The Role of Plant Latex in Virus Biology
Abstract
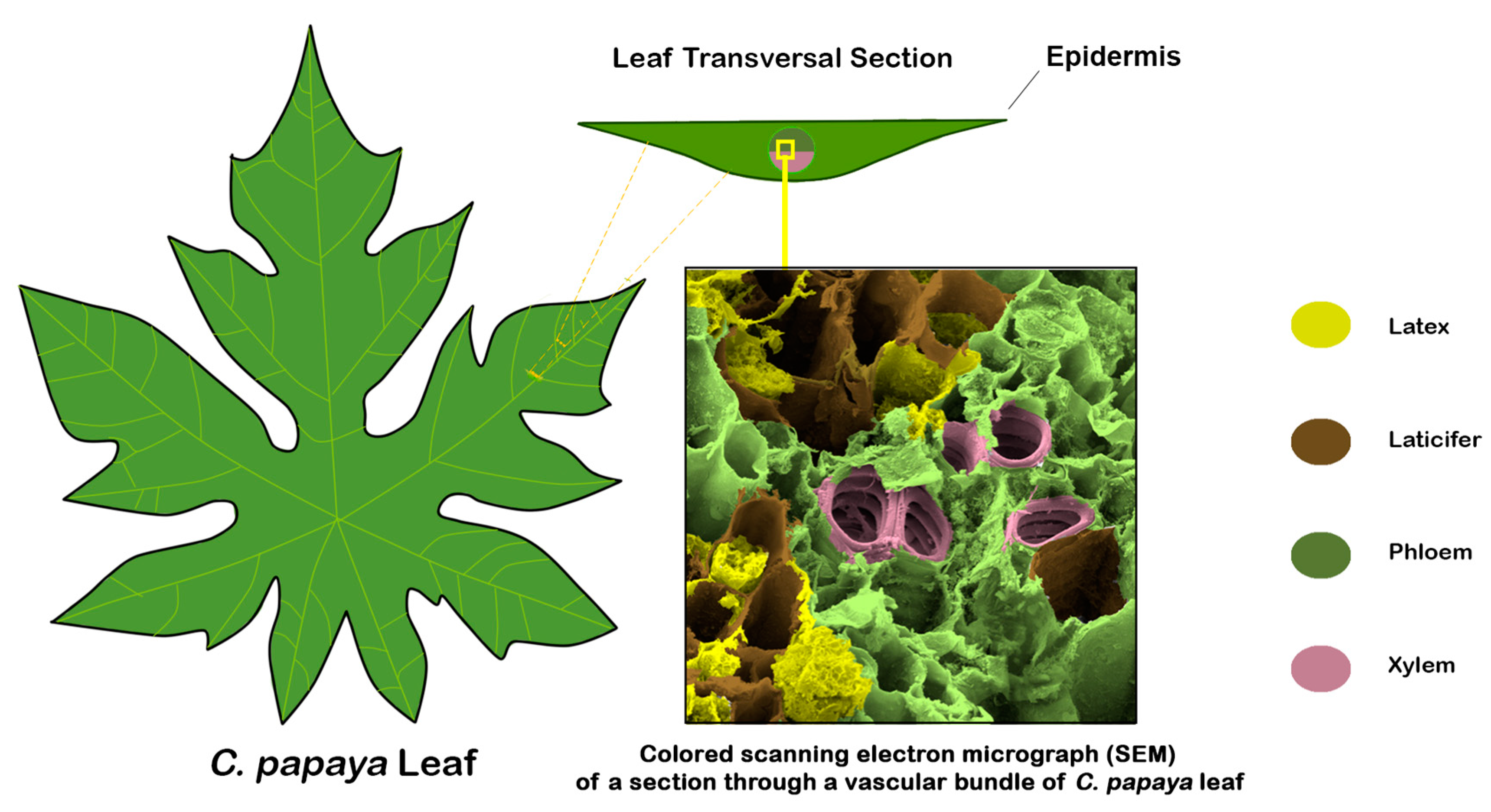
1. Introduction
2. Protein-Based Mechanisms
2.1. Proteases and Their Inhibitors
2.2. Loss-of-Susceptibility to Potyviruses (LSP1) Protein
2.3. Ubiquitin-Proteasome Degradation
2.4. Heat Shock Protein 70 (Hsp70) Isoforms
3. Oxidative Responses
4. Secondary Metabolites
4.1. Phenolics and Polyphenols
4.2. Terpenoids
4.3. Cardenolides
4.4. Alkaloids
5. Trials of the Biological Activity of Latex Constituents
5.1. Antiviral Activity
5.2. Proviral Activity
6. Papaya Meleira Virus Complex: Two Viruses Infecting Laticifers
7. Plant Genetic Editing of Laticifer’s Expressed Genes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gracz-Bernaciak, J.; Mazur, O.; Nawrot, R. Functional Studies of Plant Latex as a Rich Source of Bioactive Compounds: Focus on Proteins and Alkaloids. Int. J. Mol. Sci. 2021, 22, 12427. [Google Scholar] [CrossRef]
- Farrell, B.D.; Dussourd, D.E.; Mitter, C. Escalation of Plant Defense: Do Latex and Resin Canals Spur Plant Diversification? Am. Nat. 1991, 138, 881–900. [Google Scholar] [CrossRef]
- Lewinsohn, T.M. The Geographical Distribution of Plant Latex. Chemoecology 1991, 2, 64–68. [Google Scholar] [CrossRef]
- Nissen, S.J.; Foley, M.E. No Latex Starch Utilization in Euphorbia esula L. 1. Plant Physiol. 1986, 81, 696–698. [Google Scholar] [CrossRef]
- James, J.F. The Milkweeds. Am. Nat. 1887, 21, 605–615. [Google Scholar] [CrossRef][Green Version]
- Souza, D.P.; Freitas, C.D.T.; Pereira, D.A.; Nogueira, F.C.; Silva, F.D.A.; Salas, C.E.; Ramos, M.V. Laticifer Proteins Play a Defensive Role against Hemibiotrophic and Necrotrophic Phytopathogens. Planta 2011, 234, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Hagel, J.; Yeung, E.; Facchini, P. Got milk? The secret life of laticifers. Trends Plant Sci. 2008, 13, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Osmani, Z.; Sabet, M.S.; Nakahara, K.S. Aspartic Protease Inhibitor Enhances Resistance to Potato Virus Y and A in Transgenic Potato Plants. BMC Plant Biol. 2022, 22, 241. [Google Scholar] [CrossRef]
- Fan, Y.; Yang, W.; Yan, Q.; Chen, C.; Li, J. Genome-Wide Identification and Expression Analysis of the Protease Inhibitor Gene Families in Tomato. Genes 2019, 11, 1. [Google Scholar] [CrossRef]
- Lellis, A.D.; Kasschau, K.D.; Whitham, S.A.; Carrington, J.C. Loss-of-Susceptibility Mutants of Arabidopsis thaliana Reveal an Essential Role for EIF(Iso)4E during Potyvirus Infection. Curr. Biol. 2002, 12, 1046–1051. [Google Scholar] [CrossRef]
- Ubillas, R.; Jolad, S.D.; Bruening, R.C.; Kernan, M.R.; King, S.R.; Sesin, D.F.; Barrett, M.; Stoddart, C.A.; Flaster, T.; Kuo, J.; et al. SP-303, an Antiviral Oligomeric Proanthocyanidin from the Latex of Croton lechleri (Sangre de Drago). Phytomedicine 1994, 1, 77–106. [Google Scholar] [CrossRef]
- Bertol, J.W.; Rigotto, C.; de Pádua, R.M.; Kreis, W.; Barardi, C.R.M.; Braga, F.C.; Simões, C.M.O. Antiherpes Activity of Glucoevatromonoside, a Cardenolide Isolated from a Brazilian Cultivar of Digitalis Lanata. Antivir. Res. 2011, 92, 73–80. [Google Scholar] [CrossRef]
- Lazreg Aref, H.; Gaaliche, B.; Fekih, A.; Mars, M.; Aouni, M.; Pierre Chaumon, J.; Said, K. In Vitro Cytotoxic and Antiviral Activities of Ficus carica Latex Extracts. Nat. Prod. Res. 2011, 25, 310–319. [Google Scholar] [CrossRef]
- Tabares, P.; Avila, L.; Torres, F.; Cardona, D.; Quiñones, W.; Forero, J.E.; Rugeles, M.T.; Echeverri, F. Metabolitos secundarios y efectos antivirales de algunas especies de la familia euphorbiaceae. Sci. Et Tech. 2007, XIII, 107–110. [Google Scholar]
- Bhatti, S.T.; Carrizosa, S.; McGuire, P.E.; Young, T. Contracting for ABS: The Legal and Scientific Implications of Bioprospecting Contracts; IUCN: Gland, Switzerland, 2009. [Google Scholar]
- Chen, M.H.; Tian, G.W.; Gafni, Y.; Citovsky, V. Effects of Calreticulin on Viral Cell-to-Cell Movement. Plant Physiol. 2005, 138, 1866–1876. [Google Scholar] [CrossRef]
- Rodrigues, S.P.; Da Cunha, M.; Ventura, J.A.; Fernandes, P.M.B. Effects of the Papaya meleira virus on Papaya Latex Structure and Composition. Plant Cell Rep. 2009, 28, 861–871. [Google Scholar] [CrossRef]
- Rodrigues, S.P.; Ventura, J.A.; Aguilar, C.; Nakayasu, E.S.; Almeida, I.C.; Fernandes, P.M.B.; Zingali, R.B. Proteomic Analysis of Papaya (Carica papaya L.) Displaying Typical Sticky Disease Symptoms. Proteomics 2011, 11, 2592–2602. [Google Scholar] [CrossRef]
- Nagy, P.D.; Wang, R.Y.; Pogany, J.; Hafren, A.; Makinen, K. Emerging Picture of Host Chaperone and Cyclophilin Roles in RNA Virus Replication. Virology 2011, 411, 374–382. [Google Scholar] [CrossRef]
- MacNeil, A.; Sumba, O.P.; Lutzke, M.L.; Moormann, A.; Rochford, R. Activation of the Epstein–Barr Virus Lytic Cycle by the Latex of the Plant Euphorbia tirucalli. Br. J. Cancer 2003, 88, 1566–1569. [Google Scholar] [CrossRef]
- Daoubi, M.; Marquez, N.; Mazoir, N.; Benharref, A.; Hernández-Galán, R.; Muñoz, E.; Collado, I.G. Isolation of New Phenylacetylingol Derivatives That Reactivate HIV-1 Latency and a Novel Spirotriterpenoid from Euphorbia officinarum Latex. Bioorg. Med. Chem. 2007, 15, 4577–4584. [Google Scholar] [CrossRef]
- Martínez, M.; Cambra, I.; González-Melendi, P.; Santamaría, M.E.; Díaz, I. C1A Cysteine-proteases and Their Inhibitors in Plants. Physiol. Plant 2012, 145, 85–94. [Google Scholar] [CrossRef]
- Benucci, I.; Esti, M.; Liburdi, K. Effect of Wine Inhibitors on the Proteolytic Activity of Papain from Carica papaya L. Latex. Biotechnol. Prog. 2015, 31, 48–54. [Google Scholar] [CrossRef]
- Gagaoua, M.; Dib, A.; Lakhdara, N.; Lamri, M.; Botinestean, C.; Lorenzo, J.M. Artificial Meat Tenderization Using Plant Cysteine Proteases. Curr. Opin. Food Sci. 2021, 38, 177–188. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Cartea, M.E. Glucosinolate Induction and Resistance to the Cabbage Moth, Mamestra brassicae, Differs among Kale Genotypes with High and Low Content of Sinigrin and Glucobrassicin. Plants 2021, 10, 1951. [Google Scholar] [CrossRef]
- Konno, K.; Hirayama, C.; Nakamura, M.; Tateishi, K.; Tamura, Y.; Hattori, M.; Kohno, K. Papain Protects Papaya Trees from Herbivorous Insects: Role of Cysteine Proteases in Latex. Plant J. 2004, 37, 370–378. [Google Scholar] [CrossRef]
- Ramos, M.V.; Demarco, D.; da Costa Souza, I.C.; de Freitas, C.D.T. Laticifers, Latex, and Their Role in Plant Defense. Trends Plant Sci. 2019, 24, 553–567. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Nijs, M.; Paul, C.; Wintjens, R.; Vincentelli, J.; Azarkan, M.; Looze, Y. Revisiting the Enzymes Stored in the Laticifers of Carica papaya in the Context of Their Possible Participation in the Plant Defence Mechanism. Cell. Mol. Life Sci. 2001, 58, 556–570. [Google Scholar] [CrossRef]
- Tripathi, A.; Upadhyay, R.; Ravi, K.; Upadhyay. Anti-Termite and Antimicrobial Efficacy of Latexes from Certain Plant Families. Int. J. Green Pharm. 2022, 6, 83. [Google Scholar] [CrossRef]
- Rodrigues, S.P.; Ventura, J.A.; Aguilar, C.; Nakayasu, E.S.; Choi, H.; Sobreira, T.J.P.; Nohara, L.L.; Wermelinger, L.S.; Almeida, I.C.; Zingali, R.B.; et al. Label-Free Quantitative Proteomics Reveals Differentially Regulated Proteins in the Latex of Sticky Diseased Carica papaya L. Plants. J. Proteom. 2012, 75, 3191–3198. [Google Scholar] [CrossRef]
- Salguero-Linares, J.; Coll, N.S. Plant Proteases in the Control of the Hypersensitive Response. J. Exp. Bot. 2019, 70, 2087–2095. [Google Scholar] [CrossRef]
- Emanuele, S.; Oddo, E.; D’Anneo, A.; Notaro, A.; Calvaruso, G.; Lauricella, M.; Giuliano, M. Routes to Cell Death in Animal and Plant Kingdoms: From Classic Apoptosis to Alternative Ways to Die—A Review. Rend. Lincei Sci. Fis. Nat. 2018, 29, 397–409. [Google Scholar] [CrossRef]
- Huh, S.U. Evolutionary Diversity and Function of Metacaspases in Plants: Similar to but Not Caspases. Int. J. Mol. Sci. 2022, 23, 4588. [Google Scholar] [CrossRef]
- Woltering, E.J. Death Proteases Come Alive. Trends Plant Sci. 2004, 9, 469–472. [Google Scholar] [CrossRef]
- Liu, H.; Hu, M.; Wang, Q.; Cheng, L.; Zhang, Z. Role of Papain-Like Cysteine Proteases in Plant Development. Front. Plant Sci. 2018, 9, 1717. [Google Scholar] [CrossRef]
- Sá Antunes, T.F.; Amaral, R.J.V.; Ventura, J.A.; Godinho, M.T.; Amaral, J.G.; Souza, F.O.; Zerbini, P.A.; Zerbini, F.M.; Fernandes, P.M.B. The DsRNA Virus Papaya Meleira Virus and an SsRNA Virus Are Associated with Papaya Sticky Disease. PLoS ONE 2016, 11, e0155240. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, S.P. Fundamentals of Viruses and Their Proteases. In Viral Proteases and Their Inhibitors; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–24. [Google Scholar] [CrossRef]
- Oliver, M.E.; Hinks, T.S.C. Azithromycin in Viral Infections. Rev. Med. Virol. 2021, 31, e2163. [Google Scholar] [CrossRef]
- Cho, W.K.; Chen, X.-Y.; Uddin, N.M.; Rim, Y.; Moon, J.; Jung, J.-H.; Shi, C.; Chu, H.; Kim, S.; Kim, S.-W.; et al. Comprehensive Proteome Analysis of Lettuce Latex Using Multidimensional Protein-Identification Technology. Phytochemistry 2009, 70, 570–578. [Google Scholar] [CrossRef]
- Martínez, F.; Rodrigo, G.; Aragonés, V.; Ruiz, M.; Lodewijk, I.; Fernández, U.; Elena, S.F.; Daròs, J.-A. Interaction Network of Tobacco Etch Potyvirus NIa Protein with the Host Proteome during Infection. BMC Genom. 2016, 17, 87. [Google Scholar] [CrossRef]
- Feng, S.; Shen, Y.; Sullivan, J.A.; Rubio, V.; Xiong, Y.; Sun, T.; Deng, X.W. Arabidopsis CAND1, an Unmodified CUL1-Interacting Protein, Is Involved in Multiple Developmental Pathways Controlled by Ubiquitin/Proteasome-Mediated Protein Degradation. Plant Cell 2004, 16, 1870–1882. [Google Scholar] [CrossRef]
- Li, L.; Wang, K.; Zhou, Y.; Liu, X. Review: A Silent Concert in Developing Plants: Dynamic Assembly of Cullin-RING Ubiquitin Ligases. Plant Sci. 2023, 330, 111662. [Google Scholar] [CrossRef]
- Li, D.; Deng, Z.; Chen, C.; Xia, Z.; Wu, M.; He, P.; Chen, S. Identification and Characterization of Genes Associated with Tapping Panel Dryness from Hevea brasiliensis Latex Using Suppression Subtractive Hybridization. BMC Plant Biol. 2010, 10, 140. [Google Scholar] [CrossRef]
- Song, M.; Wang, H.; Ma, H.; Zheng, C. Genome-Wide Analysis of JAZ Family Genes Expression Patterns during Fig (Ficus carica L.) Fruit Development and in Response to Hormone Treatment. BMC Genom. 2022, 23, 170. [Google Scholar] [CrossRef]
- Poque, S.; Pagny, G.; Ouibrahim, L.; Chague, A.; Eyquard, J.-P.; Caballero, M.; Candresse, T.; Caranta, C.; Mariette, S.; Decroocq, V. Allelic Variation at the Rpv1 Locus Controls Partial Resistance to Plum pox virus Infection in Arabidopsis thaliana. BMC Plant Biol. 2015, 15, 159. [Google Scholar] [CrossRef]
- Takizawa, M.; Goto, A.; Watanabe, Y. The Tobacco Ubiquitin-Activating Enzymes NtE1A and NtE1B Are Induced by Tobacco Mosaic Virus, Wounding and Stress Hormones. Mol. Cells 2005, 19, 228–231. [Google Scholar]
- Liu, Y.; Schiff, M.; Serino, G.; Deng, X.-W.; Dinesh-Kumar, S.P. Role of SCF Ubiquitin-Ligase and the COP9 Signalosome in the N Gene–Mediated Resistance Response to Tobacco mosaic Virus. Plant Cell 2002, 14, 1483–1496. [Google Scholar] [CrossRef]
- Peart, J.R.; Lu, R.; Sadanandom, A.; Malcuit, I.; Moffett, P.; Brice, D.C.; Schauser, L.; Jaggard, D.A.W.; Xiao, S.; Coleman, M.J.; et al. Ubiquitin Ligase-Associated Protein SGT1 Is Required for Host and Nonhost Disease Resistance in Plants. Proc. Natl. Acad. Sci. USA 2002, 99, 10865–10869. [Google Scholar] [CrossRef]
- Dubiella, U.; Serrano, I. The Ubiquitin Proteasome System as a Double Agent in Plant-Virus Interactions. Plants 2021, 10, 928. [Google Scholar] [CrossRef]
- Ballut, L.; Drucker, M.; Pugnière, M.; Cambon, F.; Blanc, S.; Roquet, F.; Candresse, T.; Schmid, H.-P.; Nicolas, P.; Gall, O.L.; et al. HcPro, a Multifunctional Protein Encoded by a Plant RNA Virus, Targets the 20S Proteasome and Affects Its Enzymic Activities. J. Gen. Virol. 2005, 86, 2595–2603. [Google Scholar] [CrossRef]
- Rodriguez-Peña, R.; El Mounadi, K.; Garcia-Ruiz, H. Changes in Subcellular Localization of Host Proteins Induced by Plant Viruses. Viruses 2021, 13, 677. [Google Scholar] [CrossRef]
- Aparicio, F.; Thomas, C.L.; Lederer, C.; Niu, Y.; Wang, D.; Maule, A.J. Virus Induction of Heat Shock Protein 70 Reflects a General Response to Protein Accumulation in the Plant Cytosol. Plant Physiol. 2005, 138, 529–536. [Google Scholar] [CrossRef]
- Wang, R.Y.-L.; Stork, J.; Nagy, P.D. A Key Role for Heat Shock Protein 70 in the Localization and Insertion of Tombusvirus Replication Proteins to Intracellular Membranes. J. Virol. 2009, 83, 3276–3287. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- De Freitas, C.D.T.; da Cruz, W.T.; Silva, M.Z.R.; Vasconcelos, I.M.; Moreno, F.B.M.B.; Moreira, R.A.; Monteiro-Moreira, A.C.O.; Alencar, L.M.R.; Sousa, J.S.; Rocha, B.A.M.; et al. Proteomic Analysis and Purification of an Unusual Germin-like Protein with Proteolytic Activity in the Latex of Thevetia peruviana. Planta 2016, 243, 1115–1128. [Google Scholar] [CrossRef]
- Wu, B.; Qi, F.; Liang, Y. Fuels for ROS Signaling in Plant Immunity. Trends Plant Sci. 2023, 28, 1124–1131. [Google Scholar] [CrossRef]
- De Freitas, C.D.T.; de Souza, D.P.; Araújo, E.S.; Cavalheiro, M.G.; Oliveira, L.S.; Ramos, M.V. Anti-Oxidative and Proteolytic Activities and Protein Profile of Laticifer Cells of Cryptostegia grandiflora, Plumeria rubra and Euphorbia tirucalli. Braz. J. Plant Physiol. 2010, 22, 11–22. [Google Scholar] [CrossRef]
- Musidlak, O.; Bałdysz, S.; Krakowiak, M.; Nawrot, R. Plant Latex Proteins and Their Functions. Adv. Bot. Res. 2020, 93, 55–97. [Google Scholar] [CrossRef]
- Mali, P.Y.; Panchal, S.S. Euphorbia tirucalli L.: Review on Morphology, Medicinal Uses, Phytochemistry and Pharmacological Activities. Asian Pac. J. Trop. Biomed. 2017, 7, 603–613. [Google Scholar] [CrossRef]
- Santhosh, K.H.; Manjunatha, H.; Krishna, V.; Kumara-Swamy, B.E. Anti-Proliferative and Antioxidant Potency of Leaf Methanol Extract of Cryptostegia grandiflora R. BR. Int. J. Pharm. Pharm. Sci. 2014, 6, 156–164. [Google Scholar]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and Their Potential Role to Fight Viral Diseases: An Overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Kamboj, A. Antiviral Activity of Plant Polyphenols. J. Pharm. Res. 2012, 5, 2402–2412. [Google Scholar]
- Snook; Data, E.M.; Kays, S. Characterization and Quantification of Hexadecyl, Octadecyl, and Eicosyl Esters of p-Coumaric Acids in the Vine and Root Latex of Sweetpotato [Ipomoea batatas (L.) Lam.]. J. Agric. Food Chem. 1994, 42, 2589–2595. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chiang, W.; Chang, M.Y.; Ng, L.T.; Lin, C.C. Antiviral Activity of Plantago Major Extracts and Related Compounds in Vitro. Antivir. Res. 2002, 55, 53–62. [Google Scholar] [CrossRef]
- Sakai, A.; Watanabe, K.; Koketsu, M.; Akuzawa, K.; Yamada, R.; Li, Z.; Sadanari, H.; Matsubara, K.; Murayama, T. Anti-Human Cytomegalovirus Activity of Constituents from Sasa Albo-Marginata (Kumazasa in Japan). Antivir. Chem. Chemother. 2008, 19, 125–132. [Google Scholar] [CrossRef]
- Abarca, L.F.S.; Klinkhamer, P.G.L.; Choi, Y.H. Plant Latex, from Ecological Interests to Bioactive Chemical Resources. Planta Med. 2019, 85, 856–868. [Google Scholar] [CrossRef]
- Sessa, R.A. Metabolite Profiling of Sesquiterpene Lactones from Lactuca Species: Major Latex components Are Novel Oxalate and Sulfate Conjugates of Lactucin and Its Derivatives. J. Biol. Chem. 2000, 275, 26877–26884. [Google Scholar] [CrossRef]
- Konno, K. Plant Latex and Other Exudates as Plant Defense Systems: Roles of Various Defense Chemicals and Proteins Contained Therein. Phytochemistry 2011, 72, 1510–1530. [Google Scholar] [CrossRef]
- Abookleesh, F.L.; Al-Anzi, B.S.; Ullah, A. Potential Antiviral Action of Alkaloids. Molecules 2022, 27, 903. [Google Scholar] [CrossRef]
- Musidlak, O.; Warowicka, A.; Broniarczyk, J.; Adamczyk, D.; Goździcka-Józefiak, A.; Nawrot, R. The Activity of Chelidonium Majus L. Latex and Its Components on HPV Reveal Insights into the Antiviral Molecular Mechanism. Int. J. Mol. Sci. 2022, 23, 9241. [Google Scholar] [CrossRef]
- Parhira, S.; Yang, Z.-F.; Zhu, G.-Y.; Chen, Q.-L.; Zhou, B.-X.; Wang, Y.-T.; Liu, L.; Bai, L.-P.; Jiang, Z.-H. In Vitro Anti-Influenza Virus Activities of a New Lignan Glycoside from the Latex of Calotropis Gigantea. PLoS ONE 2014, 9, e104544. [Google Scholar] [CrossRef]
- Nothias-Scaglia, L.-F.; Dumontet, V.; Neyts, J.; Roussi, F.; Costa, J.; Leyssen, P.; Litaudon, M.; Paolini, J. LC-MS2-Based Dereplication of Euphorbia Extracts with Anti-Chikungunya Virus Activity. Fitoterapia 2015, 105, 202–209. [Google Scholar] [CrossRef]
- Van den Bosch, C.; Griffin, B.; Kazembe, P.; Dziweni, C.; Kadzamira, L. Are Plant Factors a Missing Link in the Evolution of Endemic Burkitt’s Lymphoma? Br. J. Cancer 1993, 68, 1232–1235. [Google Scholar] [CrossRef]
- Mizuno, F.; Koizumi, S.; Osato, T.; Kokwaro, J.O.; Ito, Y. Chinese and African Euphorbiaceae Plant Extracts: Markedly Enhancing Effect on Epstein-Barr Virus-Induced Transformation. Cancer Lett. 1983, 19, 199–205. [Google Scholar] [CrossRef]
- Orem, J.; Mbidde, E.K.; Lambert, B.; de Sanjose, S.; Weiderpass, E. Burkitt’s Lymphoma in Africa, a Review of the Epidemiology and Etiology. Afr. Health Sci. 2007, 7, 166–175. [Google Scholar] [CrossRef]
- Richman, D.D.; Margolis, D.M.; Delaney, M.; Greene, W.C.; Hazuda, D.; Pomerantz, R.J. The Challenge of Finding a Cure for HIV Infection. Science (1979) 2009, 323, 1304–1307. [Google Scholar] [CrossRef]
- Gulakowski, R.; Mcmahon, J.; Buckheit, R.; Gustafson, K.; Boyd, M. Antireplicative and Anticytopathic Activities of Prostratin, a Non-Tumor-Promoting Phorbol Ester, against human immunodeficiency virus (HIV)1. Antivir. Res. 1997, 33, 87–97. [Google Scholar] [CrossRef]
- Avila, L.; Perez, M.; Sanchez-Duffhues, G.; Hernández-Galán, R.; Muñoz, E.; Cabezas, F.; Quiñones, W.; Torres, F.; Echeverri, F. Effects of Diterpenes from Latex of Euphorbia lactea and Euphorbia laurifolia on human immunodeficiency virus Type 1 Reactivation. Phytochemistry 2010, 71, 243–248. [Google Scholar] [CrossRef]
- Quito-Avila, D.F.; Reyes-Proaño, E.; Cañada, G.; Cornejo-Franco, J.F.; Alvarez-Quinto, R.; Moreira, L.; Grinstead, S.; Mollov, D.; Karasev, A.V. Papaya Sticky Disease Caused by Virus “Couples”: A Challenge for Disease Detection and Management. Plant Dis. 2023, 107, 1649–1663. [Google Scholar] [CrossRef]
- Maurastoni, M.; Sá Antunes, T.F.; Abreu, E.F.M.; Ribeiro, S.G.; Mehta, A.; Sanches, M.M.; Fontes, W.; Kitajima, E.W.; Cruz, F.T.; Santos, A.M.C.; et al. A Capsid Protein Fragment of a Fusagra-like Virus Found in Carica papaya Latex Interacts with the 50S Ribosomal Protein L17. Viruses 2023, 15, 541. [Google Scholar] [CrossRef]
- Sá Antunes, T.F.; Maurastoni, M.; Madroñero, L.J.; Fuentes, G.; Santamaría, J.M.; Ventura, J.A.; Abreu, E.F.; Fernandes, A.A.R.; Fernandes, P.M.B. Battle of Three: The Curious Case of Papaya Sticky Disease. Plant Dis. 2020, 104, 2754–2763. [Google Scholar] [CrossRef]
- Wititsuwannakul, R.; Rukseree, K.; Kanokwiroon, K.; Wititsuwannakul, D. A Rubber Particle Protein Specific for Hevea Latex Lectin Binding Involved in Latex Coagulation. Phytochemistry 2008, 69, 1111–1118. [Google Scholar] [CrossRef]
- Chaykin, S.; Law, J.; Phillips, A.H.; Tchen, T.T.; Bloch, K. Phosphorylated intermediates in the synthesis of squalene. Proc. Natl. Acad. Sci. USA 1958, 44, 998–1004. [Google Scholar] [CrossRef]
- Tameling, W.I.L.; Baulcombe, D.C. Physical Association of the NB-LRR Resistance Protein Rx with a Ran GTPase–Activating Protein Is Required for Extreme Resistance to Potato virus X. Plant Cell 2007, 19, 1682–1694. [Google Scholar] [CrossRef]
- Lam, E.; Kato, N.; Lawton, M. Programmed Cell Death, Mitochondria and the Plant Hypersensitive Response. Nature 2001, 411, 848–853. [Google Scholar] [CrossRef]
- Lawton, J.A.; Estes, M.K.; Venkataram Prasad, B.V. Mechanism of Genome Transcription in Segmented DsRNA Viruses; Elsevier: Amsterdam, The Netherlands, 2000; pp. 185–229. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Zhang, H.; Jakana, J.; Lu, X.-Y.; Zhang, J.-Q. Cytoplasmic Polyhedrosis Virus Structure at 8 Å by Electron Cryomicroscopy. Structure 2003, 11, 651–663. [Google Scholar] [CrossRef]
- Pickard, W.F. Laticifers and Secretory Ducts: Two Other Tube Systems in Plants. New Phytol. 2008, 177, 877–888. [Google Scholar] [CrossRef]
- Santana, M.A.; Vásquez, V.; Matehus, J.; Aldao, R.R. Linamarase Expression in Cassava Cultivars with Roots of Low- and High-Cyanide Content. Plant Physiol. 2002, 129, 1686–1694. [Google Scholar] [CrossRef]
- Shan, S.; Soltis, P.S.; Soltis, D.E.; Yang, B. Considerations in Adapting CRISPR/Cas9 in Nongenetic Model Plant Systems. Appl. Plant Sci. 2020, 8, e11314. [Google Scholar] [CrossRef]
- Wieghaus, A.; Prüfer, D.; Schulze Gronover, C. Loss of Function Mutation of the Rapid Alkalinization Factor (RALF1)-like Peptide in the Dandelion Taraxacum koksaghyz Entails a High-Biomass Taproot Phenotype. PLoS ONE 2019, 14, e0217454. [Google Scholar] [CrossRef]
- Kwon, M.; Hodgins, C.L.; Salama, E.M.; Dias, K.R.; Parikh, A.; Mackey, A.V.; Catenza, K.F.; Vederas, J.C.; Ro, D. New Insights into Natural Rubber Biosynthesis from Rubber-deficient Lettuce Mutants Expressing Goldenrod or Guayule Cis-prenyltransferase. New Phytol. 2023, 239, 1098–1111. [Google Scholar] [CrossRef]
- Iaffaldano, B.; Zhang, Y.; Cornish, K. CRISPR/Cas9 Genome Editing of Rubber Producing Dandelion Taraxacum kok-saghyz Using Agrobacterium rhizogenes without Selection. Ind. Crops Prod. 2016, 89, 356–362. [Google Scholar] [CrossRef]
- Cankar, K.; Bundock, P.; Sevenier, R.; Häkkinen, S.T.; Hakkert, J.C.; Beekwilder, J.; van der Meer, I.M.; de Both, M.; Bosch, D. Inactivation of the Germacrene A Synthase Genes by CRISPR/Cas9 Eliminates the Biosynthesis of Sesquiterpene Lactones in Cichorium intybus L. Plant Biotechnol. J. 2021, 19, 2442–2453. [Google Scholar] [CrossRef]
- Stolze, A.; Wanke, A.; van Deenen, N.; Geyer, R.; Prüfer, D.; Gronover, C.S. Development of rubber-enriched dandelion varieties by metabolic engineering of the inulin pathway. Plant Biotechnol. J. 2017, 15, 740–753. [Google Scholar] [CrossRef]
- Cankar, K.; Hakkert, J.C.; Sevenier, R.; Campo, E.; Schipper, B.; Papastolopoulou, C.; Vahabi, K.; Tissier, A.; Bundock, P.; Bosch, D. CRISPR/Cas9 Targeted Inactivation of the Kauniolide Synthase in Chicory Results in Accumulation of Costunolide and Its Conjugates in Taproots. Front. Plant Sci. 2022, 13, 940003. [Google Scholar] [CrossRef]
- Dai, X.; Yang, X.; Wang, C.; Fan, Y.; Xin, S.; Hua, Y.; Wang, K.; Huang, H. CRISPR/Cas9-Mediated Genome Editing in Hevea brasiliensis. Ind. Crops Prod. 2021, 164, 113418. [Google Scholar] [CrossRef]
- Fan, Y.; Xin, S.; Dai, X.; Yang, X.; Huang, H.; Hua, Y. Efficient Genome Editing of Rubber Tree (Hevea brasiliensis) Protoplasts Using CRISPR/Cas9 Ribonucleoproteins. Ind. Crops Prod. 2020, 146, 112146. [Google Scholar] [CrossRef]
| ANTIVIRAL INTERACTION | |||
|---|---|---|---|
| Component | Interaction | Model | Reference |
| alkaloids | DNA and RNA synthesis inhibition and viral replication blockage | HIV-1, SARS-CoV-2 | [1] |
| cysteine protease inhibitor | inhibition of viral cysteine proteases | potato virus Y in potato plants | [8] |
| cysteine/serine protease inhibitor | inhibition of viral proteases | tomato-spotted wilt virus in tomato | [9] |
| ubiquitin–proteasome system | degradation of viral proteins | potyvirus in Arabidopsis thaliana | [10] |
| phenolics and polyphenols | binding to the viral envelope | influenza A virus in Croton lechleri | [11] |
| cardenolides | inhibition of viral protein synthesis, viral cellular release, and dispersion | herpes simplex virus type 1 in Digitalis lanata | [12] |
| latex extracts | inhibition of viral multiplication | herpes simplex virus type 1 and echovirus type 11 in Ficus carica | [13,14] |
| calanolides | inhibition of viral reverse transcriptase | Calophyllum teysmanii, a human immunodeficiency virus | [15] |
| calreticulin | binding to the virus to inhibit spread or to the virus helper protein | tobacco mosaic virus in Nicotiana tabacum | [16] |
| PRO-VIRAL INTERACTIONS | |||
| Component | Interaction | Model | Reference |
| latex particles | potential increase in latex fluidity, increasing viral dispersal | papaya meleira virus in Carica papaya L. | [17] |
| Hsp70 proteins | assembly and activation of viral proteins, reduction in plants in response to infection | tomato bushy stunt virus in Nicotiana benthamiana and Carica papaya L. | [18,19] |
| Euphorbia tirucalli latex | activation of the Epstein-Barr virus lytic cell cycle | Epstein-Barr virus in Euphorbia tirucalli | [20] |
| terpenoids | activation of the HIV-1 virus | human immunodeficiency virus type 1 in Euphorbia officinarum | [21] |
| Gene | Function | Plant Host | Reference |
|---|---|---|---|
| rapid alkalinisation factor like 1 | influences root phenotype and biomass, and inulin and natural rubber yield | Taraxacum koksaghyz | [92] |
| laticifer-specific cis-prenyltransferase 3 | involved in high-quality rubber production by laticifers | Lactuca sativa | [93] |
| 1-fructosyltransferase | encodes a key enzyme in inulin biosynthesis | Taraxacum koksaghyz | [94] |
| germacrene A synthase | involved with the degradation of sesquiterpene lactones | Cichorium intybus L. | [95] |
| rubber elongation factor and small rubber particle protein | belong to the stress-related protein superfamily involved in rubber biosynthesis and storage | Taraxacum koksaghyz | [96] |
| kauniolide synthase | disruption of sesquiterpene lactone biosynthesis in laticifers | Cichorium intybus var. sativum | [97] |
| ribonucleoprotein | belong to a family that controls plant flowering time | Hevea brasiliensis | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merchán-Gaitán, J.B.; Mendes, J.H.L.; Nunes, L.E.C.; Buss, D.S.; Rodrigues, S.P.; Fernandes, P.M.B. The Role of Plant Latex in Virus Biology. Viruses 2024, 16, 47. https://doi.org/10.3390/v16010047
Merchán-Gaitán JB, Mendes JHL, Nunes LEC, Buss DS, Rodrigues SP, Fernandes PMB. The Role of Plant Latex in Virus Biology. Viruses. 2024; 16(1):47. https://doi.org/10.3390/v16010047
Chicago/Turabian StyleMerchán-Gaitán, Julia B., João H. L. Mendes, Lucas E. C. Nunes, David S. Buss, Silas P. Rodrigues, and Patricia M. B. Fernandes. 2024. "The Role of Plant Latex in Virus Biology" Viruses 16, no. 1: 47. https://doi.org/10.3390/v16010047
APA StyleMerchán-Gaitán, J. B., Mendes, J. H. L., Nunes, L. E. C., Buss, D. S., Rodrigues, S. P., & Fernandes, P. M. B. (2024). The Role of Plant Latex in Virus Biology. Viruses, 16(1), 47. https://doi.org/10.3390/v16010047






