Assessment of a Structurally Modified Alternanthera Mosaic Plant Virus as a Delivery System for Sarcoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Purification and Coat Protein Isolation
2.2. Formation of Structurally Modified Particles and Their Labeling with Fluorescein Isothiocyanate
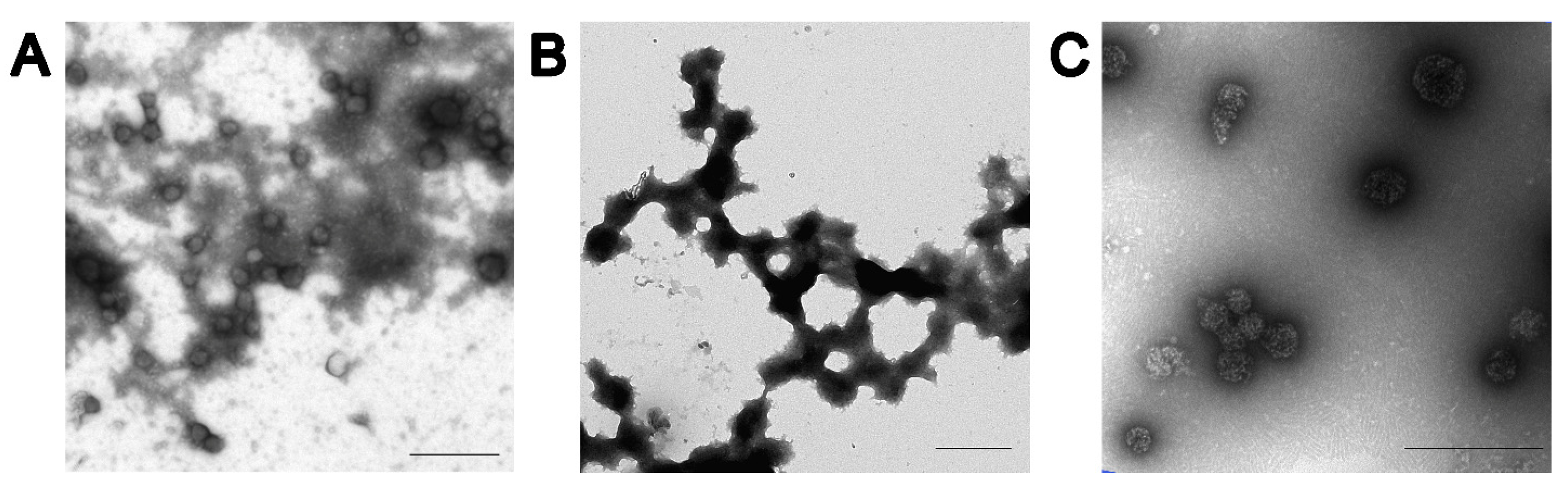
2.3. Transmission Electron Microscopy
2.4. Nanoparticle Tracking Analysis
2.5. Cells and Reagents
2.6. Adsorption of SP on Cells
2.7. Cellular Uptake
2.8. Immunofluorescence Assessment of AltMV SPV Localization inside Target Cells
2.9. Statistical Analysis
3. Results
3.1. Characterization of SP
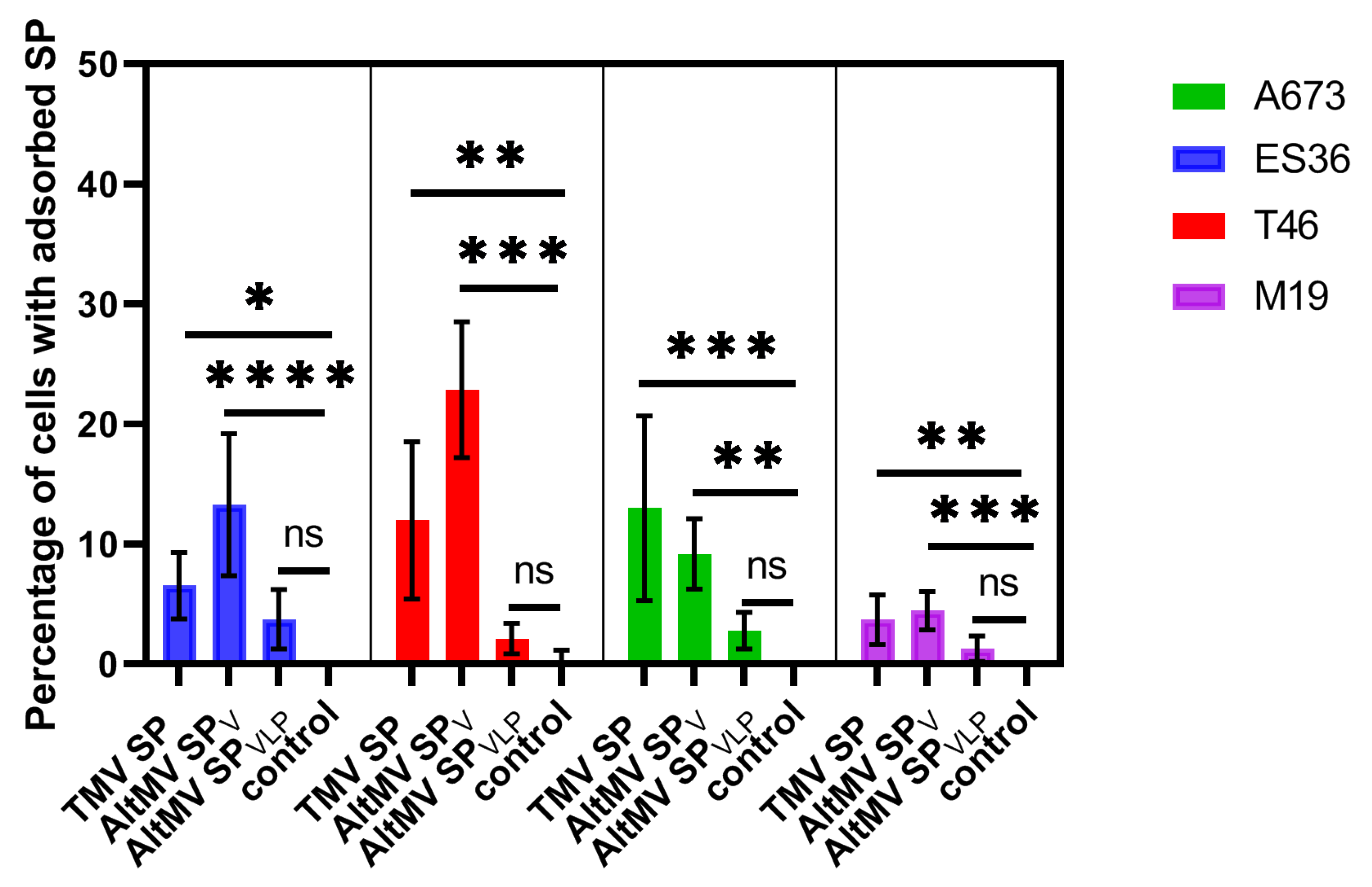
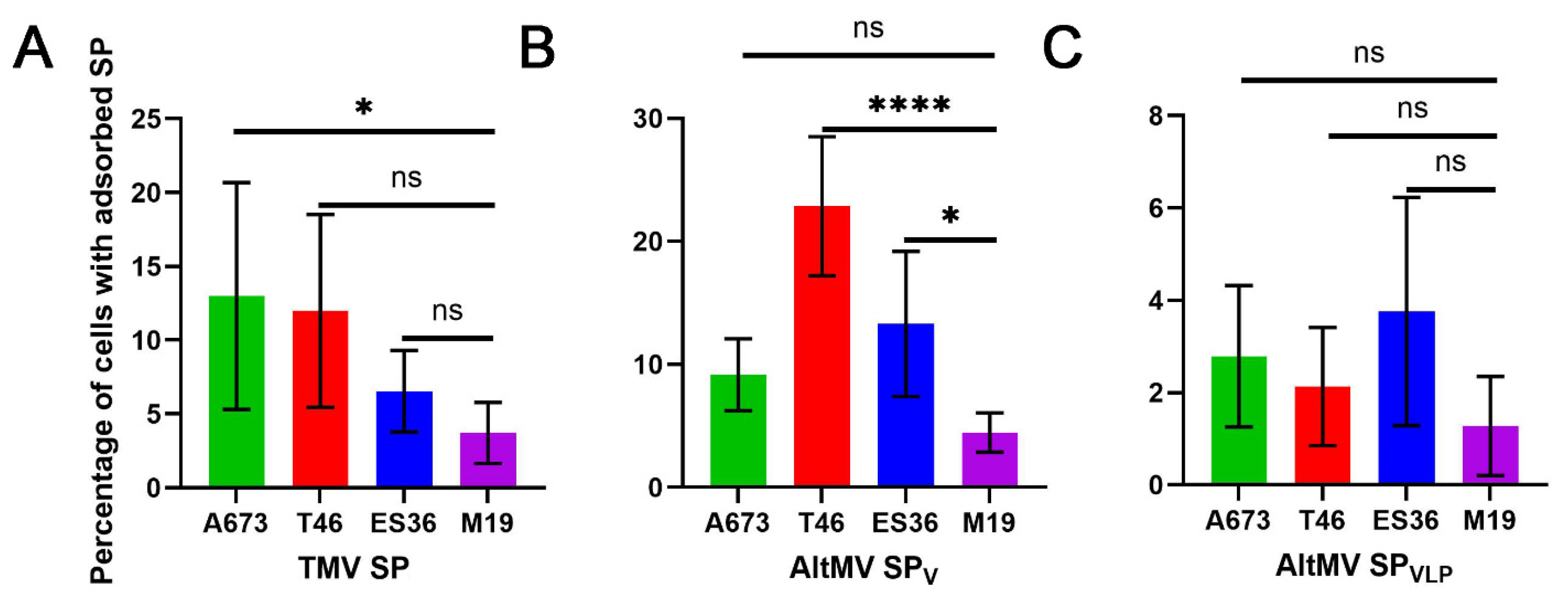
3.2. Experimental Design and SP Adsorption
3.3. Cellular Uptake and Retention of SP by Primary Tumor Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nikitin, N.A.; Trifonova, E.A.; Karpova, O.V.; Atabekov, J.G. Biosafety of Plant Viruses for Human and Animals. Moscow Univ. Biol. Sci. Bull. 2016, 71, 128–134. [Google Scholar] [CrossRef]
- Berardi, A.; Evans, D.J.; Bombelli, F.B.; Lomonossoff, G.P. Stability of Plant Virus-Based Nanocarriers in Gastrointestinal Fluids. Nanoscale 2018, 10, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Bruckman, M.A.; Randolph, L.N.; VanMeter, A.; Hern, S.; Shoffstall, A.J.; Taurog, R.E.; Steinmetz, N.F. Biodistribution, Pharmacokinetics, and Blood Compatibility of Native and PEGylated Tobacco Mosaic Virus Nano-Rods and -Spheres in Mice. Virology 2014, 449, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Blandino, A.; Lico, C.; Baschieri, S.; Barberini, L.; Cirotto, C.; Blasi, P.; Santi, L. In Vitro and in Vivo Toxicity Evaluation of Plant Virus Nanocarriers. Colloids Surf. B Biointerfaces 2015, 129, 130–136. [Google Scholar] [CrossRef]
- McCormick, A.A.; Palmer, K.E. Genetically engineered Tobacco mosaic virus as nanoparticle vaccines. Expert Rev. Vaccines 2008, 7, 33–41. [Google Scholar] [CrossRef]
- Gamper, C.; Spenlé, C.; Boscá, S.; van der Heyden, M.; Erhardt, M.; Orend, G.; Bagnard, D.; Heinlein, M. Functionalized Tobacco Mosaic Virus Coat Protein Monomers and Oligomers as Nanocarriers for Anti-Cancer Peptides. Cancers 2019, 11, 1609. [Google Scholar] [CrossRef]
- Shoeb, E.; Badar, U.; Venkataraman, S.; Hefferon, K. Frontiers in Bioengineering and Biotechnology: Plant Nanoparticles for Anti-Cancer Therapy. Vaccines 2021, 9, 830. [Google Scholar] [CrossRef]
- Murray, A.A.; Wang, C.; Fiering, S.; Steinmetz, N.F. In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol. Pharm. 2018, 15, 3700–3716. [Google Scholar] [CrossRef]
- Bruckman, M.A.; Czapar, A.E.; VanMeter, A.; Randolph, L.N.; Steinmetz, N.F. Tobacco Mosaic Virus-Based Protein Nanoparticles and Nanorods for Chemotherapy Delivery Targeting Breast Cancer. J. Control Release 2016, 231, 103–113. [Google Scholar] [CrossRef]
- Shukla, S.; Myers, J.T.; Woods, S.E.; Gong, X.; Czapar, A.E.; Commandeur, U.; Huang, A.Y.; Levine, A.D.; Steinmetz, N.F. Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials 2017, 121, 15–27. [Google Scholar] [CrossRef]
- Le, D.H.; Lee, K.L.; Shukla, S.; Commandeur, U.; Steinmetz, N.F. Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 2017, 9, 2348–2357. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Ablack, A.L.; Wen, A.M.; Lee, K.L.; Lewis, J.D.; Steinmetz, N.F. Increased tumor homing and tissue penetration of the filamentous plant viral nanoparticle Potato virus X. Mol. Pharm. 2013, 10, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Roe, A.; Liu, R.; Veliz, F.A.; Commandeur, U.; Wald, D.N.; Steinmetz, N.F. Affinity of Plant Viral Nanoparticle Potato Virus X (PVX) towards Malignant B Cells Enables Cancer Drug Delivery. Biomater. Sci. 2020, 8, 3935–3943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Beiss, V.; Steinmetz, N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93, e00129-19. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.T.; Commandeur, U.; Steinmetz, N.F. Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy. ACS Nano 2019, 13, 2501–2510. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Masarapu, H.; Gu, Y.; Zhang, Y.; Yu, X.; Steinmetz, N.F. Physalis Mottle Virus-like Nanoparticles for Targeted Cancer Imaging. ACS Appl. Mater. Interfaces 2019, 11, 18213–18223. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Alzoubi, L.; Hamzat, Y.; Alqudah, A.; Obeid, M.A.; Al Zoubi, M.S.; Ennab, R.M.; Alshaer, W.; Albatayneh, K.; Al-Trad, B.; et al. A Potential MRI Agent and an Anticancer Drug Encapsulated within CPMV Virus-Like Particles. Comb. Chem. High Throughput Screen. 2021, 24, 1557–1571. [Google Scholar] [CrossRef]
- Mukhamedzhanova, A.A.; Smirnov, A.A.; Arkhipenko, M.V.; Ivanov, P.A.; Chirkov, S.N.; Rodionova, N.P.; Karpova, O.; Atabekov, J.G. Characterization of Alternanthera Mosaic Virus and Its Coat Protein. Open Virol. J. 2011, 5, 136–140. [Google Scholar] [CrossRef]
- Manukhova, T.I.; Evtushenko, E.A.; Ksenofontov, A.L.; Arutyunyan, A.M.; Kovalenko, A.O.; Nikitin, N.A.; Karpova, O.V. Thermal Remodelling of Alternanthera Mosaic Virus Virions and Virus-like Particles into Protein Spherical Particles. PLoS ONE 2021, 16, e0255378. [Google Scholar] [CrossRef]
- Durer, S.; Gasalberti, D.P.; Shaikh, H. Ewing Sarcoma; StatPearls: Treasure Island, FL, USA, 2024; Available online: https://www.statpearls.com/point-of-care/21419 (accessed on 20 August 2024).
- Fayzullina, D.; Tsibulnikov, S.; Stempen, M.; Schroeder, B.A.; Kumar, N.; Kharwar, R.K.; Acharya, A.; Timashev, P.; Ulasov, I. Novel Targeted Therapeutic Strategies for Ewing Sarcoma. Cancers 2022, 14, 1988. [Google Scholar] [CrossRef]
- Trifonova, E.A.; Nikitin, N.A.; Kirpichnikov, M.P.; Karpova, O.V.; Atabekov, J.G. Obtaining and Characterization of Spherical Particles—New Biogenic Platforms. Moscow Univ. Biol. Sci. Bull. 2015, 70, 194–197. [Google Scholar] [CrossRef]
- Yakushov, S.; Menyailo, M.; Denisov, E.; Karlina, I.; Zainullina, V.; Kirgizov, K.; Romantsova, O.; Timashev, P.; Ulasov, I. Identification of Factors Driving Doxorubicin-Resistant Ewing Tumor Cells to Survival. Cancers 2022, 14, 5498. [Google Scholar] [CrossRef] [PubMed]
- Dudich, E.; Semenkova, L.; Gorbatova, E.; Dudich, I.; Khromykh, L.; Tatulov, E.; Grechko, G.; Sukhikh, G. Growth-Regulative Activity of Human Alpha-Fetoprotein for Different Types of Tumor and Normal Cells. Tumor Biol. 1998, 19, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Hoffmann, G.; Kushwaha, N.K.; López-González, S.; Hofius, D.; Hafrén, A. Salicylic Acid and the Viral Virulence Factor 2b Regulate the Divergent Roles of Autophagy during Cucumber Mosaic Virus Infection. Autophagy 2021, 18, 1450–1462. [Google Scholar] [CrossRef]
- Katz, D.; Baptista, J.; Azen, S.P.; Pike, M.C. Obtaining confidence intervals for the risk ratio in cohort studies. Biometrics 1978, 34, 469–474. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral Nanoparticles for Drug Delivery, Imaging, Immunotherapy, and Theranostic Applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Nikitin, N.; Vasiliev, Y.; Kovalenko, A.; Ryabchevskaya, E.; Kondakova, O.; Evtushenko, E.; Karpova, O. Plant Viruses as Adjuvants for Next-Generation Vaccines and Immunotherapy. Vaccines 2023, 11, 1372. [Google Scholar] [CrossRef]
- Chung, Y.; Zhao, Z.; Jung, E.; Omole, A.O.; Wang, H.; Sutorus, L.; Steinmetz, N.F. Systemic Administration of Cowpea Mosaic Virus Demonstrates Broad Protection against Metastatic Cancers. Adv. Sci. 2024, 11, e2308237. [Google Scholar] [CrossRef]
- Venkataraman, S.; Hefferon, K. Application of Plant Viruses in Biotechnology, Medicine, and Human Health. Viruses 2021, 13, 1697. [Google Scholar] [CrossRef]
- Singh, P.; Prasuhn, D.; Yeh, R.M.; Destito, G.; Rae, C.S.; Osborn, K.; Finn, M.G.; Manchester, M. Bio-distribution, toxicity and pathology of cowpea mosaic virus nanoparticles in vivo. J. Control. Release 2007, 120, 41–50. [Google Scholar] [CrossRef]
- Tian, Y.; Gao, S.; Wu, M.; Liu, X.; Qiao, J.; Zhou, Q.; Jiang, S.; Niu, Z. Tobacco Mosaic Virus-Based 1D Nanorod-Drug Carrier via the Integrin-Mediated Endocytosis Pathway. ACS Appl. Mater. Interfaces 2016, 8, 10800–10807. [Google Scholar] [CrossRef] [PubMed]
- Vernekar, A.A.; Berger, G.; Czapar, A.E.; Veliz, F.A.; Wang, D.I.; Steinmetz, N.F.; Lippard, S.J. Speciation of Phenanthriplatin and Its Analogs in the Core of Tobacco Mosaic Virus. J. Am. Chem. Soc. 2018, 140, 4279–4287. [Google Scholar] [CrossRef] [PubMed]
- Bruckman, M.A.; Randolph, L.N.; Gulati, N.M.; Stewart, P.L.; Steinmetz, N.F. Silica-coated Gd(DOTA)-loaded protein nanoparticles enable magnetic resonance imaging of macrophages. J. Mat. Chem. B 2015, 3, 7503–7510. [Google Scholar] [CrossRef] [PubMed]
- Pitek, A.S.; Jameson, S.A.; Veliz, F.A.; Shukla, S.; Steinmetz, N.F. Serum albumin ‘camouflage’ of plant virus based nanoparticles prevents their antibody recognition and enhances pharmacokinetics. Biomaterials 2016, 89, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Caballero, R.M.; González-Gamboa, I.; Craig, S.L.; Steinmetz, N.F. Linear and multivalent PEGylation of the tobacco mosaic virus and the effects on its biological properties. Front. Virol. 2023, 3, 1184095. [Google Scholar] [CrossRef]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef]
- Bruckman, M.A.; VanMeter, A.; Steinmetz, N.F. Nanomanufacturing of Tobacco Mosaic Virus-Based Spherical Biomaterials Using a Continuous Flow Method. ACS Biomater. Sci. Eng. 2014, 1, 13–18. [Google Scholar] [CrossRef]
- Körner, M.; Waser, B.; Reubi, J.C. High Expression of Neuropeptide Y1 Receptors in Ewing Sarcoma Tumors. Clin. Cancer Res. 2008, 14, 5043–5049. [Google Scholar] [CrossRef]
- Garofalo, C.; Manara, M.C.; Nicoletti, G.; Marino, M.T.; Lollini, P.-L.; Astolfi, A.; Pandini, G.; López-Guerrero, J.A.; Schaefer, K.-L.; Belfiore, A.; et al. Efficacy of and Resistance to Anti-IGF-1R Therapies in Ewing’s Sarcoma Is Dependent on Insulin Receptor Signaling. Oncogene 2011, 30, 2730–2740. [Google Scholar] [CrossRef]
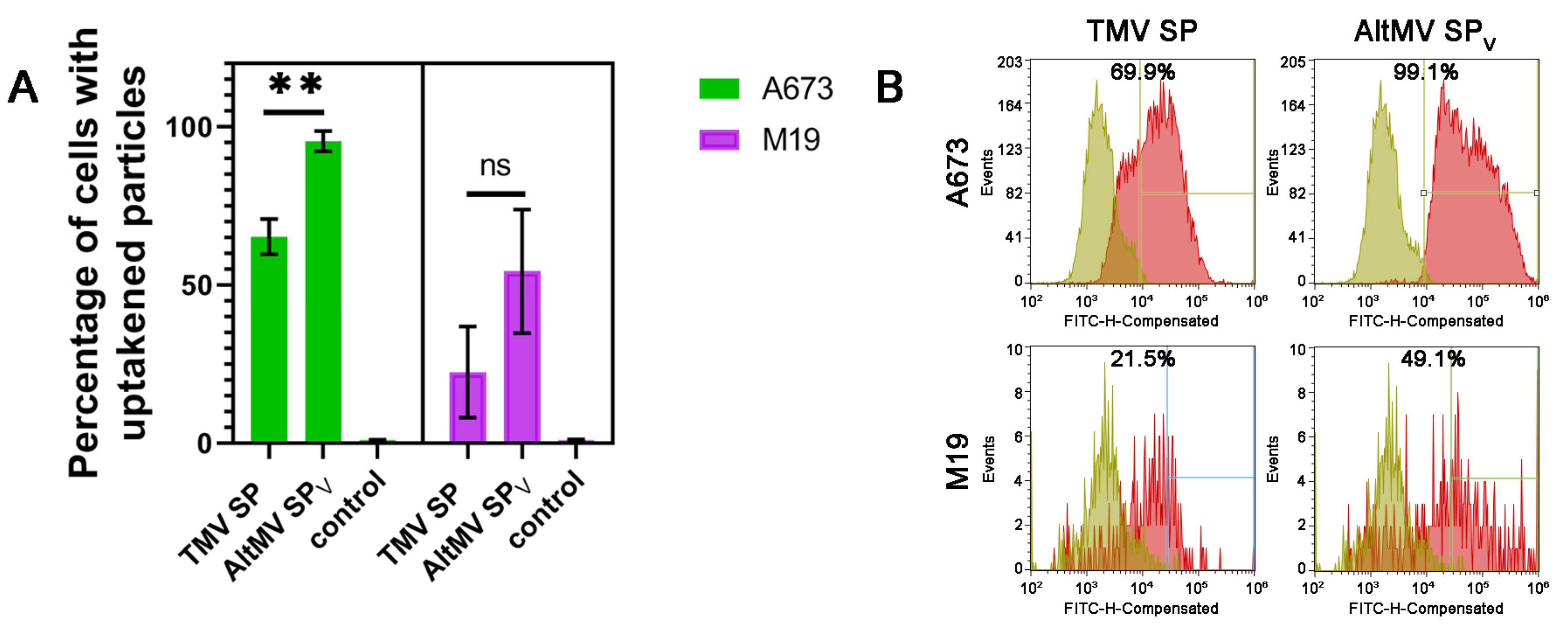
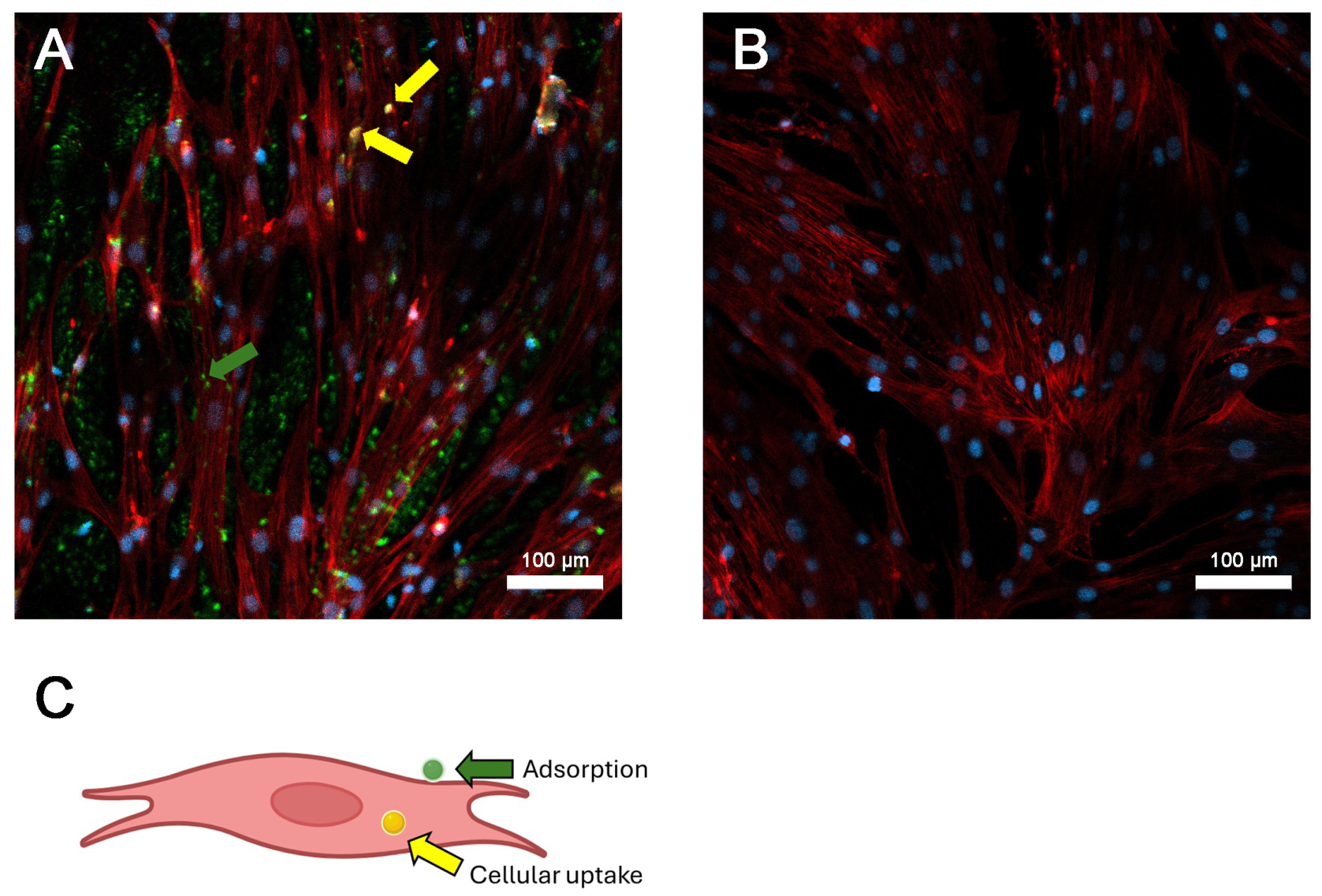
| Cell Line | Type of SP | SP Cellular Uptake |
|---|---|---|
| ES36 | TMV SP | 8.0% |
| AltMV SPV | 23.7% | |
| T46 | TMV SP | 16.3% |
| AltMV SPV | 21.5% | |
| A673 | TMV SP | 85.4% |
| AltMV SPV | 99.6% | |
| M19 | TMV SP | 4.0% |
| AltMV SPV | 20.4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fayzullina, D.; Manukhova, T.; Evtushenko, E.; Tsibulnikov, S.; Kirgizov, K.; Ulasov, I.; Nikitin, N.; Karpova, O. Assessment of a Structurally Modified Alternanthera Mosaic Plant Virus as a Delivery System for Sarcoma Cells. Viruses 2024, 16, 1621. https://doi.org/10.3390/v16101621
Fayzullina D, Manukhova T, Evtushenko E, Tsibulnikov S, Kirgizov K, Ulasov I, Nikitin N, Karpova O. Assessment of a Structurally Modified Alternanthera Mosaic Plant Virus as a Delivery System for Sarcoma Cells. Viruses. 2024; 16(10):1621. https://doi.org/10.3390/v16101621
Chicago/Turabian StyleFayzullina, Daria, Tatiana Manukhova, Ekaterina Evtushenko, Sergey Tsibulnikov, Kirill Kirgizov, Ilya Ulasov, Nikolai Nikitin, and Olga Karpova. 2024. "Assessment of a Structurally Modified Alternanthera Mosaic Plant Virus as a Delivery System for Sarcoma Cells" Viruses 16, no. 10: 1621. https://doi.org/10.3390/v16101621
APA StyleFayzullina, D., Manukhova, T., Evtushenko, E., Tsibulnikov, S., Kirgizov, K., Ulasov, I., Nikitin, N., & Karpova, O. (2024). Assessment of a Structurally Modified Alternanthera Mosaic Plant Virus as a Delivery System for Sarcoma Cells. Viruses, 16(10), 1621. https://doi.org/10.3390/v16101621





