COVID-19 in Relation to Polypharmacy and Immunization (2020–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Variables
2.2. COVID-19 Variants
- -
- -
- 20E (EU1)—Spanish clade [17]: 17 August–21 December 2020;
- -
- 20I (Alpha): 15 March–24 April 2020;
- -
- 21I + 21J (Delta): 19 July–6 December 2021;
- -
- 21K (Omicron): 3 January–14 February 2022;
- -
- 21L (Omicron): 28 March–23 May 2022;
- -
- 22B (Omicron): 4 July–26 September 2022;
- -
- 22E (Omicron): 18 December 2022–2 January 2023;
- -
- 24A (Omicron): 1 January–25 March 2024;
- -
- 24C (Omicron): 29 Julyto August 2024.
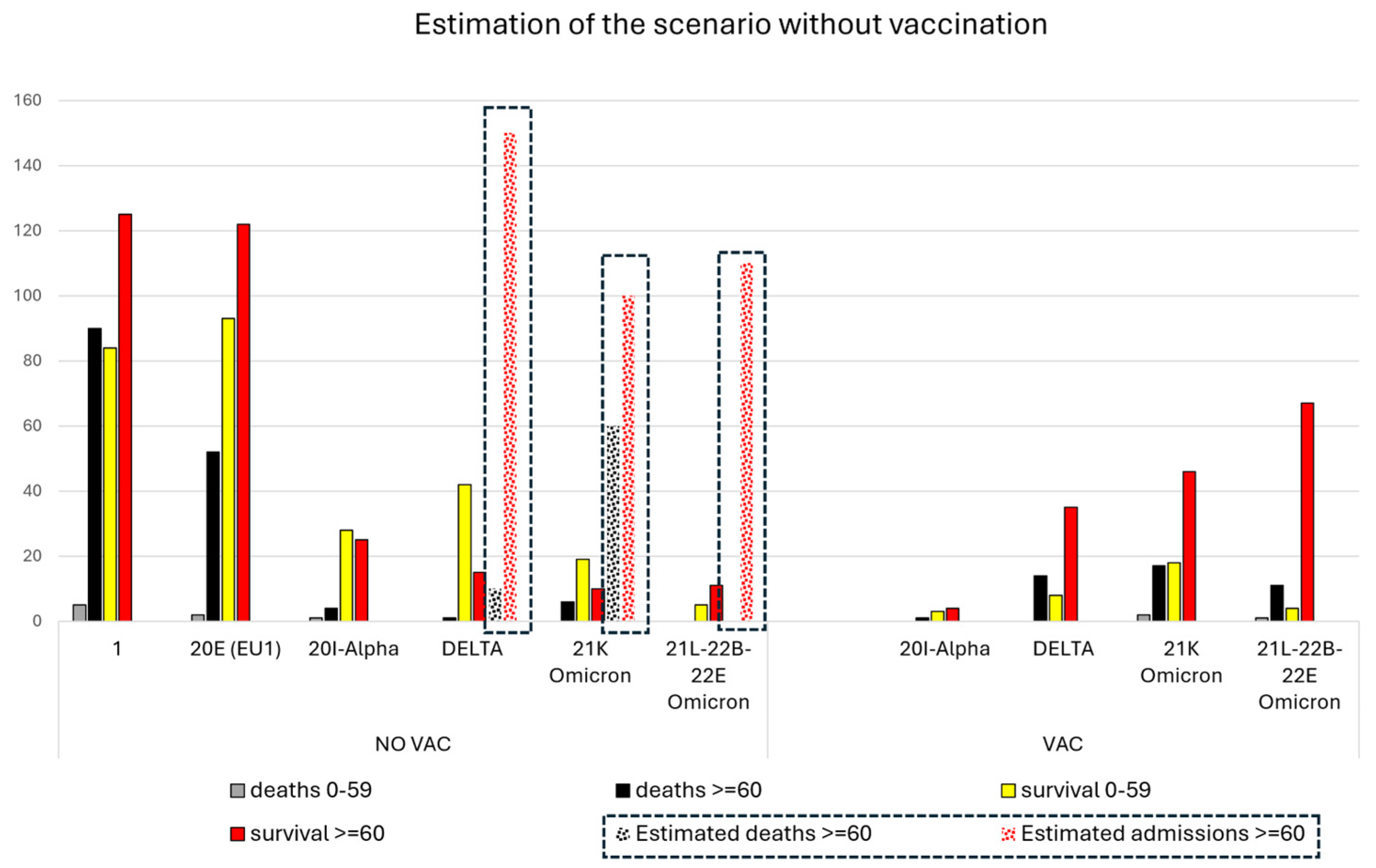
2.3. Calculation of a Theoretical Scenario without Vaccines during the Pandemic
3. Results
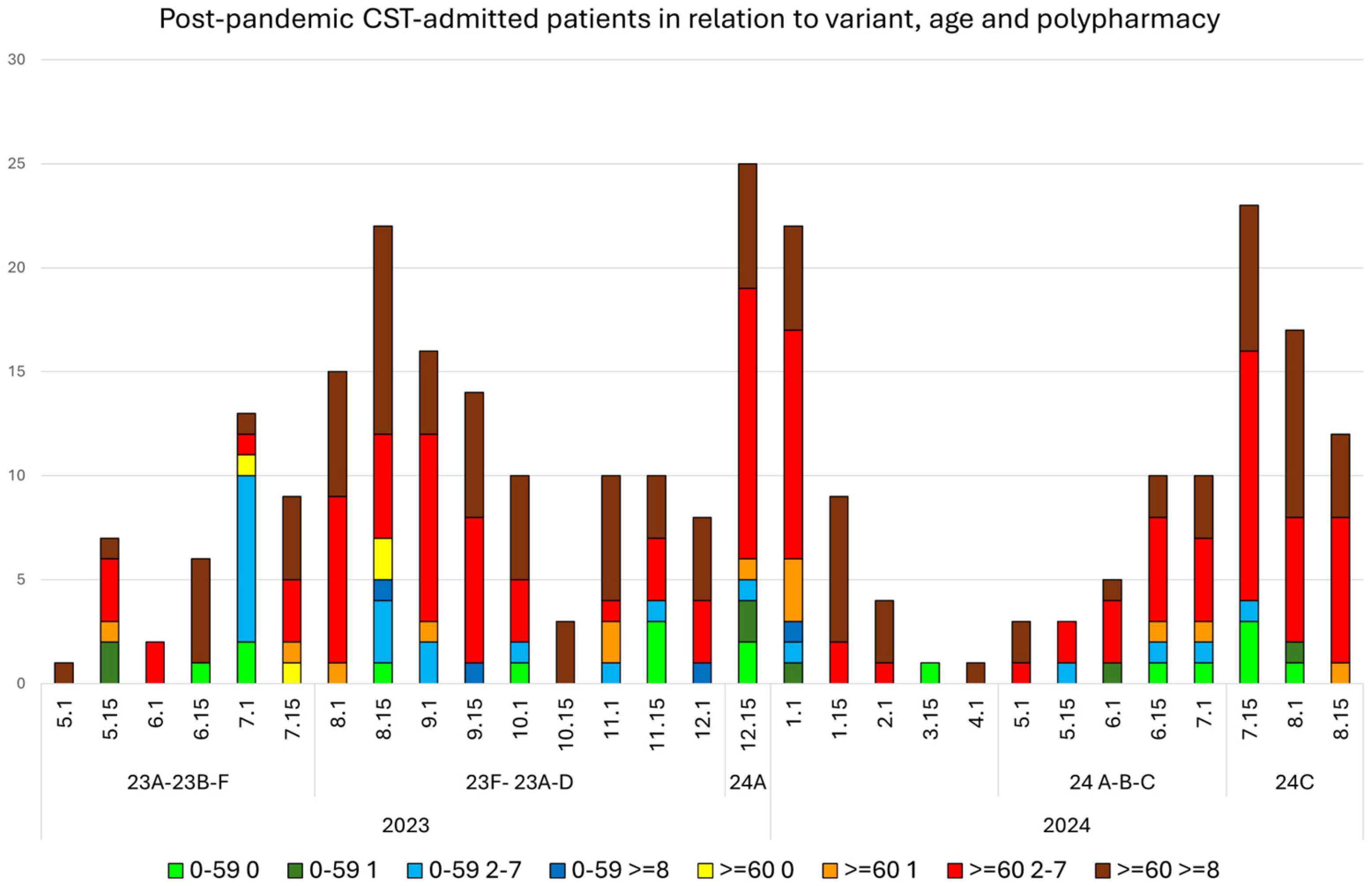
3.1. Distribution of Hospital-Admitted Patients per Period
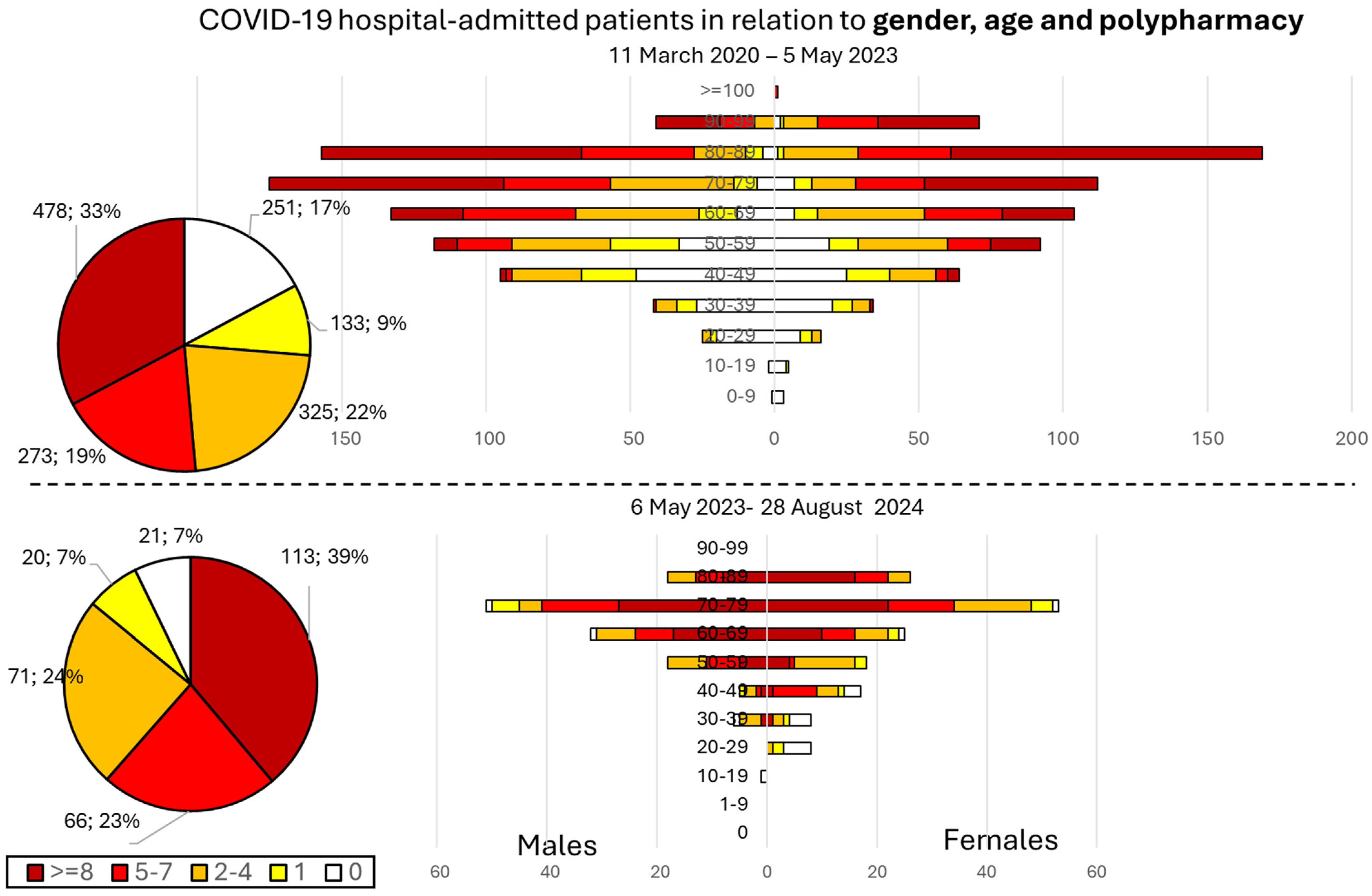
3.2. Profile of Hospital-Admitted Patients in Terms of Age, Gender, and Polypharmacy after 5 May 2023
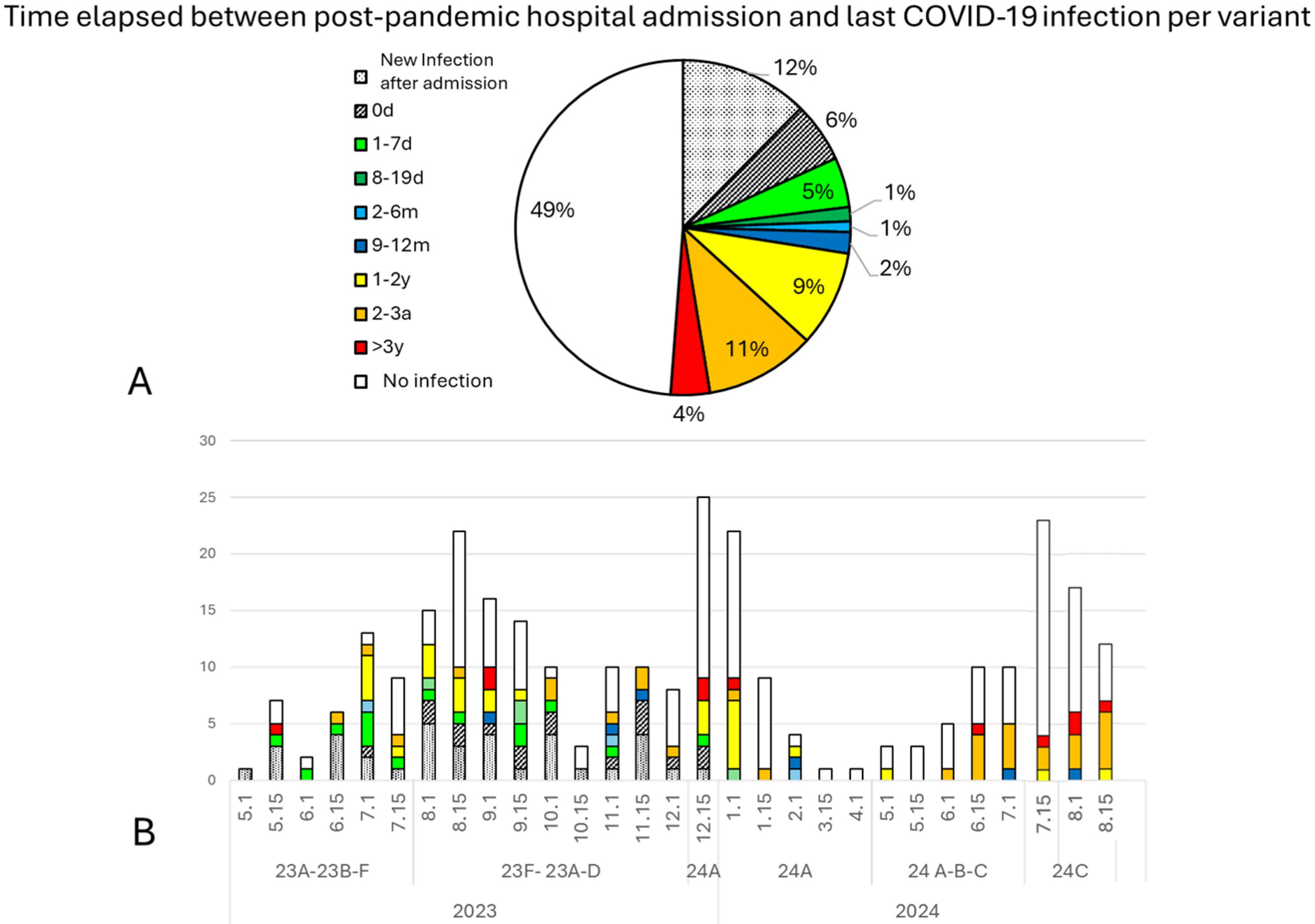
3.3. Hospital-Admitted Patients and Their Immunization Status
4. Discussion
4.1. Estimated Hospital Admissions in a Theoretical Scenario without Vaccination
4.2. Immunization: Vaccination and Previous COVID-19 Infection
4.3. Chronic Prescription and Polypharmacy
4.4. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- University of Oxford-Oxford Martin School. Official Data Collated by Our World in Data—Last Updated August 7th 2024—Processed by Our World in Data. Available online: https://ourworldindata.org/grapher/current-covid-hospitalizations-per-million (accessed on 4 July 2024).
- Fadel, R.; Morrison, A.R.; Vahia, A.; Smith, A.R.; Chaudhry, Z.; Bhargava, P.; Miller, J.; Kenney, R.M.; Alangaden, G.; Ramesh, M.S. Early Short-Course Corticosteroids in Hospitalized Patients with COVID-19. Clin. Infect. Dis. 2020, 71, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Elalamy, I.; Kastritis, E.; Sergentanis, T.N.; Politou, M.; Psaltopoulou, T.; Gerotziafas, G.; Dimopoulos, M.A. Hematological findings and complications of COVID-19. Am. J. Hematol. 2020, 95, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Fountaine, R.J.; Yunis, C.; Fleishaker, D.; Almas, M.; Bao, W.; Wisemandle, W.; Baniecki, M.L.; Hendrick, V.M.; Kalfov, V.; et al. Nirmatrelvir for Vaccinated or Unvaccinated Adult Outpatients with COVID-19. N. Engl. J. Med. 2024, 390, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Taghioff, S.M.; Slavin, B.R.; Holton, T.; Singh, D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 75754 patients. PLoS ONE 2021, 16, e0255541. [Google Scholar] [CrossRef] [PubMed]
- Ragni, P.; Marino, M.; Formisano, D.; Bisaccia, E.; Scaltriti, S.; Bedeschi, E.; Grilli, R. Association between exposure to influenza vaccination and COVID-19 diagnosis and outcomes. Vaccines 2020, 8, 675. [Google Scholar] [CrossRef]
- Juanes-González, M.; Calderón-Valdiviezo, A.; Losa-Puig, H.; Valls-Foix, R.; González-Salvador, M.; León-Pérez, M.; Pueyo-Antón, L.; Lozano-Paz, C.; Franco-Romero, M.; Vidal-Alaball, J.; et al. COVID-19 first and delta waves in relation to ACEI, ARB, Influenza vaccination, and comorbidity in a North Metropolitan Barcelona Health Consortium. medRxiv 2021. [Google Scholar] [CrossRef]
- Mehta, N.; Kalra, A.; Nowacki, A.S.; Anjewierden, S.; Han, Z.; Bhat, P.; Carmona-Rubio, A.E.; Jacob, M.; Procop, G.W.; Harrington, S.; et al. Association of Use of Angiotensin-Converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1020–1026. [Google Scholar] [CrossRef]
- Owen, R.K.; Conroy, S.P.; Taub, N.; Jones, W.; Bryden, D.; Pareek, M.; Faull, C.; Abrams, K.R.; Davis, D.; Banerjee, J. Comparing associations between frailty and mortality in hospitalised older adults with or without COVID-19 infection: A retrospective observational study using electronic health records. Age Ageing 2020, 50, 307–316. [Google Scholar] [CrossRef]
- Aryal, K.; Mowbray, F.I.; Miroshnychenko, A.; Strum, R.P.; Dash, D.; Hillmer, M.P.; Malikov, K.; Costa, A.P.; Jones, A. Evaluating methods for risk prediction of COVID-19 mortality in nursing home residents before and after vaccine availability: A retrospective cohort study. BMC Med. Res. Methodol. 2024, 24, 77. [Google Scholar] [CrossRef]
- Morán-Blanco, J.I.; Alvarenga-Bonilla, J.A.; Homma, S.; Suzuki, K.; Fremont-Smith, P.; Villar-Gómez de Las Heras, K. Antihistamines and azithromycin as a treatment for COVID-19 on primary health care—A retrospective observational study in elderly patients. Pulm. Pharmacol. Ther. 2021, 67, 101989. [Google Scholar] [CrossRef]
- Morán-Blanco, J.I.; Alvarenga-Bonilla, J.A.; Fremont-Smith, P.; Villar-Gómez de Las Heras, K. Antihistamines as an early treatment for COVID-19. Heliyon 2023, 9, e15772. [Google Scholar] [CrossRef] [PubMed]
- Puigdellívol-Sánchez, A.; Juanes-González, M.; Calderón-Valdiviezo, A.; Losa-Puig, H.; Valls-Foix, R.; González-Salvador, M.; Lozano-Paz, C.; Vidal-Alaball, J. COVID-19 in Relation to Chronic Antihistamine Prescription. In Review in Pharmacological Reports. Available online: https://www.researchsquare.com/article/rs-4686775/v1 (accessed on 30 August 2024). [CrossRef]
- CoVariants; Hodcroft, E. Institute of Social and Preventive Medicine University of Bern, Switzerland & SIB Swiss Insitute of Bioinformatics. Available online: https://covariants.org/ (accessed on 30 August 2024).
- López, M.G.; Chiner-Oms, Á.; García de Viedma, D.; Ruiz-Rodriguez, P.; Bracho, M.A.; Cancino-Muñoz, I.; D’Auria, G.; de Marco, G.; García-González, N.; Goig, G.A.; et al. The first wave of the COVID-19 epidemic in Spain was associated with early introductions and fast spread of a dominating genetic variant. Nat. Genet. 2021, 53, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Carballa, A.; Bello, X.; Pardo-Seco, J.; Pérez del Molino, M.L.; Martiñón-Torres, F.; Salas, A. Phylogeography of SARS-CoV-2 pandemic in Spain: A story of multiple introductions, micro-geographic stratification, founder effects, and super-spreaders. Zool. Res. 2020, 41, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hodcroft, E.B.; Zuber, M.; Nadeau, S.; Vaughan, T.G.; Crawford, K.H.; Althaus, C.L.; Reichmuth, M.L.; Bowen, J.E.; Walls, A.C.; Corti, D.; et al. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature 2021, 595, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M. OpenEpi: Open Source Epidemiologic Statistics for Public Health, Versión. Actualized 6 March 2013. Available online: www.openepi.com (accessed on 1 June 2024).
- Vacunas y Programa de Vacunación. Datos de Vacunación COVID-19 en España. Ministerio de Sanidad. Available online: https://www.sanidad.gob.es/areas/alertasEmergenciasSanitarias/alertasActuales/nCov/documentos/Informe_GIV_Comunicacion_12042024.pdf (accessed on 30 August 2024).
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Gonzalo-Pérez, M.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, R.L.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa-Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Corchado-Garcia, J.; Zemmour, D.; Hughes, T.; Bandi, H.; Cristea-Platon, T.; Lenehan, P.; Pawlowski, C.; Bade, S.; O’Horo, J.C.; Gores, G.J.; et al. Analysis of the Effectiveness of the Ad26.COV2.S Adenoviral Vector Vaccine for Preventing COVID-19. JAMA Netw. Open 2021, 4, e2132540. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://iris.who.int/bitstream/handle/10665/360580/WHO-2019-nCoV-SurveillanceGuidance-2022.2-eng.pdf (accessed on 18 September 2024).
- Kucirka, L.M.; Lauer, S.A.; Laeyendecker, O.; Boon, D.; Lessler, J. Variation in False-Negative Rate of Reverse Transcriptase Polymerase Chain Reaction-Based SARS-CoV-2 Tests by Time Since Exposure. Ann. Intern. Med. 2020, 173, 262–267. [Google Scholar] [CrossRef]
- Rasmussen, M.; Møller, F.T.; Gunalan, V.; Baig, S.; Bennedbæk, M.; Christiansen, L.E.; Cohen, A.S.; Ellegaard, K.; Fomsgaard, A.; Franck, K.T.; et al. First cases of SARS-CoV-2 BA.2.86 in Denmark, 2023. Eurosurveillance 2023, 28, 2300460. [Google Scholar] [CrossRef]
- Nextrain.org. Genomic Epidemiology of SARS-CoV-2 with Subsampling Focused Globally over the Past 6 Months. Available online: https://nextstrain.org/ncov/gisaid/global/6m (accessed on 29 August 2024).
- Hadfield, J.; Megill, C.; Bell, S.M.; Huddleston, J.; Potter, B.; Callender, C.; Sagulenko, P.; Bedford, T.; Neher, R.A. Nextstrain: Real-time tracking of pathogen evolution. Bioinformatics 2018, 34, 4121–4123. [Google Scholar] [CrossRef] [PubMed]
- Faksova, K.; Walsh, D.; Jiang, Y.; Griffin, J.; Phillips, A.; Gentile, A.; Kwong, J.C.; Macartney, K.; Naus, M.; Grange, Z.; et al. COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals. Vaccine 2024, 42, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Nextrain.org. Tree of SARS-CoV-2 Clades. Available online: https://github.com/nextstrain/ncov-clades-schema (accessed on 29 August 2024).
- Takefuji, Y. Exploring the connection between frailty and cardiovascular diseases. Arch. Gerontol. Geriatr. 2024, 124, 105449. [Google Scholar] [CrossRef]
- Ariza, M.; Cano, N.; Segura, B.; Adan, A.; Bargalló, N.; Caldú, X.; Campabadal, A.; Jurado, M.A.; Mataró, M.; Pueyo, R.; et al. Neuropsychological impairment in post-COVID condition individuals with and without cognitive complaents. Front. Aging Neurosci. 2022, 14, 1029842. [Google Scholar] [CrossRef] [PubMed]
- Man, M.A.; Rosca, D.; Bratosin, F.; Fira-Mladinescu, O.; Ilie, A.C.; Burtic, S.R.; Fildan, A.P.; Fizedean, C.M.; Jianu, A.M.; Negrean, R.A.; et al. Impact of Pre-Infection COVID-19 Vaccination on the Incidence and Severity of Post-COVID Syndrome: A Systematic Review and Meta-Analysis. Vaccines 2024, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kuang, T.; Liu, X. Advances in researches on long coronavirus disease in children: A narrative review. Transl. Pediatr. 2024, 13, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Borra, A.; Aranda-Abreu, G.E. Amantadine in the prevention of clinical symptoms caused by SARS-CoV-2. Pharmacol. Rep. 2021, 73, 962–965. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, M.J.; Pezeshkian, F.; Ranjbar, K.; Javaheri, R.; Shahriarirad, R. Evaluation of the predictors and frequency of silent hypoxemia in COVID-19 patients and the gap between pulse oximeter and arterial blood gas levels: A cross-sectional study. Health Care Sci. 2024, 3, 172–180. [Google Scholar] [CrossRef]
- Colaneri, M.; Matone, M.; Fassio, F.; Lai, A.; Bergna, A.; Della Ventura, C.; Galli, L.; Scaglione, G.; Gori, A.; Schiavini, M. Exploring early COVID-19 therapies, variants, and viral clearance dynamics: Insights from a high-risk outpatients study. Diagn. Microbiol. Infect. Dis. 2024, 110, 116452. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puigdellívol-Sánchez, A.; Juanes-González, M.; Calderón-Valdiviezo, A.; Valls-Foix, R.; González-Salvador, M.; Lozano-Paz, C.; Vidal-Alaball, J. COVID-19 in Relation to Polypharmacy and Immunization (2020–2024). Viruses 2024, 16, 1533. https://doi.org/10.3390/v16101533
Puigdellívol-Sánchez A, Juanes-González M, Calderón-Valdiviezo A, Valls-Foix R, González-Salvador M, Lozano-Paz C, Vidal-Alaball J. COVID-19 in Relation to Polypharmacy and Immunization (2020–2024). Viruses. 2024; 16(10):1533. https://doi.org/10.3390/v16101533
Chicago/Turabian StylePuigdellívol-Sánchez, Anna, Marta Juanes-González, Ana Calderón-Valdiviezo, Roger Valls-Foix, Marta González-Salvador, Celia Lozano-Paz, and Josep Vidal-Alaball. 2024. "COVID-19 in Relation to Polypharmacy and Immunization (2020–2024)" Viruses 16, no. 10: 1533. https://doi.org/10.3390/v16101533
APA StylePuigdellívol-Sánchez, A., Juanes-González, M., Calderón-Valdiviezo, A., Valls-Foix, R., González-Salvador, M., Lozano-Paz, C., & Vidal-Alaball, J. (2024). COVID-19 in Relation to Polypharmacy and Immunization (2020–2024). Viruses, 16(10), 1533. https://doi.org/10.3390/v16101533





