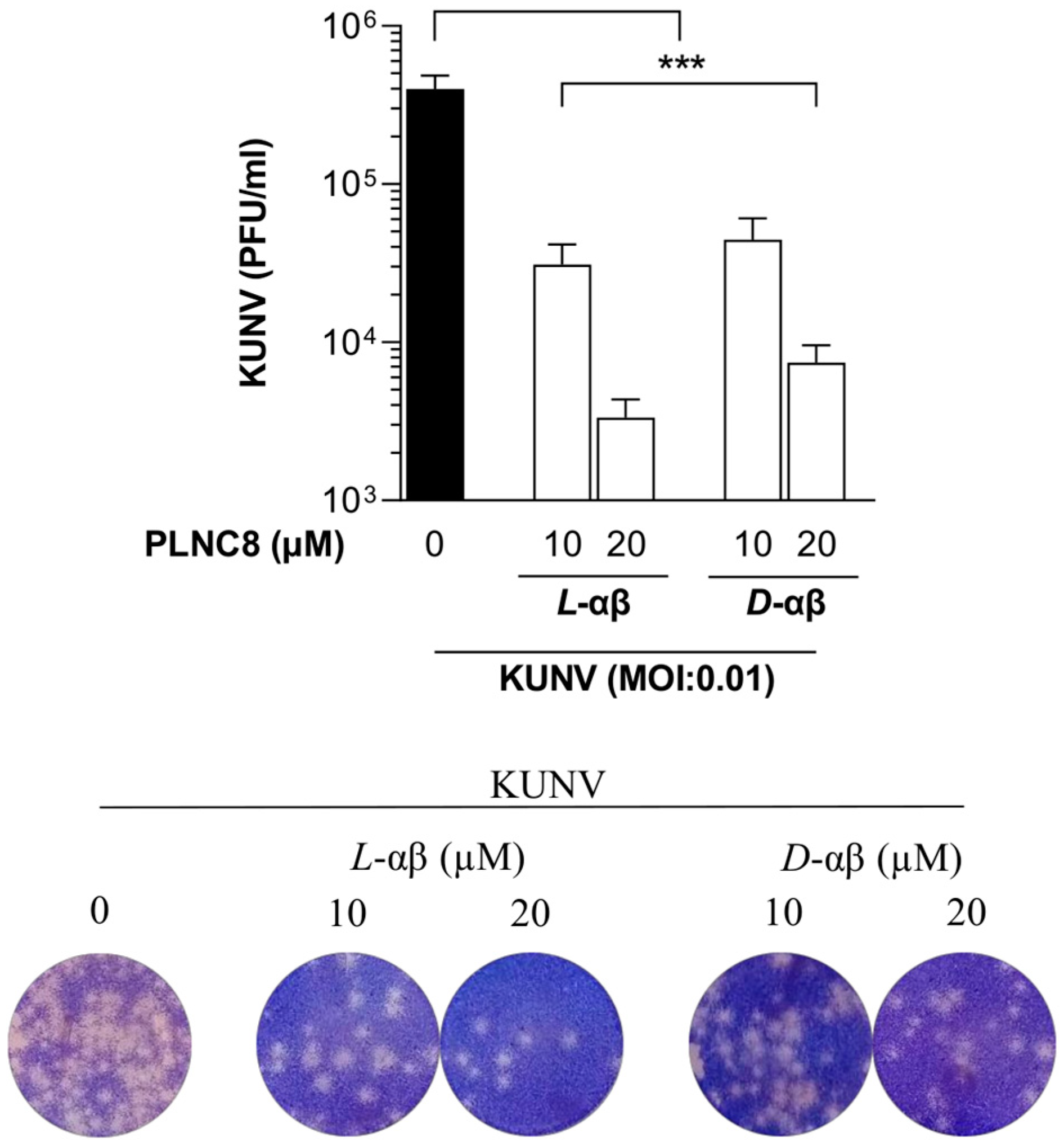
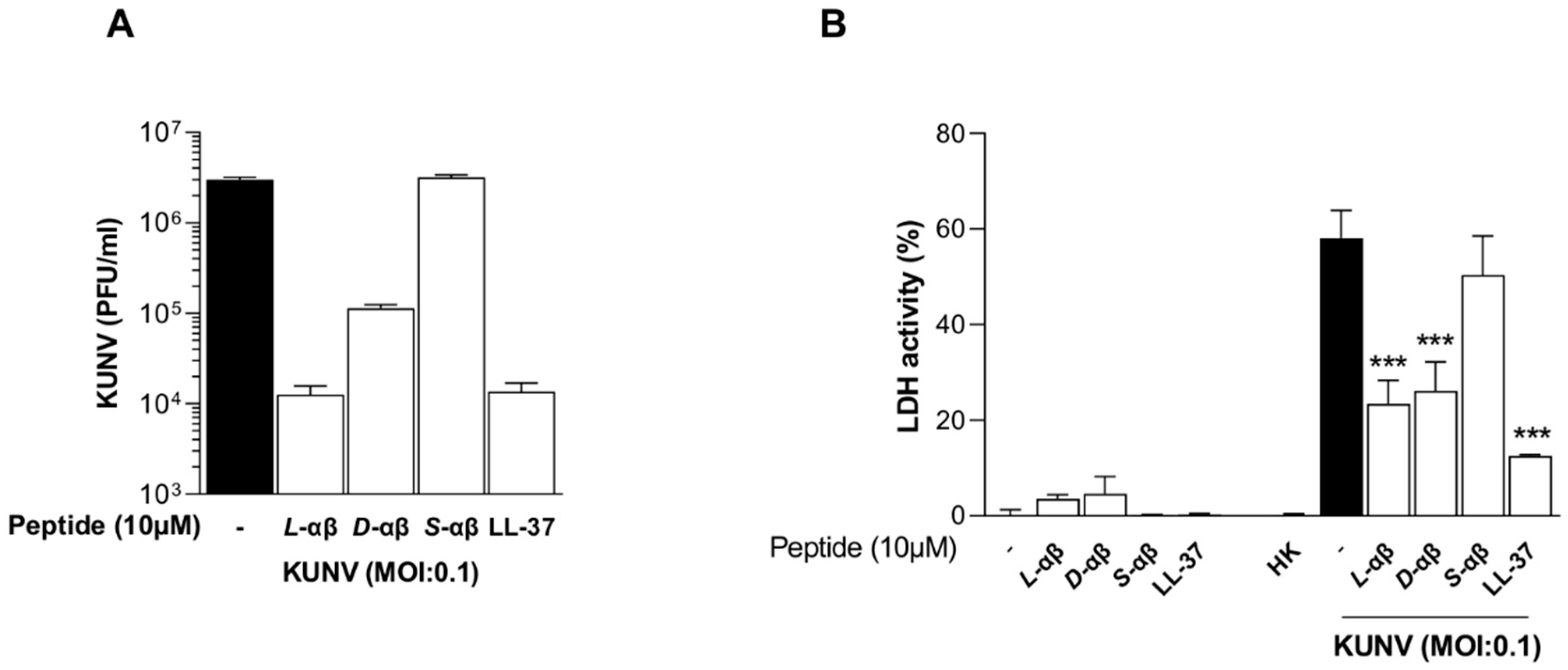
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, D.W.; Ramamoorthy, A. Studies on anticancer activities of antimicrobial peptides. Biochim. Biophys. Acta (BBA)-Biomembr. 2008, 1778, 357–375. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef] [PubMed]
- Buda De Cesare, G.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Pushpanathan, M.; Gunasekaran, P.; Rajendhran, J. Antimicrobial peptides: Versatile biological properties. Int. J. Pept. 2013, 2013, 675391. [Google Scholar] [CrossRef]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef]
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Håkansson, J.; Ringstad, L.; Björn, C. Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef]
- Chou, H.-T.; Wen, H.-W.; Kuo, T.-Y.; Lin, C.-C.; Chen, W.-J. Interaction of cationic antimicrobial peptides with phospholipid vesicles and their antibacterial activity. Peptides 2010, 31, 1811–1820. [Google Scholar] [CrossRef]
- Luteijn, R.D.; Praest, P.; Thiele, F.; Sadasivam, S.M.; Singethan, K.; Drijfhout, J.W.; Bach, C.; de Boer, S.M.; Lebbink, R.J.; Tao, S.; et al. A Broad-Spectrum Antiviral Peptide Blocks Infection of Viruses by Binding to Phosphatidylserine in the Viral Envelope. Cells 2020, 9, 1989. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.G.; Akbulut, B.S.; Ozkirimli, E. Membrane Active Peptides and Their Biophysical Characterization. Biomolecules 2018, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Duarte-Mata, D.I.; Salinas-Carmona, M.C. Antimicrobial peptides’ immune modulation role in intracellular bacterial infection. Front. Immunol. 2023, 14, 1119574. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef]
- Auvynet, C.; Rosenstein, Y. Multifunctional host defense peptides: Antimicrobial peptides, the small yet big players in innate and adaptive immunity. FEBS J. 2009, 276, 6497–6508. [Google Scholar] [CrossRef]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Innate immunity to virus infection. Immunol. Rev. 2009, 227, 75–86. [Google Scholar] [CrossRef]
- Daffis, S.; Samuel, M.A.; Suthar, M.S.; Gale, M., Jr.; Diamond, M.S. Toll-like receptor 3 has a protective role against West Nile virus infection. J. Virol. 2008, 82, 10349–10358. [Google Scholar] [CrossRef]
- Szretter, K.J.; Daffis, S.; Patel, J.; Suthar, M.S.; Klein, R.S.; Gale, M., Jr.; Diamond, M.S. The innate immune adaptor molecule MyD88 restricts West Nile virus replication and spread in neurons of the central nervous system. J. Virol. 2010, 84, 12125–12138. [Google Scholar] [CrossRef]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Lee, E.Y.; Lee, M.W.; Wong, G.C.L. Modulation of toll-like receptor signaling by antimicrobial peptides. Semin. Cell Dev. Biol. 2019, 88, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Sharma, N.; Arina, P.; Defilippi, P. Molecular Insights into the MAPK Cascade during Viral Infection: Potential Crosstalk between HCQ and HCQ Analogues. Biomed. Res. Int. 2020, 2020, 8827752. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Xie, Y.; Gao, X.; Wang, Q.; Zhu, W.Y. Inflammatory response and MAPK and NF-κB pathway activation induced by natural street rabies virus infection in the brain tissues of dogs and humans. Virol. J. 2020, 17, 157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Regulation of NF-κB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Peng, H.; Shi, M.; Zhang, L.; Li, Y.; Sun, J.; Zhang, L.; Wang, X.; Xu, X.; Zhang, X.; Mao, Y.; et al. Activation of JNK1/2 and p38 MAPK signaling pathways promotes enterovirus 71 infection in immature dendritic cells. BMC Microbiol. 2014, 14, 147. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Pathological roles of MAPK signaling pathways in human diseases. Biochim. Biophys. Acta 2010, 1802, 396–405. [Google Scholar] [CrossRef]
- Jang, M.; Kim, J.; Choi, Y.; Bang, J.; Kim, Y. Antiseptic Effect of Ps-K18: Mechanism of Its Antibacterial and Anti-Inflammatory Activities. Int. J. Mol. Sci. 2019, 20, 4895. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Van Wetering, S.; Mannesse-Lazeroms, S.P.; Van Sterkenburg, M.A.; Daha, M.R.; Dijkman, J.H.; Hiemstra, P.S. Effect of defensins on interleukin-8 synthesis in airway epithelial cells. Am. J. Physiol. 1997, 272, L888–L896. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.G.; Davidson, D.J.; Gold, M.R.; Bowdish, D.; Hancock, R.E. The human antimicrobial peptide LL-37 is a multifunctional modulator of innate immune responses. J. Immunol. 2002, 169, 3883–3891. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef] [PubMed]
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Scherret, J.H.; Poidinger, M.; Mackenzie, J.S.; Broom, A.K.; Deubel, V.; Lipkin, W.I.; Briese, T.; Gould, E.A.; Hall, R.A. The relationships between West Nile and Kunjin viruses. Emerg. Infect. Dis. 2001, 7, 697–705. [Google Scholar] [CrossRef]
- Hall, R.A.; Broom, A.K.; Smith, D.W.; Mackenzie, J.S. The Ecology and Epidemiology of Kunjin Virus. In Japanese Encephalitis and West Nile Viruses; Mackenzie, J.S., Barrett, A.D.T., Deubel, V., Eds.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 253–269. [Google Scholar] [CrossRef]
- Hoffmann, H.H.; Schneider, W.M.; Rozen-Gagnon, K.; Miles, L.A.; Schuster, F.; Razooky, B.; Jacobson, E.; Wu, X.; Yi, S.; Rudin, C.M.; et al. TMEM41B Is a Pan-flavivirus Host Factor. Cell 2021, 184, 133–148.e120. [Google Scholar] [CrossRef]
- Boldescu, V.; Behnam, M.A.M.; Vasilakis, N.; Klein, C.D. Broad-spectrum agents for flaviviral infections: Dengue, Zika and beyond. Nat. Rev. Drug Discov. 2017, 16, 565–586. [Google Scholar] [CrossRef]
- Khalaf, H.; Nakka, S.S.; Sandén, C.; Svärd, A.; Hultenby, K.; Scherbak, N.; Aili, D.; Bengtsson, T. Antibacterial effects of Lactobacillus and bacteriocin PLNC8 αβ on the periodontal pathogen Porphyromonas gingivalis. BMC Microbiol. 2016, 16, 188. [Google Scholar] [CrossRef]
- Bengtsson, T.; Selegård, R.; Musa, A.; Hultenby, K.; Utterström, J.; Sivlér, P.; Skog, M.; Nayeri, F.; Hellmark, B.; Söderquist, B.; et al. Plantaricin NC8 αβ exerts potent antimicrobial activity against Staphylococcus spp. and enhances the effects of antibiotics. Sci. Rep. 2020, 10, 3580. [Google Scholar] [CrossRef]
- Omer, A.A.M.; Hinkula, J.; Tran, P.T.; Melik, W.; Zattarin, E.; Aili, D.; Selegård, R.; Bengtsson, T.; Khalaf, H. Plantaricin NC8 αβ rapidly and efficiently inhibits flaviviruses and SARS-CoV-2 by disrupting their envelopes. PLoS ONE 2022, 17, e0278419. [Google Scholar] [CrossRef]
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704. [Google Scholar] [CrossRef] [PubMed]
- Alagarasu, K.; Patil, P.S.; Shil, P.; Seervi, M.; Kakade, M.B.; Tillu, H.; Salunke, A. In-vitro effect of human cathelicidin antimicrobial peptide LL-37 on dengue virus type 2. Peptides 2017, 92, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Zidek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI Input Generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM Simulations Using the CHARMM36 Additive Force Field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Davila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Lee, J.; Patel, D.S.; Stahle, J.; Park, S.J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI Membrane Builder for Complex Biological Membrane Simulations with Glycolipids and Lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Huang, J.; Rauscher, S.; Nawrocki, G.; Ran, T.; Feig, M.; de Groot, B.L.; Grubmuller, H.; MacKerell, A.D., Jr. CHARMM36m: An improved force field for folded and intrinsically disordered proteins. Nat. Methods 2017, 14, 71–73. [Google Scholar] [CrossRef]
- Schrödinger, L. The PyMOL Molecular Graphics System; Version 2.6.0a0; Schrödinger: New York, NY, USA, 2022. [Google Scholar]
- Touw, W.G.; Baakman, C.; Black, J.; Te Beek, T.A.; Krieger, E.; Joosten, R.P.; Vriend, G. A series of PDB-related databanks for everyday needs. Nucleic Acids Res. 2015, 43, D364–D368. [Google Scholar] [CrossRef]
- Smith, P.; Lorenz, C.D. LiPyphilic: A Python Toolkit for the Analysis of Lipid Membrane Simulations. J. Chem. Theory Comput. 2021, 17, 5907–5919. [Google Scholar] [CrossRef] [PubMed]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Selegård, R.; Musa, A.; Nyström, P.; Aili, D.; Bengtsson, T.; Khalaf, H. Plantaricins markedly enhance the effects of traditional antibiotics against Staphylococcus epidermidis. Future Microbiol. 2019, 14, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lee, M.; Fairn, G.D. Phospholipid subcellular localization and dynamics. J. Biol. Chem. 2018, 293, 6230–6240. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, D.Y.; Savushkin, E.V.; Dergunov, A.D. Intracellular and Plasma Membrane Events in Cholesterol Transport and Homeostasis. J. Lipids 2018, 2018, 3965054. [Google Scholar] [CrossRef]
- Findlay, F.; Proudfoot, L.; Stevens, C.; Barlow, P.G. Cationic host defense peptides; novel antimicrobial therapeutics against Category A pathogens and emerging infections. Pathog. Glob. Health 2016, 110, 137–147. [Google Scholar] [CrossRef]
- Sebők, C.; Tráj, P.; Mackei, M.; Márton, R.A.; Vörösházi, J.; Kemény, Á.; Neogrády, Z.; Mátis, G. Modulation of the immune response by the host defense peptide IDR-1002 in chicken hepatic cell culture. Sci. Rep. 2023, 13, 14530. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chow, L.N.; Mookherjee, N. Cationic host defence peptides: Multifaceted role in immune modulation and inflammation. J. Innate Immun. 2012, 4, 361–370. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2563–2582. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Lauder, S.N.; Jones, E.; Smart, K.; Bloom, A.; Williams, A.S.; Hindley, J.P.; Ondondo, B.; Taylor, P.R.; Clement, M.; Fielding, C.; et al. Interleukin-6 limits influenza-induced inflammation and protects against fatal lung pathology. Eur. J. Immunol. 2013, 43, 2613–2625. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chang, L.; Wu, L.; Yuan, Y.F. IL-6 Plays a Crucial Role in HBV Infection. J. Clin. Transl. Hepatol. 2015, 3, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Ito, M.; Chikuma, S.; Akanuma, T.; Nakatsukasa, H. Negative Regulation of Cytokine Signaling in Immunity. Cold Spring Harb. Perspect. Biol. 2018, 10, a028571. [Google Scholar] [CrossRef] [PubMed]
- Babon, J.J.; Varghese, L.N.; Nicola, N.A. Inhibition of IL-6 family cytokines by SOCS3. Semin. Immunol. 2014, 26, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Abdelsam, S.; Ghanem, S.; Zahid, M.; Abunada, H.; Bader, L.; Raïq, H.; Khan, A.; Parray, A.; Djouhri, L.; Agouni, A. Human antigen R: Exploring its inflammatory response impact and significance in cardiometabolic disorders. J. Cell. Physiol. 2024, 239, e31229. [Google Scholar] [CrossRef]
- Cheng, Y.; King, N.J.C.; Kesson, A.M. The role of tumor necrosis factor in modulating responses of murine embryo fibroblasts by flavivirus, West Nile. Virology 2004, 329, 361–370. [Google Scholar] [CrossRef][Green Version]
- Peper, R.L.; Van Campen, H. Tumor necrosis factor as a mediator of inflammation in influenza A viral pneumonia. Microb. Pathog. 1995, 19, 175–183. [Google Scholar] [CrossRef]
- Tyner, J.W.; Uchida, O.; Kajiwara, N.; Kim, E.Y.; O’Sullivan, M.P.; Walter, M.J.; Cook, D.N.; Danoff, T.M.; Holtzman, M.J. Chemokine CCL5 signals required for survival during viral infection. J. Allergy Clin. Immunol. 2004, 113, S49. [Google Scholar] [CrossRef]
- Culley, F.J.; Pennycook, A.M.; Tregoning, J.S.; Dodd, J.S.; Walzl, G.; Wells, T.N.; Hussell, T.; Openshaw, P.J. Role of CCL5 (RANTES) in viral lung disease. J. Virol. 2006, 80, 8151–8157. [Google Scholar] [CrossRef]
- Silva, T.; Temerozo, J.R.; do Vale, G.; Ferreira, A.C.; Soares, V.C.; Dias, S.S.G.; Sardella, G.; Bou-Habib, D.C.; Siqueira, M.; Souza, T.M.L.; et al. The Chemokine CCL5 Inhibits the Replication of Influenza A Virus Through SAMHD1 Modulation. Front. Cell. Infect. Microbiol. 2021, 11, 549020. [Google Scholar] [CrossRef]
- Mirzaei, H.; Khodadad, N.; Karami, C.; Pirmoradi, R.; Khanizadeh, S. The AP-1 pathway; A key regulator of cellular transformation modulated by oncogenic viruses. Rev. Med. Virol. 2020, 30, e2088. [Google Scholar] [CrossRef] [PubMed]
- Gazon, H.; Barbeau, B.; Mesnard, J.-M.; Peloponese, J.-M. Hijacking of the AP-1 Signaling Pathway during Development of ATL. Front. Microbiol. 2018, 8, 2686. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, S.; Ehrhardt, C.; Neumeier, E.R.; Kracht, M.; Rapp, U.R.; Pleschka, S. Influenza virus-induced AP-1-dependent gene expression requires activation of the JNK signaling pathway. J. Biol. Chem. 2001, 276, 10990–10998. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Wang, H.; Su, A.; Xu, S.; Chu, Y. Herpes simplex virus type 2 infection triggers AP-1 transcription activity through TLR4 signaling in genital epithelial cells. Virol. J. 2018, 15, 173. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lin, Y.; Jia, R.; Geng, Y.; Liang, C.; Tan, J.; Qiao, W. HIV-1 Vpr stimulates NF-κB and AP-1 signaling by activating TAK1. Retrovirology 2014, 11, 45. [Google Scholar] [CrossRef]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-kappaB signaling: Balancing life and death—A new approach to cancer therapy. J. Clin. Invest. 2005, 115, 2625–2632. [Google Scholar] [CrossRef]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef]
- Majzoub, K.; Wrensch, F.; Baumert, T.F. The Innate Antiviral Response in Animals: An Evolutionary Perspective from Flagellates to Humans. Viruses 2019, 11, 758. [Google Scholar] [CrossRef]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef]
- Boehme, K.W.; Compton, T. Innate sensing of viruses by toll-like receptors. J. Virol. 2004, 78, 7867–7873. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.H.; Wang, M.Y.; Huang, C.Y.; Wu, C.H.; Hung, L.F.; Yang, C.Y.; Ke, P.Y.; Luo, S.F.; Liu, S.J.; Ho, L.J. Infection with the dengue RNA virus activates TLR9 signaling in human dendritic cells. EMBO Rep. 2018, 19, e46182. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.J.; Potje, S.R.; Fraga-Silva, T.F.C.; da Silva-Neto, J.A.; Barros, P.R.; Rodrigues, D.; Machado, M.R.; Martins, R.B.; Santos-Eichler, R.A.; Benatti, M.N.; et al. Mitochondrial DNA and TLR9 activation contribute to SARS-CoV-2-induced endothelial cell damage. Vasc. Pharmacol. 2022, 142, 106946. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef] [PubMed]
- Limonta, D.; Dyna-Dagman, L.; Branton, W.; Mancinelli, V.; Makio, T.; Wozniak, R.W.; Power, C.; Hobman, T.C. Nodosome Inhibition as a Novel Broad-Spectrum Antiviral Strategy against Arboviruses, Enteroviruses, and SARS-CoV-2. Antimicrob. Agents Chemother. 2021, 65, e0049121. [Google Scholar] [CrossRef]
- Wu, X.M.; Zhang, J.; Li, P.W.; Hu, Y.W.; Cao, L.; Ouyang, S.; Bi, Y.H.; Nie, P.; Chang, M.X. NOD1 Promotes Antiviral Signaling by Binding Viral RNA and Regulating the Interaction of MDA5 and MAVS. J. Immunol. 2020, 204, 2216–2231. [Google Scholar] [CrossRef]
- Khoufache, K.; LeBouder, F.; Morello, E.; Laurent, F.; Riffault, S.; Andrade-Gordon, P.; Boullier, S.; Rousset, P.; Vergnolle, N.; Riteau, B. Protective Role for Protease-Activated Receptor-2 against Influenza Virus Pathogenesis via an IFN-γ-Dependent Pathway. J. Immunol. 2009, 182, 7795–7802. [Google Scholar] [CrossRef]
- Shpacovitch, V.; Feld, M.; Bunnett, N.W.; Steinhoff, M. Protease-activated receptors: Novel PARtners in innate immunity. Trends Immunol. 2007, 28, 541–550. [Google Scholar] [CrossRef]

| Name | Sequence | MW | Net Charge at pH 7 |
|---|---|---|---|
| L-PLNC8 α | DLTTKLWSSWGYYLGKKARWNLKHPYVQF | 3587 | 4.1 |
| D-PLNC8 α | DLTTKLWSSWGYYLGKKARWNLKHPYVQF | 3587 | 4.1 |
| S-PLNC8 α | TWLKYGHGDAKLWSWSKPLNLTFRYQYRK | 3587 | 4.1 |
| L-PLNC8 β | SVPTSVYTLGIKILWSAYKHRKTIEKSFNKGFYH | 4001 | 5.2 |
| D-PLNC8 β | SVPTSVYTLGIKILWSAYKHRKTIEKSFNKGFYH | 4001 | 5.2 |
| S-PLNC8 β | LKLWNTYGTFSRFYTSKSEVKIAHGIKSIHVPYK | 4001 | 5.2 |
| LL-37 | LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES | 4493 | 6.0 |
| Gene | Oligo | Primer Sequence |
|---|---|---|
| GAPDH | Forward | GTCTCCTCTGACTTCAACAGCG |
| Reverse | ACCACCCTGTTGCTGTAGCCAA | |
| KUNV | Forward | CACCACTACAGAGAGTGGAAAG |
| Reverse | ATCATGTCTCTGTGGCCTAATC | |
| TLR1 | Forward | CAGCGATGTGTTCGGTTTTCCG |
| Reverse | GATGGGCAAAGCATGTGGACCA | |
| TLR2 | Forward | CTTCACTCAGGAGCAGCAAGCA |
| Reverse | ACACCAGTGCTGTCCTGTGACA | |
| TLR3 | Forward | GCGCTAAAAAGTGAAGAACTGGAT |
| Reverse | GCTGGACATTGTTCAGAAAGAGG | |
| TLR4 | Forward | CCCTGAGGCATTTAGGCAGCTA |
| Reverse | AGGTAGAGAGGTGGCTTAGGCT | |
| TLR6 | Forward | ACTGACCTTCCTGGATGTGGCA |
| Reverse | TGACCTCATCTTCTGGCAGCTC | |
| TLR9 | Forward | TGAGCCACAACTGCATCTCGCA |
| Reverse | CAGTCGTGGTAGCTCCGTGAAT | |
| PAR1 | Forward | GTTTCTGGCTGTGGTGTATCCC |
| Reverse | CCTGGATGGTTTGCTCCTTGAG | |
| PAR2 | Forward | CTCCTCTCTGTCATCTGGTTCC |
| Reverse | TGCACACTGAGGCAGGTCATGA | |
| PAR3 | Forward | CTTGGCAAAGCCAACCTTACCC |
| Reverse | CAGTAATCGTGGCTCCTGTCCA | |
| NOD1 | Forward | CAACGGCATCTCCACAGAAGGA |
| Reverse | CCAAACTCTCTGCCACTTCATCG | |
| NOD2 | Forward | GCACTGATGCTGGCAAAGAACG |
| Reverse | CTTCAGTCCTTCTGCGAGAGAAC | |
| RANKL | Forward | GCCTTTCAAGGAGCTGTGCAAAA |
| Reverse | GAGCAAAAGGCTGAGCTTGAAGC | |
| RELA | Forward | TGAACCGAAACTCTGGCAGCTG |
| Reverse | CATCAGCTTGCGAAAAGGAGCC | |
| JUN | Forward | CCTTGAAAGCTCAGAACTCGGAG |
| Reverse | TGCTGCGTTAGCATGAGTTGGC | |
| FOS | Forward | GCCTCTCTTACTACCACTCACC |
| Reverse | AGATGGCAGTGACCGTGGGAAT | |
| STAT5 | Forward | GTTCAGTGTTGGCAGCAATGAGC |
| Reverse | AGCACAGTAGCCGTGGCATTGT | |
| IL1B | Forward | CCACAGACCTTCCAGGAGAATG |
| Reverse | GTGCAGTTCAGTGATCGTACAGG | |
| CXCL8 | Forward | GAGAGTGATTGAGAGTGGACCAC |
| Reverse | CACAACCCTCTGCACCCAGTTT | |
| IL6 | Forward | AGACAGCCACTCACCTCTTCAG |
| Reverse | TTCTGCCAGTGCCTCTTTGCTG | |
| TNF | Forward | CTCTTCTGCCTGCTGCACTTTG |
| Reverse | ATGGGCTACAGGCTTGTCACTC | |
| CCL5 | Forward | CCTGCTGCTTTGCCTACATTGC |
| Reverse | ACACACTTGGCGGTTCTTTCGG | |
| IFNA | Forward | AGCCATCTCTGTCCTCCATGA |
| Reverse | CATGATTTCTGCTCTGACAACC | |
| TNFB1 | Forward | TACCTGAACCCGTGTTGCTCTC |
| Reverse | GTTGCTGAGGTATCGCCAGGAA |
| Un-Infected | KUNV (MOI:0.1) | ||||
|---|---|---|---|---|---|
| Gene | L-αβ | D-αβ | - | L-αβ | D-αβ |
| IL1B | 1.9 ± 1.0 * | 1.7 ± 0.8 * | 10.2 ± 3.6 * | 5.1 ± 3.2 *# | 1.7 ± 0.7 *# |
| IL6 | 1.3 ± 1.7 | 2.8 ± 1.0 * | 120.4 ± 42.2 * | 556.9 ± 295.1 *# | 276.1 ± 165.6 * |
| TNF | 0.8 ± 0.6 | 1.5 ± 2.9 | 90.4 ± 73.4 | 36.7 ± 30.2 | 6.5 ± 4.5 |
| CXCL8 | 0.8 ± 0.6 | 0.9 ± 0.6 | 68.5 ± 11.3 * | 24.9 ± 17.1 *# | 7.3 ± 8.1 *# |
| CCL5 | 0.9 ± 0.2 | 0.9 ± 0.2 | 7.1 ± 3.2 * | 25.5 ± 11.9 *# | 31.3 ± 8.8 *# |
| IFNA | 3.0 ± 3.8 | 2.7 ± 1.9 | 8.2 ± 5.6 | 4.5 ± 3.2 | 0.6 ± 0.4 |
| TGFB1 | 1.8 ± 1.9 | 1.7 ± 1.2 | 1.4 ± 1.6 | 2.0 ± 1.9 | 2.1 ± 1.9 |
| Un-INFECTED | KUNV (MOI:0.1) | ||||
|---|---|---|---|---|---|
| Gene | L-αβ | D-αβ | - | L-αβ | D-αβ |
| RANKL | 1.0 ± 0.6 | 1.9 ± 0.9 * | 0.7 ± 0.2 * | 4.2 ± 2.0 *# | 2.1 ± 1.1 # |
| RELA | 0.8 ± 0.5 | 0.9 ± 0.5 | 1.7 ± 0.9 | 2.2 ± 1.4 * | 1.3 ± 0.8 |
| FOS | 0.6 ± 0.4 * | 0.9 ± 0.5 | 19.9 ± 8.6 * | 4.9 ± 3.2 *# | 0.9 ± 0.4 # |
| JUN | 0.7 ± 0.3 * | 1.3 ± 0.4 | 9.5 ± 2.9 * | 3.4 ± 1.6 *# | 2.5 ± 1.0 *# |
| STAT1 | 1.0 ± 0.3 | 0.8 ± 0.5 | 2.9 ± 1.6 | 2.6 ± 1.1 | 2.3 ± 0.8 * |
| Un-Infected | KUNV (MOI:0.1) | ||||
|---|---|---|---|---|---|
| Gene | L-αβ | D-αβ | - | L-αβ | D-αβ |
| TLR1 | 1.2 ± 0.6 | 0.2 ± 0.1 * | 0.4 ± 0.2 * | 0.9 ± 0.5 # | 0.6 ± 0.2 * |
| TLR3 | 0.9 ± 0.5 | 0.4 ± 0.1 * | 9.5 ± 3.3 * | 26.7 ± 4.7 *# | 18.3 ± 5.9 *# |
| TLR6 | 1.2 ± 0.5 | 0.4 ± 0.2 * | 0.4 ± 0.2 * | 0.8 ± 0.4 # | 0.7 ± 0.2 *# |
| TLR9 | 2.9 ± 1.8 | 3.1 ± 2.1 | 11.3 ± 6.6 * | 0.9 ± 0.6 # | 3.6 ± 1.7 *# |
| PAR1 | 0.8 ± 0.3 | 1.2 ± 0.4 | 0.4 ± 0.3 * | 0.6 ± 0.4 * | 2.2 ± 1.7 # |
| PAR2 | 1.3 ± 0.5 | 1.5 ± 0.7 | 1.6 ± 0.7 | 1.1 ± 0.4 | 0.9 ± 0.3 |
| PAR3 | 0.5 ± 0.2 * | 0.4 ± 0.1 * | 0.2 ± 0.2 * | 0.4 ± 0.5 * | 0.5 ± 0.5 |
| NOD1 | 0.9 ± 0.6 | 1.0 ± 0.7 | 0.7 ± 0.2 * | 1.5 ± 0.4 *# | 2.1 ± 0.5 *# |
| NOD2 | 0.9 ± 0.3 | 0.2 ± 0.1 * | 88.1 ± 37.5 * | 27.7 ± 8.0 *# | 22.8 ± 6.7 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omer, A.A.M.; Kumar, S.; Söderquist, B.; Melik, W.; Bengtsson, T.; Khalaf, H. PLNC8 αβ Potently Inhibits the Flavivirus Kunjin and Modulates Inflammatory and Intracellular Signaling Responses of Alveolar Epithelial Cells. Viruses 2024, 16, 1770. https://doi.org/10.3390/v16111770
Omer AAM, Kumar S, Söderquist B, Melik W, Bengtsson T, Khalaf H. PLNC8 αβ Potently Inhibits the Flavivirus Kunjin and Modulates Inflammatory and Intracellular Signaling Responses of Alveolar Epithelial Cells. Viruses. 2024; 16(11):1770. https://doi.org/10.3390/v16111770
Chicago/Turabian StyleOmer, Abubakr A. M., Sanjiv Kumar, Bo Söderquist, Wessam Melik, Torbjörn Bengtsson, and Hazem Khalaf. 2024. "PLNC8 αβ Potently Inhibits the Flavivirus Kunjin and Modulates Inflammatory and Intracellular Signaling Responses of Alveolar Epithelial Cells" Viruses 16, no. 11: 1770. https://doi.org/10.3390/v16111770
APA StyleOmer, A. A. M., Kumar, S., Söderquist, B., Melik, W., Bengtsson, T., & Khalaf, H. (2024). PLNC8 αβ Potently Inhibits the Flavivirus Kunjin and Modulates Inflammatory and Intracellular Signaling Responses of Alveolar Epithelial Cells. Viruses, 16(11), 1770. https://doi.org/10.3390/v16111770







