B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Assessment of Vaccine-Related Side Effects
2.3. Procedures
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics
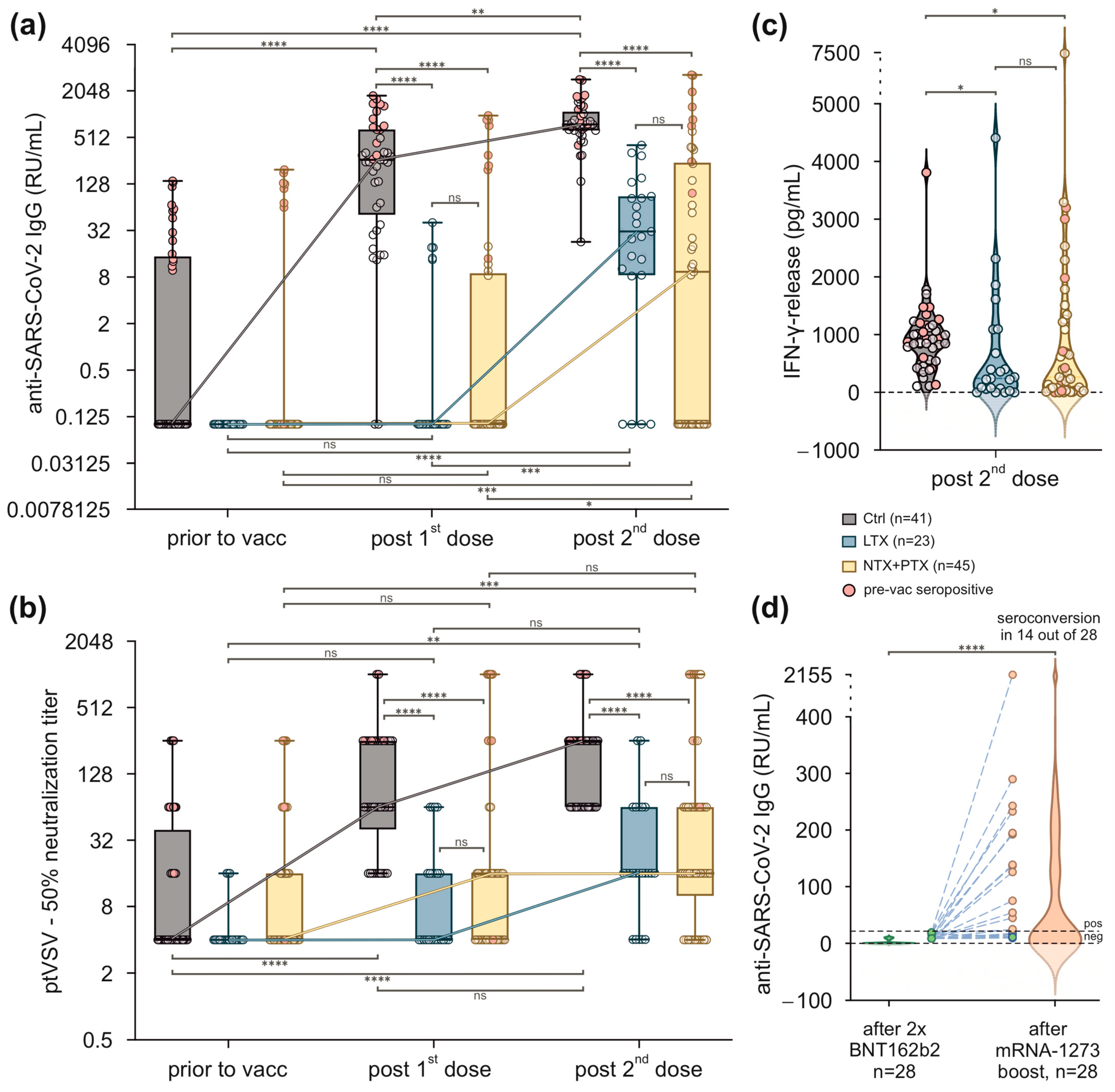
3.2. Vaccination with BNT162b2 Results in Reduced Humoral and Cellular Immune Responses in SOT Recipients
3.3. Transplant Patients Who Failed to Achieve Protective Antibody Titres after Two Immunisations with BNT162b2 Benefit from a Booster Vaccination with mRNA-1273
3.4. In SOT Recipients, Humoral Responses after Two Vaccinations with BNT162b2 Are Most Negatively Affected by Mycophenolate
3.5. SOT Recipients Experience Fewer but More Severe Vaccine-Related Side Effects Than Healthy Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nordham, K.D.; Ninokawa, S. The history of organ transplantation. Bayl. Univ. Med. Cent. Proc. 2022, 35, 124–128. [Google Scholar] [CrossRef]
- Pilch, N.A.; Bowman, L.J.; Taber, D.J. Immunosuppression trends in solid organ transplantation: The future of individualization, monitoring, and management. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.D.; Goff, C.; Kamepalli, S.; Montgomery, A.E.; Miggins, J.J.; Goss, J.A.; Rana, A. Survival Benefit of Solid-Organ Transplantation: 10-Year Update. Dig. Dis. Sci. 2023, 68, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Amaeshi, L.C. Navigating Through the Complications of Chronic Immunosuppression in Transplant Patients. Ann. Intern. Med. Clin. Cases 2022, 1, e220940C. [Google Scholar] [CrossRef]
- Green, M.; Blumberg, E.A.; Danziger-Isakov, L.; Huprikar, S.; Kotton, C.N.; Kumar, D. Foreword: 4th edition of the American Society of Transplantation Infectious Diseases Guidelines. Clin. Transplant. 2019, 33, e13642. [Google Scholar] [CrossRef] [PubMed]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.T.; Francisco, J.A.T.S.; Freitas, J.C.; Carvalho, R.V.; Vilela, S.R.B.; Ribeiro, C.I.C.D.; Silvano, J.L.C.S.L.; Pedroso, S.; Almeida, M.; Martins, L.S.; et al. Excess Mortality in Kidney and Kidney-Pancreas Transplant Recipients in the COVID-19 Pandemic in Portugal—A Cohort Study. Transpl. Int. 2023, 36, 11655. [Google Scholar] [CrossRef]
- Moreno-Torres, V.; Martínez-Urbistondo, M.; Calderón-Parra, J.; Mills, P.; Muñoz-Serrano, A.; Arias-Milla, A.; Benítez, L.; Aguilar-Pérez, M.; Múñez-Rubio, E.; Ramos-Martínez, A.; et al. COVID-19 in hospitalized solid organ transplant recipients in a nationwide registry study. Int. J. Infect. Dis. 2023, 134, 154–159. [Google Scholar] [CrossRef]
- Kulkarni, A.V.; Tevethia, H.V.; Premkumar, M.; Arab, J.P.; Candia, R.; Kumar, K.; Kumar, P.; Sharma, M.; Rao, P.N.; Reddy, D.N. Impact of COVID-19 on liver transplant recipients—A systematic review and meta-analysis. eClinicalMedicine 2021, 38, 101025. [Google Scholar] [CrossRef]
- Chen, X.; Luo, D.; Mei, B.; Du, J.; Liu, X.; Xie, H.; Liu, L.; Su, S.; Mai, G. Immunogenicity of COVID-19 vaccines in solid organ transplant recipients: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2023, 29, 441–456. [Google Scholar] [CrossRef]
- Manothummetha, K.; Chuleerarux, N.; Sanguankeo, A.; Kates, O.S.; Hirankarn, N.; Thongkam, A.; Dioverti-Prono, M.V.; Torvorapanit, P.; Langsiri, N.; Worasilchai, N.; et al. Immunogenicity and Risk Factors Associated with Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e226822. [Google Scholar] [CrossRef] [PubMed]
- Naylor, K.L.; Kim, S.J.; Smith, G.; McArthur, E.; Kwong, J.C.; Dixon, S.N.; Treleaven, D.; Knoll, G.A. Effectiveness of first, second, and third COVID-19 vaccine doses in solid organ transplant recipients: A population-based cohort study from Canada. Am. J. Transplant. 2022, 22, 2228–2236. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.P.-Y.; Abedon, A.T.; Alejo, J.L.; Segev, D.L.; Massie, A.B.; Werbel, W.A. Incident COVID-19 and Hospitalizations by Variant Era Among Vaccinated Solid Organ Transplant Recipients. JAMA Netw. Open 2023, 6, e2329736. [Google Scholar] [CrossRef] [PubMed]
- Zollner, A.; Watschinger, C.; Rössler, A.; Farcet, M.R.; Penner, A.; Böhm, V.; Kiechl, S.J.; Stampfel, G.; Hintenberger, R.; Tilg, H.; et al. B and T cell response to SARS-CoV-2 vaccination in health care professionals with and without previous COVID-19. EBioMedicine 2021, 70, 103539. [Google Scholar] [CrossRef] [PubMed]
- Riepler, L.; Rössler, A.; Falch, A.; Volland, A.; Borena, W.; von Laer, D.; Kimpel, J. Comparison of Four SARS-CoV-2 Neutralization Assays. Vaccines 2020, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Eckerle, I.; Rosenberger, K.D.; Zwahlen, M.; Junghanss, T. Serologic vaccination response after solid organ transplantation: A systematic review. PLoS ONE 2013, 8, e56974. [Google Scholar] [CrossRef]
- Walti, L.N.; Mugglin, C.; Mombelli, M.; Manuel, O.; Hirsch, H.H.; Khanna, N.; Mueller, N.J.; Berger, C.; Boggian, K.; Garzoni, C.; et al. Vaccine-Preventable Infections Among Solid Organ Transplant Recipients in Switzerland. JAMA Netw. Open 2023, 6, e2310687. [Google Scholar] [CrossRef]
- Danziger-Isakov, L.; Kumar, D. Vaccination of solid organ transplant candidates and recipients: Guidelines from the American society of transplantation infectious diseases community of practice. Clin. Transplant. 2019, 33, e13563. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2020, 384, 403–416. [Google Scholar] [CrossRef]
- Sakuraba, A.; Luna, A.; Micic, D. A Systematic Review and Meta-Analysis of Serologic Response following Coronavirus Disease 2019 (COVID-19) Vaccination in Solid Organ Transplant Recipients. Viruses 2022, 14, 1822. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Marking, U.; Greilert-Norin, N.; Ng, H.; Gordon, M.; Salomonsson, A.-C.; Hellström, C.; Pin, E.; Blom, K.; Mangsbo, S.; et al. Antibody responses after a single dose of ChAdOx1 nCoV-19 vaccine in healthcare workers previously infected with SARS-CoV-2. eBioMedicine 2021, 70, 103523. [Google Scholar] [CrossRef] [PubMed]
- Higashimoto, Y.; Kozawa, K.; Miura, H.; Kawamura, Y.; Ihira, M.; Hiramatsu, H.; Suzuki, R.; Haga, K.; Takai-Todaka, R.; Sawada, A.; et al. Correlation between anti-S IgG and neutralizing antibody titers against three live SARS-CoV-2 variants in BNT162b2 vaccine recipients. Hum. Vaccines Immunother. 2022, 18, 2105611. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Schlub, T.E.; Cromer, D.; Steain, M.; Fong, Y.; Gilbert, P.B.; Subbarao, K.; Triccas, J.A.; Kent, S.J.; Davenport, M.P. Correlates of Protection, Thresholds of Protection, and Immunobridging among Persons with SARS-CoV-2 Infection. Emerg. Infect. Dis. 2023, 29, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Yanis, A.; Haddadin, Z.; Spieker, A.J.; Waqfi, D.; Rankin, D.A.; Talj, R.; Thomas, L.; Birdwell, K.A.; Ezzell, L.; Blair, M.; et al. Humoral and cellular immune responses to the SARS-CoV-2 BNT162b2 vaccine among a cohort of solid organ transplant recipients and healthy controls. Transpl. Infect. Dis. 2022, 24, e13772. [Google Scholar] [CrossRef]
- Miele, M.; Busà, R.; Russelli, G.; Sorrentino, M.C.; Di Bella, M.; Timoneri, F.; Mularoni, A.; Panarello, G.; Vitulo, P.; Conaldi, P.G.; et al. Impaired anti-SARS-CoV-2 humoral and cellular immune response induced by Pfizer-BioNTech BNT162b2 mRNA vaccine in solid organ transplanted patients. Am. J. Transplant. 2021, 21, 2919–2921. [Google Scholar] [CrossRef]
- Williams, W.W.; Ingelfinger, J.R. Third Time’s a Charm—COVID-19 Vaccine Hope for Solid-Organ Transplant Recipients. N. Engl. J. Med. 2021, 385, 1233–1234. [Google Scholar] [CrossRef]
- Hulme, W.J.; Horne, E.M.F.; Parker, E.P.K.; Keogh, R.H.; Williamson, E.J.; Walker, V.; Palmer, T.M.; Curtis, H.J.; Walker, A.J.; Andrews, C.D.; et al. Comparative effectiveness of BNT162b2 versus mRNA-1273 COVID-19 vaccine boosting in England: Matched cohort study in OpenSAFELY-TPP. BMJ 2023, 380, e072808. [Google Scholar] [CrossRef]
- Hall, V.G.; Ferreira, V.H.; Ku, T.; Ierullo, M.; Majchrzak-Kita, B.; Chaparro, C.; Selzner, N.; Schiff, J.; McDonald, M.; Tomlinson, G.; et al. Randomized Trial of a Third Dose of mRNA-1273 Vaccine in Transplant Recipients. N. Engl. J. Med. 2021, 385, 1244–1246. [Google Scholar] [CrossRef]
- Bailey, A.J.M.; Maganti, H.B.; Cheng, W.; Shorr, R.; Arianne Buchan, C.; Allan, D.S. Humoral and Cellular Response of Transplant Recipients to a Third Dose of mRNA SARS-CoV-2 Vaccine: A Systematic Review and Meta-analysis. Transplantation 2023, 107, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Meziyerh, S.; Bouwmans, P.; van Gelder, T.; van der Helm, D.; Messchendorp, L.; van der Boog, P.J.M.; de Fijter, J.W.; Moes, D.; de Vries, A.P.J. Mycophenolic Acid Exposure Determines Antibody Formation Following SARS-CoV-2 Vaccination in Kidney Transplant Recipients: A Nested Cohort Study. Clin. Pharmacol. Ther. 2023, 114, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Zecca, E.; Rizzi, M.; Tonello, S.; Matino, E.; Costanzo, M.; Rizzi, E.; Casciaro, G.F.; Manfredi, G.F.; Acquaviva, A.; Gagliardi, I.; et al. Ongoing Mycophenolate Treatment Impairs Anti-SARS-CoV-2 Vaccination Response in Patients Affected by Chronic Inflammatory Autoimmune Diseases or Liver Transplantation Recipients: Results of the RIVALSA Prospective Cohort. Viruses 2022, 14, 1766. [Google Scholar] [CrossRef] [PubMed]
- Kantauskaite, M.; Müller, L.; Kolb, T.; Fischer, S.; Hillebrandt, J.; Ivens, K.; Andree, M.; Luedde, T.; Orth, H.M.; Adams, O.; et al. Intensity of mycophenolate mofetil treatment is associated with an impaired immune response to SARS-CoV-2 vaccination in kidney transplant recipients. Am. J. Transplant. 2022, 22, 634–639. [Google Scholar] [CrossRef]

| Baseline Characteristics | |||||
|---|---|---|---|---|---|
| Unit | All SOT Recipients | NTX + PTX Recipients | LTX Recipients | Healthy Controls | |
| N | 68 | 45 | 23 | 41 | |
| Post COVID, N (%) | 8 (11.8) | 8 (17.8) | 0 (0) | 14 (34.1) | |
| Sex female, N (%) | 21 (30.9) | 15 (33.3) | 6 (26.1) | 21 (51.2) | |
| Age, N (SD) | years | 58.5 (18.6) | 58.4 (11.2) | 58.8 (28.2) | 36.7 (10.3) |
| BMI, mean (SD) | kg/m2 | 26.0 (4.0) | 26.0 (4.0) | 26.2 (4.0) | 23.6 (5.9) |
| Time since Tx, mean (SD) | 13.7 (6.5) | 13.9 (6.1) | 13.3 (7.2) | - | |
| Hb, mean (SD) | g/dL | 13.2 (2.2) | 13.0 (2.4) | 13.7 (1.6) | - |
| GFR, mean (SD) | mL/min | 52.1 (22.4) | 51.7 (24.9) | 52.9 (15.2) | - |
| GOT, mean (SD) | U/I | 23.8 (7.2) | 22.6 (6.0) | 26.3 (9.1) | - |
| GPT, mean (SD) | U/I | 22.2 (10.0) | 21.1 (9.4) | 24.8 (11.2) | - |
| Bilirubin, mean (SD) | mg/dL | 0.6 (0.3) | 0.6 (0.3) | 0.6 (0.2) | - |
| Albumin, mean (SD) | mg/dL | 4215.9 (627.4) | 4233.1 (322.1) | 4175.3 (1059.1) | - |
| Immunosuppressants, N (%) | |||||
| GC | 19 (28) | 16 (36) | 3 (13) | - | |
| CNI | 57 (84) | 37 (82) | 20 (87) | - | |
| MMF/MPA | 49 (72) | 37 (82) | 12 (52) | - | |
| AZA | 3 (4) | 3 (7) | 0 (0) | - | |
| mTOR | 6 (9) | 5 (11) | 1 (4) | - | |
| Other | 1 (1) | 1 (2) | 0 (0) | - | |
| Vaccine related side effects | |||||
| p-Value | All SOT recipients | NTX + PTX recipients | LTX recipients | Healthy controls | |
| N | <0.01 | 68 | 45 | 23 | 41 |
| Any, N (%) | <0.01 | 33 (48.5) | 20 (44.4) | 13 (56.5) | 31 (75.6) |
| Local, N (%) | 0.65 | 22 (32.4) | 14 (31.1) | 8 (34.8) | 15 (36.6) |
| Systemic, N (%) | <0.01 | 22 (32.4) | 14 (31.1) | 8 (34.8) | 27 (65.9) |
| Severity, mean | <0.01 | 0.50 | 0.47 | 0.57 | 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watschinger, C.; Stampfel, G.; Zollner, A.; Hoog, A.M.; Rössler, A.; Reiter, S.; Dax, K.; Kimpel, J.; Tilg, H.; Antlanger, M.; et al. B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19. Viruses 2024, 16, 1. https://doi.org/10.3390/v16010001
Watschinger C, Stampfel G, Zollner A, Hoog AM, Rössler A, Reiter S, Dax K, Kimpel J, Tilg H, Antlanger M, et al. B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19. Viruses. 2024; 16(1):1. https://doi.org/10.3390/v16010001
Chicago/Turabian StyleWatschinger, Christina, Gerald Stampfel, Andreas Zollner, Anna M. Hoog, Annika Rössler, Silvia Reiter, Kristina Dax, Janine Kimpel, Herbert Tilg, Marlies Antlanger, and et al. 2024. "B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19" Viruses 16, no. 1: 1. https://doi.org/10.3390/v16010001
APA StyleWatschinger, C., Stampfel, G., Zollner, A., Hoog, A. M., Rössler, A., Reiter, S., Dax, K., Kimpel, J., Tilg, H., Antlanger, M., Schwaiger, E., & Moschen, A. R. (2024). B and T Cell Responses to SARS-CoV-2 Vaccination in Kidney and Liver Transplant Recipients with and without Previous COVID-19. Viruses, 16(1), 1. https://doi.org/10.3390/v16010001






