B-Cell Epitopes-Based Chimeric Protein from SARS-CoV-2 N and S Proteins Is Recognized by Specific Antibodies in Serum and Urine Samples from Patients
Abstract
:1. Introduction
2. Material and Methods
2.1. Research Subjects
2.2. Biological Samples
2.3. Prediction of B-Cell Epitopes and Construction of Chimeric Protein
2.4. Production of Recombinant N4S11-SC2 Protein
2.5. Immunoblottings
2.6. ELISA
2.7. Statistical Analysis
3. Results
3.1. Construction of the Chimeric Protein
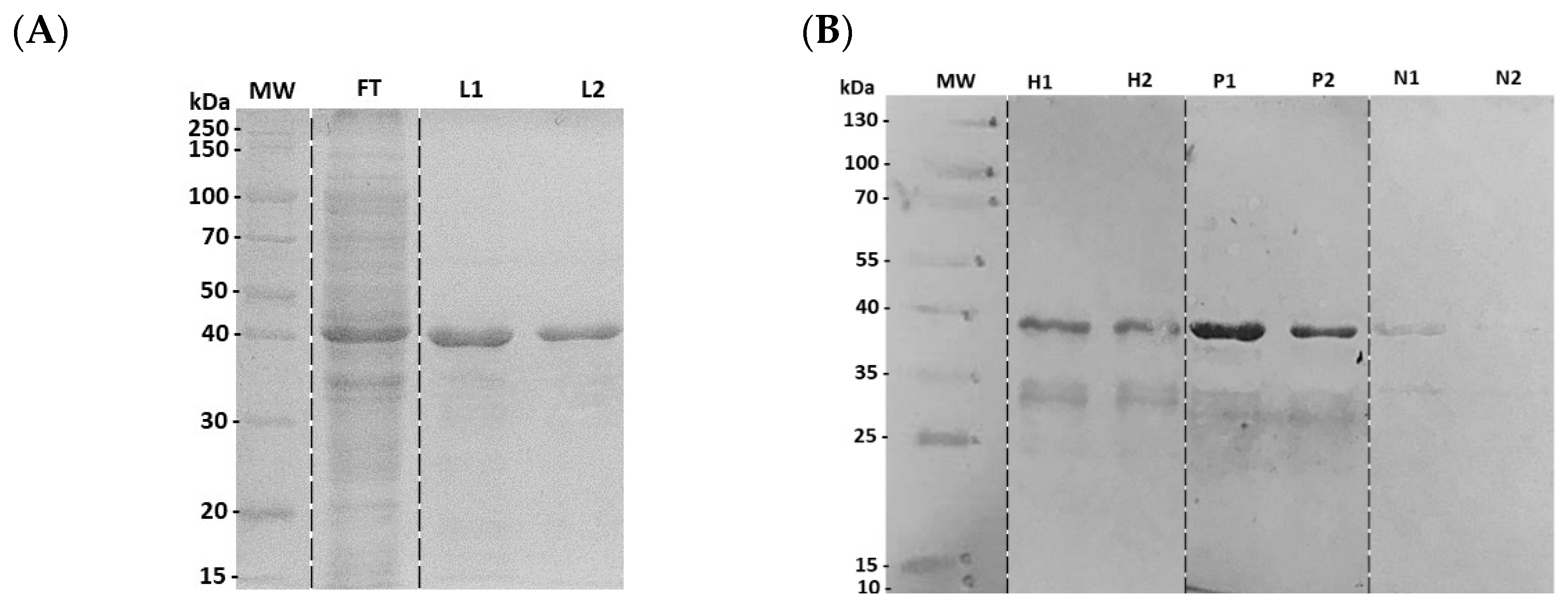
3.2. Characterization of Recombinant N4S11-SC2 Protein
3.3. Diagnostic Evaluation of Chimeric N4S11-SC2 Protein
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FDA. EUA Authorized Serology Test Performance. Food and Drug Administration. Available online: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance (accessed on 22 August 2022).
- CDC. COVID-19 Testing: What You Need to Know. Available online: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/testing.html (accessed on 16 August 2023).
- Matta, J.; Wiernik, E.; Robineau, O.; Carrat, F.; Touvier, M.; Severi, G.; de Lamballerie, X.; Blanché, H.; Deleuze, J.-F.; Gouraud, C.; et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results with Persistent Physical Symptoms among French Adults during the COVID-19 Pandemic. JAMA Intern. Med. 2022, 182, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef] [PubMed]
- FDA. SARS-CoV-2 Viral Mutations: Impact on COVID-19 Tests. Food and Drug Administration. Available online: https://www.fda.gov/medical-devices/coronavirus-covid-19-and-medical-devices/sars-cov-2-viral-mutations-impact-covid-19-tests (accessed on 16 August 2023).
- Kumar, S.; Saxena, S.K. Structural and molecular perspectives of SARS-CoV-2. Methods 2021, 195, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Verheije, M.H.; Hagemeijer, M.C.; Ulasli, M.; Reggiori, F.; Rottier, P.J.M.; Masters, P.S.; de Haan, C.A.M. The coronavirus nucleocapsid protein is dynamically associated with the replication-transcription complexes. J. Virol. 2010, 84, 11575–11579. [Google Scholar] [CrossRef]
- Grzelak, L.; Temmam, S.; Planchais, C.; Demeret, C.; Tondeur, L.; Huon, C.; Guivel-Benhassine, F.; Staropoli, I.; Chazal, M.; Dufloo, J.; et al. A comparison of four serological assays for detecting anti-SARS-CoV-2 antibodies in human serum samples from different populations. Sci. Transl. Med. 2020, 12, eabc3103. [Google Scholar] [CrossRef]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of nucleocapsid and spike protein-based enzyme-linked immunosorbent assays for detecting antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00461-20. [Google Scholar] [CrossRef]
- Li, D.; Li, J. Immunologic Testing for SARS-CoV-2 Infection from the Antigen Perspective. J. Clin. Microbiol. 2021, 59, e02160-20. [Google Scholar] [CrossRef]
- Ramos, F.F.; Bagno, F.F.; Vassallo, P.F.; Oliveira-Da-Silva, J.A.; Reis, T.A.R.; Bandeira, R.S.; Machado, A.S.; Lage, D.P.; Martins, V.T.; Fernandes, A.P.; et al. A urine-based ELISA with recombinant non-glycosylated SARS-CoV-2 spike protein for detecting anti-SARS-CoV-2 spike antibodies. Sci. Rep. 2023, 13, 4345. [Google Scholar] [CrossRef]
- Ludolf, F.; Ramos, F.F.; Bagno, F.F.; Oliveira-Da-Silva, J.A.; Reis, T.A.R.; Christodoulides, M.; Vassallo, P.F.; Ravetti, C.G.; Nobre, V.; da Fonseca, F.G.; et al. Detecting anti-SARS-CoV-2 antibodies in urine samples: A noninvasive and sensitive way to assay COVID-19 immune conversion. Sci Adv. 2022, 8, eabn7424. [Google Scholar] [CrossRef]
- Mamaghani, A.J.; Arab-Mazar, Z.; Heidarzadeh, S.; Ranjbar, M.M.; Molazadeh, S.; Rashidi, S.; Niazpour, F.; Vishteh, M.N.; Bashiri, H.; Bozorgomid, A.; et al. In-silico design of a multi-epitope for developing sero-diagnosis detection of SARS-CoV-2 using spike glycoprotein and nucleocapsid antigens. Netw. Model. Anal. Health Inform. Bioinform. 2021, 10, 61. [Google Scholar] [CrossRef]
- Vengesai, A.; Naicker, T.; Midzi, H.; Kasambala, M.; Muleya, V.; Chipako, I.; Choto, E.; Moyo, P.; Mduluza, T. Peptide microarray analysis of in-silico predicted B-cell epitopes in SARS-CoV-2 sero-positive healthcare workers in Bulawayo, Zimbabwe. Acta Trop. 2023, 238, 106781. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.; de Magalhães, M.T.Q.; Homan, E.J. Immunoinformatic Analysis of SARS-CoV-2 Nucleocapsid Protein and Identification of COVID-19 Vaccine Targets. Front. Immunol. 2020, 11, 587615. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, M.A.; Pachón, D.; Maier, A.; Bermúdez, H.; Losada, S.; Toledo, M.; Pujol, F.H.; de Noya, B.A.; Noya, O.; Serrano, M.L. Immunoinformatics and Pepscan strategies on the path of a peptide-based serological diagnosis of COVID-19. J. Immunol. Methods 2021, 495, 113071. [Google Scholar] [CrossRef] [PubMed]
- Musicò, A.; Frigerio, R.; Mussida, A.; Barzon, L.; Sinigaglia, A.; Riccetti, S.; Gobbi, F.; Piubelli, C.; Bergamaschi, G.; Chiari, M.; et al. SARS-CoV-2 Epitope Mapping on Microarrays Highlights Strong Immune-Response to N Protein Region. Vaccines 2021, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, T.; Heiss, K.; Mahendran, Y.; Casilag, F.; Kurth, F.; Sander, L.E.; Wendtner, C.-M.; Hoechstetter, M.A.; Müller, M.A.; Sekul, R.; et al. SARS-CoV-2 Proteome-Wide Analysis Revealed Significant Epitope Signatures in COVID-19 Patients. Front. Immunol. 2021, 12, 629185. [Google Scholar] [CrossRef]
- Gomes, L.R.; Durans, A.M.; Napoleão-Pêgo, P.; Waterman, J.A.; Freitas, M.S.; De Sá, N.B.R.; Pereira, L.V.; Furtado, J.S.; Aquino, R.G.; Machado, M.C.R.; et al. Multiepitope Proteins for the Differential Detection of IgG Antibodies against RBD of the Spike Protein and Non-RBD Regions of SARS-CoV-2. Vaccines 2021, 9, 986. [Google Scholar] [CrossRef]
- Abraham Peele, K.; Srihansa, T.; Krupanidhi, S.; Ayyagari, V.S.; Venkateswarulu, T.C. Design of multi-epitope vaccine candidate against SARS-CoV-2: A in-silico study. J. Biomol. Struct. Dyn. 2021, 39, 3793–3801. [Google Scholar] [CrossRef]
- Ludolf, F.; Ramos, F.F.; Bagno, F.; Coelho, E.A.; Fonseca, F.G. Urine-based Enzyme-Linked Immunosorbent Assay to detect anti-SARS-CoV-2 nucleocapsid protein antibodies. Bio Protocol. 2022, preprint. [Google Scholar] [CrossRef]
- Phan, I.Q.; Subramanian, S.; Kim, D.; Murphy, M.; Pettie, D.; Carter, L.; Anishchenko, I.; Barrett, L.K.; Craig, J.; Tillery, L.; et al. In silico detection of SARS-CoV-2 specific B-cell epitopes and validation in ELISA for serological diagnosis of COVID-19. Sci. Rep. 2021, 11, 4290. [Google Scholar] [CrossRef]
- Polyiam, K.; Phoolcharoen, W.; Butkhot, N.; Srisaowakarn, C.; Thitithanyanont, A.; Auewarakul, P.; Hoonsuwan, T.; Ruengjitchatchawalya, M.; Mekvichitsaeng, P.; Roshorm, Y.M. Immunodominant linear B cell epitopes in the spike and membrane proteins of SARS-CoV-2 identified by immunoinformatics prediction and immunoassay. Sci. Rep. 2021, 11, 20383. [Google Scholar] [CrossRef]
- Rodrigues-Da-Silva, R.N.; Conte, F.P.; da Silva, G.; Carneiro-Alencar, A.L.; Gomes, P.R.; Kuriyama, S.N.; Neto, A.A.F.; Lima-Junior, J.C. Identification of B-Cell Linear Epitopes in the Nucleocapsid (N) Protein B-Cell Linear Epitopes Conserved among the Main SARS-CoV-2 Variants. Viruses 2023, 15, 923. [Google Scholar] [CrossRef] [PubMed]
- Scussel, R.; Feuser, P.E.; Luiz, G.P.; Galvani, N.C.; Fagundes, M.; Dal-Bó, A.G.; de Araújo, P.H.H.; Coelho, E.A.F.; Chávez-Olórtegui, C.; Machado-De-Ávila, R.A. Peptide-Integrated Superparamagnetic Nanoparticles for the Identification of Epitopes from SARS-CoV-2 Spike and Nucleocapsid Proteins. ACS Appl. Nano Mater. 2022, 5, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lai, D.-Y.; Lei, Q.; Xu, Z.-W.; Wang, F.; Hou, H.; Chen, L.; Wu, J.; Ren, Y.; Ma, M.-L.; et al. Systematic evaluation of IgG responses to SARS-CoV-2 spike protein-derived peptides for monitoring COVID-19 patients. Cell. Mol. Immunol. 2021, 18, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Le Gars, M.; Hendriks, J.; Sadoff, J.; Ryser, M.; Struyf, F.; Douoguih, M.; Schuitemaker, H. Immunogenicity and efficacy of Ad26.COV2.S: An adenoviral vector-based COVID-19 vaccine. Immunol. Rev. 2022, 310, 47–60. [Google Scholar] [CrossRef]
- Naranbhai, V.; Garcia-Beltran, W.F.; Chang, C.C.; Mairena, C.B.; Thierauf, J.C.; Kirkpatrick, G.; Onozato, M.L.; Cheng, J.; Denis, K.J.S.; Lam, E.C.; et al. Comparative Immunogenicity and Effectiveness of mRNA-1273, BNT162b2, and Ad26.COV2.S COVID-19 Vaccines. J. Infect. Dis. 2022, 225, 1141–1150. [Google Scholar] [CrossRef]
- WHO. Interim Statement on Hybrid Immunity and Increasing Population Seroprevalence Rates. 2022. Available online: https://www.who.int/news/item/01-06-2022-interim-statement-on-hybrid-immunity-and-increasing-population-seroprevalence-rates (accessed on 16 August 2023).
- Zhang, B.; Tian, J.; Zhang, Q.; Xie, Y.; Wang, K.; Qiu, S.; Lu, K.; Liu, Y. Comparing the Nucleocapsid Proteins of Human Coronaviruses: Structure, Immunoregulation, Vaccine, and Targeted Drug. Front. Mol. Biosci. 2022, 9, 761173. [Google Scholar] [CrossRef]
- Channappanavar, R.; Fett, C.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014, 88, 11034–11044. [Google Scholar] [CrossRef]
- Alcantara, L.C.J.; Nogueira, E.; Shuab, G.; Tosta, S.; Fristch, H.; Pimentel, V.; Souza-Neto, J.A.; Coutinho, L.L.; Fukumasu, H.; Sampaio, S.C.; et al. SARS-CoV-2 epidemic in Brazil: How the displacement of variants has driven distinct epidemic waves. Virus Res. 2022, 315, 198785. [Google Scholar] [CrossRef]
- Thakur, S.; Sasi, S.; Pillai, S.G.; Nag, A.; Shukla, D.; Singhal, R.; Phalke, S.; Velu, G.S.K. SARS-CoV-2 Mutations and Their Impact on Diagnostics, Therapeutics and Vaccines. Front. Med. 2022, 9, 815389. [Google Scholar] [CrossRef]
- Sanches, P.R.S.; Charlie-Silva, I.; Braz, H.L.B.; Bittar, C.; Freitas Calmon, M.; Rahal, P.; Cilli, E.M. Recent advances in SARS-CoV-2 Spike protein and RBD mutations comparison between new variants Alpha (B.1.1.7, United Kingdom), Beta (B.1.351, South Africa), Gamma (P.1, Brazil) and Delta (B.1.617.2, India). J. Virus Erad. 2021, 7, 100054. [Google Scholar] [CrossRef]



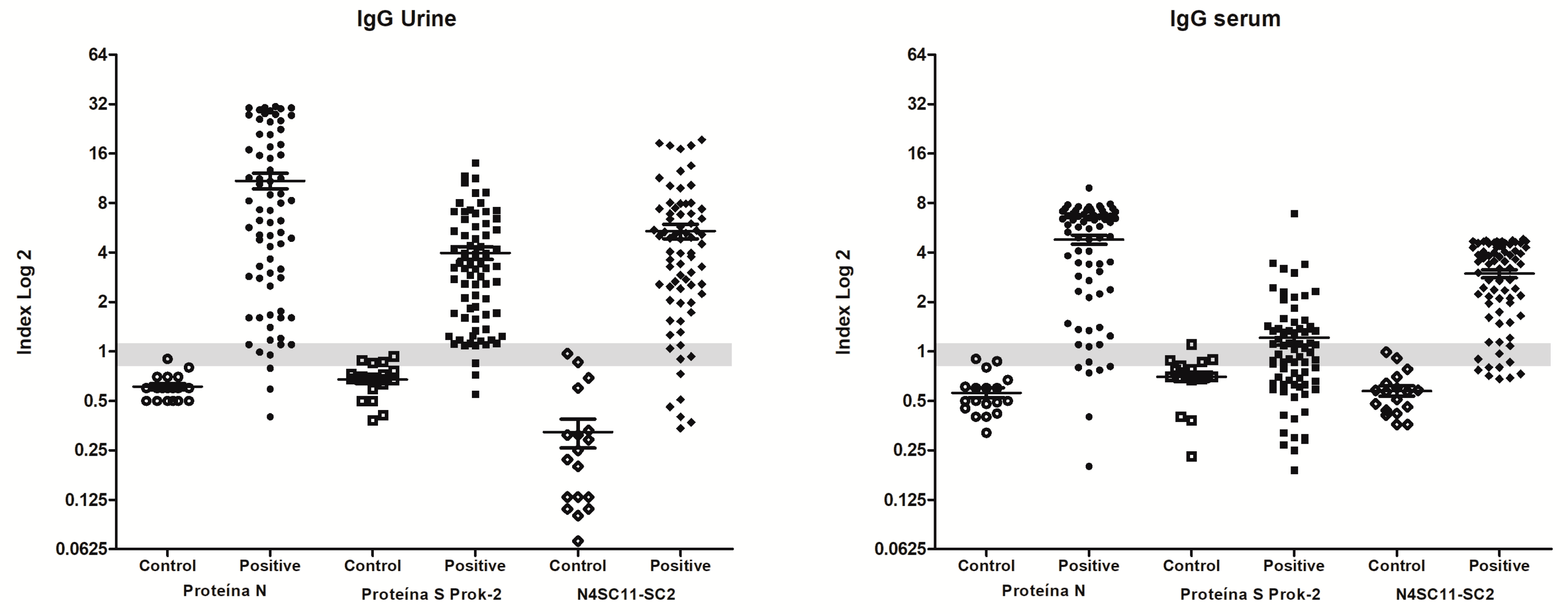
| Sample | AUC | p-Value | Cut-Off (Abs) | Se | 95%CI | Sp | 95%CI | J | PPV | NPV |
|---|---|---|---|---|---|---|---|---|---|---|
| URINE | 0.9841 | <0.0001 | >0.1900 | 91.11 | 84.99% to 95.32% | 100.0 | 83.89% to 100.0% | 0.9111 | 1.000 | 0.679 |
| SERUM | 0.9722 | <0.0001 | >0.7525 | 83.70 | 76.37% to 89.50% | 100.0 | 83.16% to 100.0% | 0.7525 | 1.000 | 0.607 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, F.F.; Pereira, I.A.G.; Cardoso, M.M.; Bandeira, R.S.; Lage, D.P.; Scussel, R.; Anastacio, R.S.; Freire, V.G.; Melo, M.F.N.; Oliveira-da-Silva, J.A.; et al. B-Cell Epitopes-Based Chimeric Protein from SARS-CoV-2 N and S Proteins Is Recognized by Specific Antibodies in Serum and Urine Samples from Patients. Viruses 2023, 15, 1877. https://doi.org/10.3390/v15091877
Ramos FF, Pereira IAG, Cardoso MM, Bandeira RS, Lage DP, Scussel R, Anastacio RS, Freire VG, Melo MFN, Oliveira-da-Silva JA, et al. B-Cell Epitopes-Based Chimeric Protein from SARS-CoV-2 N and S Proteins Is Recognized by Specific Antibodies in Serum and Urine Samples from Patients. Viruses. 2023; 15(9):1877. https://doi.org/10.3390/v15091877
Chicago/Turabian StyleRamos, Fernanda F., Isabela A. G. Pereira, Mariana M. Cardoso, Raquel S. Bandeira, Daniela P. Lage, Rahisa Scussel, Rafaela S. Anastacio, Victor G. Freire, Marina F. N. Melo, Joao A. Oliveira-da-Silva, and et al. 2023. "B-Cell Epitopes-Based Chimeric Protein from SARS-CoV-2 N and S Proteins Is Recognized by Specific Antibodies in Serum and Urine Samples from Patients" Viruses 15, no. 9: 1877. https://doi.org/10.3390/v15091877
APA StyleRamos, F. F., Pereira, I. A. G., Cardoso, M. M., Bandeira, R. S., Lage, D. P., Scussel, R., Anastacio, R. S., Freire, V. G., Melo, M. F. N., Oliveira-da-Silva, J. A., Martins, V. T., Tavares, G. S. V., Vale, D. L., Freitas, C. S., Chaves, A. T., Caporali, J. F. M., Vassallo, P. F., Ravetti, C. G., Nobre, V., ... Ludolf, F. (2023). B-Cell Epitopes-Based Chimeric Protein from SARS-CoV-2 N and S Proteins Is Recognized by Specific Antibodies in Serum and Urine Samples from Patients. Viruses, 15(9), 1877. https://doi.org/10.3390/v15091877







