SARS-CoV-2 Outbreaks on Mink Farms—A Review of Current Knowledge on Virus Infection, Spread, Spillover, and Containment
Abstract
1. Introduction to SARS-CoV-2
2. Virus Transmission between Humans and Animals
3. Fur Farming Industry
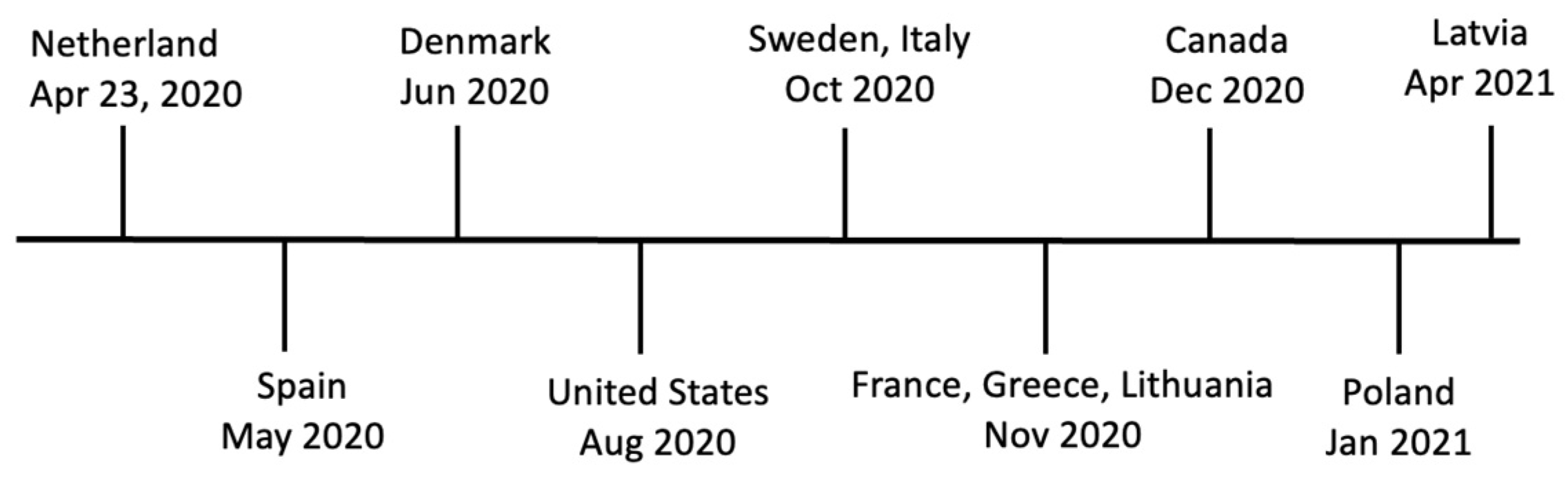
4. SARS-CoV-2 Outbreaks in Farmed Mink
4.1. The Netherlands
4.2. Spain
4.3. Denmark
4.4. Poland
4.5. United States
4.6. Canada
5. Mink–Mink and Mink–Human Viral Transmission Dynamics
6. Mink as a Reservoir
7. Control Measures and Biosecurity
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pomorska-Mól, M.; Włodarek, J.; Gogulski, M.; Rybska, M. Review: SARS-CoV-2 Infection in Farmed Minks—An Overview of Current Knowledge on Occurrence, Disease and Epidemiology. Anim. Int. J. Anim. Biosci. 2021, 15, 100272. [Google Scholar] [CrossRef]
- Tang, Q.; Song, Y.; Shi, M.; Cheng, Y.; Zhang, W.; Xia, X.-Q. Inferring the Hosts of Coronavirus Using Dual Statistical Models Based on Nucleotide Composition. Sci. Rep. 2015, 5, 17155. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Q.; Guo, D. Emerging Coronaviruses: Genome Structure, Replication, and Pathogenesis. J. Med. Virol. 2020, 92, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Turlewicz-Podbielska, H.; Pomorska-Mól, M. Porcine Coronaviruses: Overview of the State of the Art. Virol. Sin. 2021, 36, 833–851. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.S.; Sircar, S.; Bhat, S.; Sharun, K.; Dhama, K.; Dadar, M.; Tiwari, R.; Chaicumpa, W. Emerging Novel Coronavirus (2019-nCoV)-Current Scenario, Evolutionary Perspective Based on Genome Analysis and Recent Developments. Vet. Q. 2020, 40, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jiang, J.-Z.; Wan, X.-F.; Hua, Y.; Li, L.; Zhou, J.; Wang, X.; Hou, F.; Chen, J.; Zou, J.; et al. Are Pangolins the Intermediate Host of the 2019 Novel Coronavirus (SARS-CoV-2)? PLoS Pathog. 2020, 16, e1008421. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Yip, C.C.Y.; Huang, Y.; Tsoi, H.-W.; Chan, K.-H.; Yuen, K.-Y. Comparative Analysis of 22 Coronavirus HKU1 Genomes Reveals a Novel Genotype and Evidence of Natural Recombination in Coronavirus HKU1. J. Virol. 2006, 80, 7136–7145. [Google Scholar] [CrossRef]
- Porter, A.F.; Purcell, D.F.J.; Howden, B.P.; Duchene, S. Evolutionary Rate of SARS-CoV-2 Increases during Zoonotic Infection of Farmed Mink. Virus Evol. 2023, 9, vead002. [Google Scholar] [CrossRef]
- Tan, W.; Zhao, X.; Ma, X.; Wang, W.; Niu, P.; Xu, W.; Gao, G.F.; Wu, G. A Novel Coronavirus Genome Identified in a Cluster of Pneumonia Cases—Wuhan, China 2019−2020. China CDC Wkly. 2020, 2, 61–62. [Google Scholar] [CrossRef]
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821. [Google Scholar] [CrossRef]
- COVID—Coronavirus Statistics—Worldometer. Available online: https://www.worldometers.info/coronavirus/ (accessed on 14 June 2023).
- Eckstrand, C.D.; Baldwin, T.J.; Rood, K.A.; Clayton, M.J.; Lott, J.K.; Wolking, R.M.; Bradway, D.S.; Baszler, T. An Outbreak of SARS-CoV-2 with High Mortality in Mink (Neovison vison) on Multiple Utah Farms. PLoS Pathog. 2021, 17, e1009952. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Ou, X.; Xu, G.; Li, P.; Liu, Y.; Zan, F.; Liu, P.; Hu, J.; Lu, X.; Dong, S.; Zhou, Y.; et al. Host Susceptibility and Structural and Immunological Insight of S Proteins of Two SARS-CoV-2 Closely Related Bat Coronaviruses. Cell Discov. 2023, 9, 78. [Google Scholar] [CrossRef]
- Temmam, S.; Vongphayloth, K.; Baquero, E.; Munier, S.; Bonomi, M.; Regnault, B.; Douangboubpha, B.; Karami, Y.; Chrétien, D.; Sanamxay, D.; et al. Bat Coronaviruses Related to SARS-CoV-2 and Infectious for Human Cells. Nature 2022, 604, 330–336. [Google Scholar] [CrossRef]
- Munir, K.; Ashraf, S.; Munir, I.; Khalid, H.; Muneer, M.A.; Mukhtar, N.; Amin, S.; Ashraf, S.; Imran, M.A.; Chaudhry, U.; et al. Zoonotic and Reverse Zoonotic Events of SARS-CoV-2 and Their Impact on Global Health. Emerg. Microbes Infect. 2020, 9, 2222–2235. [Google Scholar] [CrossRef]
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-Related Coronaviruses in Malayan Pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef]
- Li, X.; Xiao, K.; Chen, X.; Liang, X.; Zhang, X.; Zhang, Z.; Zhai, J.; Wang, R.; Zhou, N.; Chen, Z.-J.; et al. Pathogenicity, Tissue Tropism and Potential Vertical Transmission of SARSr-CoV-2 in Malayan Pangolins. PLoS Pathog. 2020, 19, e1011384. [Google Scholar] [CrossRef]
- Lee, J.; Hughes, T.; Lee, M.-H.; Field, H.; Rovie-Ryan, J.J.; Sitam, F.T.; Sipangkui, S.; Nathan, S.K.S.S.; Ramirez, D.; Kumar, S.V.; et al. No Evidence of Coronaviruses or Other Potentially Zoonotic Viruses in Sunda Pangolins (Manis javanica) Entering the Wildlife Trade via Malaysia. EcoHealth 2020, 17, 406–418. [Google Scholar] [CrossRef]
- Oude Munnink, B.B.; Sikkema, R.S.; Nieuwenhuijse, D.F.; Molenaar, R.J.; Munger, E.; Molenkamp, R.; van der Spek, A.; Tolsma, P.; Rietveld, A.; Brouwer, M.; et al. Transmission of SARS-CoV-2 on Mink Farms between Humans and Mink and Back to Humans. Science 2021, 371, 172–177. [Google Scholar] [CrossRef]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef]
- Shi, J.; Wen, Z.; Zhong, G.; Yang, H.; Wang, C.; Huang, B.; Liu, R.; He, X.; Shuai, L.; Sun, Z.; et al. Susceptibility of Ferrets, Cats, Dogs, and Other Domesticated Animals to SARS-Coronavirus 2. Science 2020, 368, 1016–1020. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Natesan, S.; Dhama, K. SARS-CoV-2 Infection in Farmed Minks, Associated Zoonotic Concerns, and Importance of the One Health Approach during the Ongoing COVID-19 Pandemic. Vet. Q. 2021, 41, 50–60. [Google Scholar] [CrossRef]
- Low-Gan, J.; Huang, R.; Kelley, A.; Jenkins, G.W.; McGregor, D.; Smider, V.V. Diversity of ACE2 and Its Interaction with SARS-CoV-2 Receptor Binding Domain. Biochem. J. 2021, 478, 3671–3684. [Google Scholar] [CrossRef]
- Zhao, J.; Cui, W.; Tian, B. The Potential Intermediate Hosts for SARS-CoV-2. Front. Microbiol. 2020, 11, 580137. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.; Hua, Y.; Liu, P.; Zhou, J.; Chen, J.; An, F.; Hou, F.; Huang, W.; Chen, J. Epidemiological Study of Betacoronaviruses in Captive Malayan Pangolins. Front. Microbiol. 2021, 12, 657439. [Google Scholar] [CrossRef]
- Gollakner, R.; Capua, I. Is COVID-19 the First Pandemic That Evolves into a Panzootic? Vet. Ital. 2020, 56, 11–12. [Google Scholar]
- Bosco-Lauth, A.M.; Walker, A.; Guilbert, L.; Porter, S.; Hartwig, A.; McVicker, E.; Bielefeldt-Ohmann, H.; Bowen, R.A. Susceptibility of Livestock to SARS-CoV-2 Infection. Emerg. Microbes Infect. 2021, 10, 2199–2201. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Larsen, C.S.; Paludan, S.R. Corona’s New Coat: SARS-CoV-2 in Danish Minks and Implications for Travel Medicine. Travel Med. Infect. Dis. 2020, 38, 101922. [Google Scholar] [CrossRef]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. SARS-CoV-2 in Fruit Bats, Ferrets, Pigs, and Chickens: An Experimental Transmission Study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef]
- Ulrich, L.; Wernike, K.; Hoffmann, D.; Mettenleiter, T.C.; Beer, M. Experimental Infection of Cattle with SARS-CoV-2. Emerg. Infect. Dis. 2020, 26, 2979–2981. [Google Scholar] [CrossRef]
- Adney, D.R.; Lovaglio, J.; Schulz, J.E.; Yinda, C.K.; Avanzato, V.A.; Haddock, E.; Port, J.R.; Holbrook, M.G.; Hanley, P.W.; Saturday, G.; et al. Severe Acute Respiratory Disease in American Mink Experimentally Infected with SARS-CoV-2. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Virtanen, J.; Aaltonen, K.; Kegler, K.; Venkat, V.; Niamsap, T.; Kareinen, L.; Malmgren, R.; Kivelä, O.; Atanasova, N.; Österlund, P.; et al. Experimental Infection of Mink with SARS-COV-2 Omicron Variant and Subsequent Clinical Disease. Emerg. Infect. Dis. 2022, 28, 1286–1288. [Google Scholar] [CrossRef]
- Chan, J.F.-W.; Zhang, A.J.; Yuan, S.; Poon, V.K.-M.; Chan, C.C.-S.; Lee, A.C.-Y.; Chan, W.-M.; Fan, Z.; Tsoi, H.-W.; Wen, L.; et al. Simulation of the Clinical and Pathological Manifestations of Coronavirus Disease 2019 (COVID-19) in Golden Syrian Hamster Model: Implications for Disease Pathogenesis and Transmissibility. Clin. Infect. Dis. 2020, 71, 2428–2446. [Google Scholar] [CrossRef]
- Munster, V.J.; Feldmann, F.; Williamson, B.N.; van Doremalen, N.; Pérez-Pérez, L.; Schulz, J.; Meade-White, K.; Okumura, A.; Callison, J.; Brumbaugh, B.; et al. Respiratory Disease in Rhesus Macaques Inoculated with SARS-CoV-2. Nature 2020, 585, 268–272. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, J.; Kuang, D.; Xu, J.; Yang, M.; Ma, C.; Zhao, S.; Li, J.; Long, H.; Ding, K.; et al. Susceptibility of Tree Shrew to SARS-CoV-2 Infection. Sci. Rep. 2020, 10, 16007. [Google Scholar] [CrossRef]
- Rockx, B.; Kuiken, T.; Herfst, S.; Bestebroer, T.; Lamers, M.M.; Oude Munnink, B.B.; de Meulder, D.; van Amerongen, G.; van den Brand, J.; Okba, N.M.A.; et al. Comparative Pathogenesis of COVID-19, MERS, and SARS in a Nonhuman Primate Model. Science 2020, 368, 1012–1015. [Google Scholar] [CrossRef]
- Woolsey, C.; Borisevich, V.; Prasad, A.N.; Agans, K.N.; Deer, D.J.; Dobias, N.S.; Heymann, J.C.; Foster, S.L.; Levine, C.B.; Medina, L.; et al. Establishment of an African Green Monkey Model for COVID-19 and Protection against Re-Infection. Nat. Immunol. 2021, 22, 86–98. [Google Scholar] [CrossRef]
- Sailleau, C.; Dumarest, M.; Vanhomwegen, J.; Delaplace, M.; Caro, V.; Kwasiborski, A.; Hourdel, V.; Chevaillier, P.; Barbarino, A.; Comtet, L.; et al. First Detection and Genome Sequencing of SARS-CoV-2 in an Infected Cat in France. Transbound. Emerg. Dis. 2020, 67, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Sit, T.H.C.; Brackman, C.J.; Ip, S.M.; Tam, K.W.S.; Law, P.Y.T.; To, E.M.W.; Yu, V.Y.T.; Sims, L.D.; Tsang, D.N.C.; Chu, D.K.W.; et al. Infection of Dogs with SARS-CoV-2. Nature 2020, 586, 776–778. [Google Scholar] [CrossRef] [PubMed]
- Hale, V.L.; Dennis, P.M.; McBride, D.S.; Nolting, J.M.; Madden, C.; Huey, D.; Ehrlich, M.; Grieser, J.; Winston, J.; Lombardi, D.; et al. SARS-CoV-2 Infection in Free-Ranging White-Tailed Deer. Nature 2022, 602, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Gisbert, J.; Padilla-Blanco, M.; Lizana, V.; Maiques, E.; Muñoz-Baquero, M.; Chillida-Martínez, E.; Cardells, J.; Rubio-Guerri, C. First Description of SARS-CoV-2 Infection in Two Feral American Mink (Neovison vison) Caught in the Wild. Animals 2021, 11, 1422. [Google Scholar] [CrossRef] [PubMed]
- SARS-CoV-2 Exposure in Escaped Mink, Utah, USA. Pediatr. Infect. Dis. J. 2021, 40, 549. [CrossRef]
- USDA APHIS Cases of SARS-CoV-2 in Animals in the United States. Available online: https://www.aphis.usda.gov/aphis/dashboards/tableau/sars-dashboard (accessed on 5 July 2023).
- Oreshkova, N.; Molenaar, R.J.; Vreman, S.; Harders, F.; Munnink, B.B.O.; der Honing, R.W.H.; Gerhards, N.; Tolsma, P.; Bouwstra, R.; Sikkema, R.S.; et al. SARS-CoV-2 Infection in Farmed Minks, the Netherlands, April and May 2020. Eurosurveillance 2020, 25, 2001005. [Google Scholar] [CrossRef]
- Badiola, J.J.; Otero, A.; Sevilla, E.; Marín, B.; García Martínez, M.; Betancor, M.; Sola, D.; Pérez Lázaro, S.; Lozada, J.; Velez, C.; et al. SARS-CoV-2 Outbreak on a Spanish Mink Farm: Epidemiological, Molecular, and Pathological Studies. Front. Vet. Sci. 2022, 8, 805004. [Google Scholar] [CrossRef]
- Berguido, F.J.; Burbelo, P.D.; Bortolami, A.; Bonfante, F.; Wernike, K.; Hoffmann, D.; Balkema-Buschmann, A.; Beer, M.; Dundon, W.G.; Lamien, C.E.; et al. Serological Detection of SARS-CoV-2 Antibodies in Naturally-Infected Mink and Other Experimentally-Infected Animals. Viruses 2021, 13, 1649. [Google Scholar] [CrossRef]
- Boklund, A.; Hammer, A.S.; Quaade, M.L.; Rasmussen, T.B.; Lohse, L.; Strandbygaard, B.; Jørgensen, C.S.; Olesen, A.S.; Hjerpe, F.B.; Petersen, H.H.; et al. SARS-CoV-2 in Danish Mink Farms: Course of the Epidemic and a Descriptive Analysis of the Outbreaks in 2020. Animals 2021, 11, 164. [Google Scholar] [CrossRef]
- Paiero, A.; Newhouse, E.; Chan, E.; Clair, V.; Russell, S.; Zlonsnik, J.; Prystajecky, N.; Fraser, E. SARS-CoV-2 in Mink Farms in British Columbia, Canada: A Report of Two Outbreaks in 2020–2021, CCDR 48(6). Available online: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2022-48/issue-6-june-2022/sars-cov-2-mink-farms-british-columbia-two-outbreaks-2020-2021.html (accessed on 12 June 2023).
- Chaintoutis, S.C.; Thomou, Z.; Mouchtaropoulou, E.; Tsiolas, G.; Chassalevris, T.; Stylianaki, I.; Lagou, M.; Michailidou, S.; Moutou, E.; Koenen, J.J.H.; et al. Outbreaks of SARS-CoV-2 in Naturally Infected Mink Farms: Impact, Transmission Dynamics, Genetic Patterns, and Environmental Contamination. PLoS Pathog. 2021, 17, e1009883. [Google Scholar] [CrossRef]
- Dall Schmidt, T.; Mitze, T. SARS-CoV-2 Outbreaks on Danish Mink Farms and Mitigating Public Health Interventions. Eur. J. Public Health 2022, 32, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Blicharz, K.; Orłowska, A.; Smreczak, M.; Niemczuk, K.; Iwan, E.; Bomba, A.; Lisowska, A.; Opolska, J.; Trębas, P.; Potyrało, P.; et al. Mink SARS-CoV-2 Infection in Poland—Short Communication. J. Vet. Res. 2021, 65, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Blicharz, K.; Munnink, B.B.O.; Orłowska, A.; Smreczak, M.; Opolska, J.; Lisowska, A.; Trębas, P.; Socha, W.; Giza, A.; Bomba, A.; et al. Cryptic SARS-CoV-2 Lineage Identified on Two Mink Farms as a Possible Result of Long-Term Undetected Circulation in an Unknown Animal Reservoir, Poland, November 2022 to January 2023. Eurosurveillance 2023, 28, 2300188. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, M. SARS-CoV-2 and the Human-Animal Interface: Outbreaks on Mink Farms. Lancet Infect. Dis. 2021, 21, 18–19. [Google Scholar] [CrossRef]
- Larsen, H.D.; Fonager, J.; Lomholt, F.K.; Dalby, T.; Benedetti, G.; Kristensen, B.; Urth, T.R.; Rasmussen, M.; Lassaunière, R.; Rasmussen, T.B.; et al. Preliminary Report of an Outbreak of SARS-CoV-2 in Mink and Mink Farmers Associated with Community Spread, Denmark, June to November 2020. Eurosurveillance 2021, 26, 2100009. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Sikkema, R.S.; Velkers, F.C.; Nieuwenhuijse, D.F.; Fischer, E.A.J.; Meijer, P.A.; Bouwmeester-Vincken, N.; Rietveld, A.; Wegdam-Blans, M.C.A.; Tolsma, P.; et al. Adaptation, Spread and Transmission of SARS-CoV-2 in Farmed Minks and Associated Humans in the Netherlands. Nat. Commun. 2021, 12, 6802. [Google Scholar] [CrossRef] [PubMed]
- Kok, K.-H.; Wong, S.-C.; Chan, W.-M.; Wen, L.; Chu, A.W.-H.; Ip, J.D.; Lee, L.-K.; Wong, I.T.-F.; Lo, H.W.-H.; Cheng, V.C.-C.; et al. Co-Circulation of Two SARS-CoV-2 Variant Strains within Imported Pet Hamsters in Hong Kong. Emerg. Microbes Infect. 2022, 11, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.; Lelli, D.; Trogu, T.; Lavazza, A.; Barbieri, I.; Boniotti, M.; Pezzoni, G.; Salogni, C.; Giovannini, S.; Alborali, G.; et al. SARS-CoV-2 in a Mink Farm in Italy: Case Description, Molecular and Serological Diagnosis by Comparing Different Tests. Viruses 2022, 14, 1738. [Google Scholar] [CrossRef]
- Pappas, G.; Vokou, D.; Sainis, I.; Halley, J.M. SARS-CoV-2 as a Zooanthroponotic Infection: Spillbacks, Secondary Spillovers, and Their Importance. Microorganisms 2022, 10, 2166. [Google Scholar] [CrossRef]
- Linder, A.; McCarthy, V.W.; Green, C.; Nadzam, B.; Jamieson, D.; Stilt, K. Animal Markets and Zoonotic Disease in the United States; Harward Law School & New York University, 2023; Available online: https://animal.law.harvard.edu/wp-content/uploads/Animal-Markets-and-Zoonotic-Disease-in-the-United-States.pdf (accessed on 29 December 2023).
- Fur Farming. Available online: https://www.fourpawsusa.org/campaigns-topics/topics/fur/fur-farming (accessed on 24 July 2023).
- Fenollar, F.; Mediannikov, O.; Maurin, M.; Devaux, C.; Colson, P.; Levasseur, A.; Fournier, P.-E.; Raoult, D. Mink, SARS-CoV-2, and the Human-Animal Interface. Front. Microbiol. 2021, 12, 663815. [Google Scholar] [CrossRef]
- Tomson, F.N. Mink. Vet. Clin. N. Am. Small Anim. Pract. 1987, 17, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Kim, H. Mink Farms: What Are Mink Farmed for and How Are Mink Killed? Available online: https://sentientmedia.org/mink-farms/ (accessed on 17 June 2023).
- Clair, V.; Chan, E.; Paiero, A.; Fraser, E.; Gunvaldsen, R.; Newhouse, E. One Health Response to SARS-CoV-2-Associated Risk from Mink Farming in British Columbia, Canada, October 2020 to October 2021. Available online: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2022-48/issue-6-june-2022/one-health-response-sars-cov-2-risk-mink-farming-british-columbia-2020-2021.html (accessed on 12 June 2023).
- Lyhs, U.; Frandsen, H.; Andersen, B.; Nonnemann, B.; Hjulsager, C.; Pedersen, K.; Chriél, M. Microbiological Quality of Mink Feed Raw Materials and Feed Production Area. Acta Vet. Scand. 2019, 61, 56. [Google Scholar] [CrossRef] [PubMed]
- Chelsea Mink Farming: The Reality of the Mink Fur Trade. Available online: https://veganuary.com/mink-farming/ (accessed on 19 June 2023).
- Wang, L.; Didelot, X.; Bi, Y.; Gao, G.F. Assessing the Extent of Community Spread Caused by Mink-Derived SARS-CoV-2 Variants. Innov. Camb. Mass 2021, 2, 100128. [Google Scholar] [CrossRef] [PubMed]
- OIE (World Organisation for Animal Health) SARS-CoV-2 in Animals—Situation Report 21. Available online: https://www.woah.org/app/uploads/2023/06/sars-cov-2-situation-report-21.pdf (accessed on 29 December 2023).
- WOAH Cases of SARS-CoV-2 Infection in Animals Reported to WOAH since March 2020. Available online: https://www.woah.org/en/disease/sars-cov-2/#ui-id-2 (accessed on 9 November 2023).
- WOAH SARS-CoV-2 in Animals—Situation Report 22. Available online: https://www.woah.org/app/uploads/2023/07/sars-cov-2-situation-report-22.pdf (accessed on 29 December 2023).
- Hammer, A.S.; Quaade, M.L.; Rasmussen, T.B.; Fonager, J.; Rasmussen, M.; Mundbjerg, K.; Lohse, L.; Strandbygaard, B.; Jørgensen, C.S.; Alfaro-Núñez, A.; et al. SARS-CoV-2 Transmission between Mink (Neovison vison) and Humans, Denmark. Emerg. Infect. Dis. 2021, 27, 547–551. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control Detection of New SARS-CoV-2 Variants Related to Mink. ECDC: Stockholm 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/detection-new-sars-cov-2-variants-mink (accessed on 29 December 2023).
- OIE (World Organisation for Animal Health) Cases of SARS-CoV-2 Infection in Animals Reported to WOAH since March 2020. Available online: https://www.woah.org/en/what-we-offer/emergency-preparedness/covid-19/ (accessed on 18 July 2023).
- European Food Safety Authority and European Centre for Disease Prevention and Control; Boklund, A.; Gortázar, C.; Pasquali, P.; Roberts, H.; Nielsen, S.S.; Stahl, K.; Stegeman, A.; Baldinelli, F.; Broglia, A.; et al. Monitoring of SARS-CoV-2 Infection in Mustelids. EFSA J. 2021, 19, e06459. [Google Scholar] [CrossRef] [PubMed]
- ProMED Coronavirus Disease 2019 Update (319): Spain (Aragon) Animal, Farmed Mink, First Report. ProMED-mail 2020.
- Villanueva-Saz, S.; Giner, J.; Palomar, A.M.; Gómez, M.A.; Põdra, M.; Aranda, M.d.C.; Jiménez, M.d.l.Á.; Lizarraga, P.; Hernández, R.; Portillo, A.; et al. No Evidence of SARS-CoV-2 Infection in Wild Mink (Mustela lutreola and Neogale vison) from Northern Spain during the First Two Years of Pandemic. Animals 2022, 12, 1971. [Google Scholar] [CrossRef] [PubMed]
- Apaa, T.; Withers, A.J.; Mackenzie, L.; Staley, C.; Dessi, N.; Blanchard, A.; Bennett, M.; Bremner-Harrison, S.; Chadwick, E.A.; Hailer, F.; et al. Lack of Detection of SARS-CoV-2 in British Wildlife 2020–21 and First Description of a Stoat (Mustela Erminea) Minacovirus. J. Gen. Virol. 2023, 104, 001917. [Google Scholar] [CrossRef]
- Enserink, M. Coronavirus Rips through Dutch Mink Farms, Triggering Culls. Science 2020, 368, 1169. [Google Scholar] [CrossRef]
- Rabalski, L.; Kosinski, M.; Smura, T.; Aaltonen, K.; Kant, R.; Sironen, T.; Szewczyk, B.; Grzybek, M. Severe Acute Respiratory Syndrome Coronavirus 2 in Farmed Mink (Neovison vison), Poland. Emerg. Infect. Dis. 2021, 27, 2333–2339. [Google Scholar] [CrossRef]
- WHO SARS-CoV-2 Mink- Associated Variant Strain—Denmark. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2020-DON301 (accessed on 6 July 2023).
- Domańska-Blicharz, K.; Orłowska, A.; Smreczak, M.; Munnink, B.O.; Trębas, P.; Socha, W.; Niemczuk, K.; Kawiak-Sadurska, M.; Opolska, J.; Lisowska, A.; et al. SARS-CoV-2 Monitoring on Mink Farms in Poland. J. Vet. Res. 2022, 66, 449–458. [Google Scholar] [CrossRef]
- USDA Cases of SARS-CoV-2 in Animals in the United States. Available online: https://www.aphis.usda.gov/animal_health/one_health/downloads/sars-cov2-in-animals.pdf (accessed on 3 July 2023).
- USDA APHIS | USDA Confirms SARS-CoV-2 in Mink in Utah. Available online: https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2020/sa-08/sare-cov-2-mink (accessed on 5 July 2023).
- Cossaboom, C.M.; Wendling, N.M.; Lewis, N.M.; Rettler, H.; Harvey, R.R.; Amman, B.R.; Towner, J.S.; Spengler, J.R.; Erickson, R.; Burnett, C.; et al. One Health Investigation of SARS-CoV-2 in People and Animals on Multiple Mink Farms in Utah. Viruses 2022, 15, 96. [Google Scholar] [CrossRef]
- Amman, B.R.; Cossaboom, C.M.; Wendling, N.M.; Harvey, R.R.; Rettler, H.; Taylor, D.; Kainulainen, M.H.; Ahmad, A.; Bunkley, P.; Godino, C.; et al. GPS Tracking of Free-Roaming Cats (Felis Catus) on SARS-CoV-2-Infected Mink Farms in Utah. Viruses 2022, 14, 2131. [Google Scholar] [CrossRef] [PubMed]
- Canadian Food Inspection Agency (CFIA); Public Health Agency of Canada. Canadian Wildlife Health Cooperative and Guidance for Managing SARS-CoV-2 Infections in Farmed Mink in Canada. 2021. Available online: https://www.cezd.ca/CAHSS/Assets/SharedDocuments/Guidance-for-managing-SARS-CoV-2-infections-in-farmed-mink-in-Canada-March-2021.pdf (accessed on 29 December 2023).
- Strang, T.; Flockhart, L.; Thacker, C.; Schwantje, H.; Soos, C.; Dibernardo, A.; Lindsay, L.R.; Toledo, N.; Beauclerc, K.; Fraser, E.; et al. SARS-CoV-2 Wildlife Surveillance Surrounding Mink Farms in British Columbia, Canada, CCDR 48(6). Available online: https://www.canada.ca/en/public-health/services/reports-publications/canada-communicable-disease-report-ccdr/monthly-issue/2022-48/issue-6-june-2022/sars-cov-2-wildlife-surveillance-mink-farms-british-columbia.html (accessed on 12 June 2023).
- OIE (World Organisation for Animal Health) Guidance on Working with Farmed Animals of Species Susceptible to Infection with SARS-CoV-2. 2021. Available online: https://www.woah.org/app/uploads/2021/06/en-oie-guidance-farmed-animals.pdf (accessed on 29 December 2023).
- Ren, W.; Lan, J.; Ju, X.; Gong, M.; Long, Q.; Zhu, Z.; Yu, Y.; Wu, J.; Zhong, J.; Zhang, R.; et al. Mutation Y453F in the Spike Protein of SARS-CoV-2 Enhances Interaction with the Mink ACE2 Receptor for Host Adaption. PLoS Pathog. 2021, 17, e1010053. [Google Scholar] [CrossRef] [PubMed]
- Douam, F.; Gaska, J.M.; Winer, B.Y.; Ding, Q.; Von Schaewen, M.; Ploss, A. Genetic Dissection of the Host Tropism of Human-Tropic Pathogens. Annu. Rev. Genet. 2015, 49, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Alshishani, A.; Al-ebini, Y. Genome Similarities between Human-Derived and Mink-Derived SARS-CoV-2 Make Mink a Potential Reservoir of the Virus. Vaccines 2022, 10, 1352. [Google Scholar] [CrossRef] [PubMed]
- THE EUROPEAN COMMISSION Commission Implementating Decision (EU) 2021/788. Available online: https://www.stradalex.com/en/sl_src_publ_leg_eur_jo/toc/leg_eur_jo_3_20210517_173/doc/ojeu_2021.173.01.0006.01 (accessed on 26 December 2023).
- USDA APHIS Response & Containment Guidelines: Interim Guidance for Animal Health and Public Health Officials Managing Farmed Mink and Other Farmed Mustelids with SARS-CoV-2. 2020. Available online: https://www.aphis.usda.gov/publications/animal_health/sars-cov-2-mink-guidance.pdf (accessed on 29 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahid, M.J.; Bowman, A.S.; Nolting, J.M. SARS-CoV-2 Outbreaks on Mink Farms—A Review of Current Knowledge on Virus Infection, Spread, Spillover, and Containment. Viruses 2024, 16, 81. https://doi.org/10.3390/v16010081
Jahid MJ, Bowman AS, Nolting JM. SARS-CoV-2 Outbreaks on Mink Farms—A Review of Current Knowledge on Virus Infection, Spread, Spillover, and Containment. Viruses. 2024; 16(1):81. https://doi.org/10.3390/v16010081
Chicago/Turabian StyleJahid, Mohammad Jawad, Andrew S. Bowman, and Jacqueline M. Nolting. 2024. "SARS-CoV-2 Outbreaks on Mink Farms—A Review of Current Knowledge on Virus Infection, Spread, Spillover, and Containment" Viruses 16, no. 1: 81. https://doi.org/10.3390/v16010081
APA StyleJahid, M. J., Bowman, A. S., & Nolting, J. M. (2024). SARS-CoV-2 Outbreaks on Mink Farms—A Review of Current Knowledge on Virus Infection, Spread, Spillover, and Containment. Viruses, 16(1), 81. https://doi.org/10.3390/v16010081





