Predicting Factors of Plasma HIV RNA Undetectability after Switching to Co-Formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Experienced HIV-1 Patients: A Multicenter Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Variables and Assessment Methods
2.3. Statistical Analysis
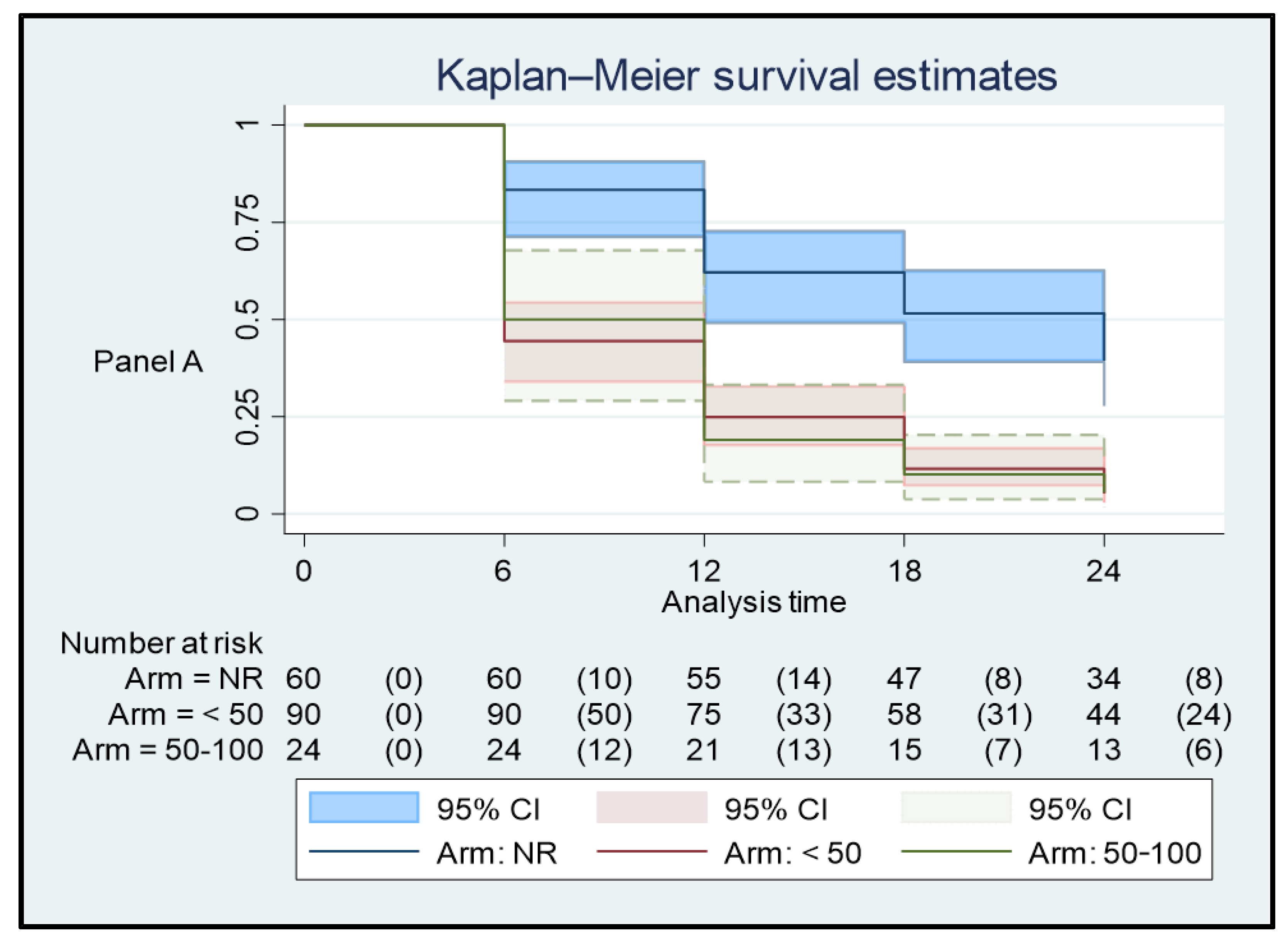
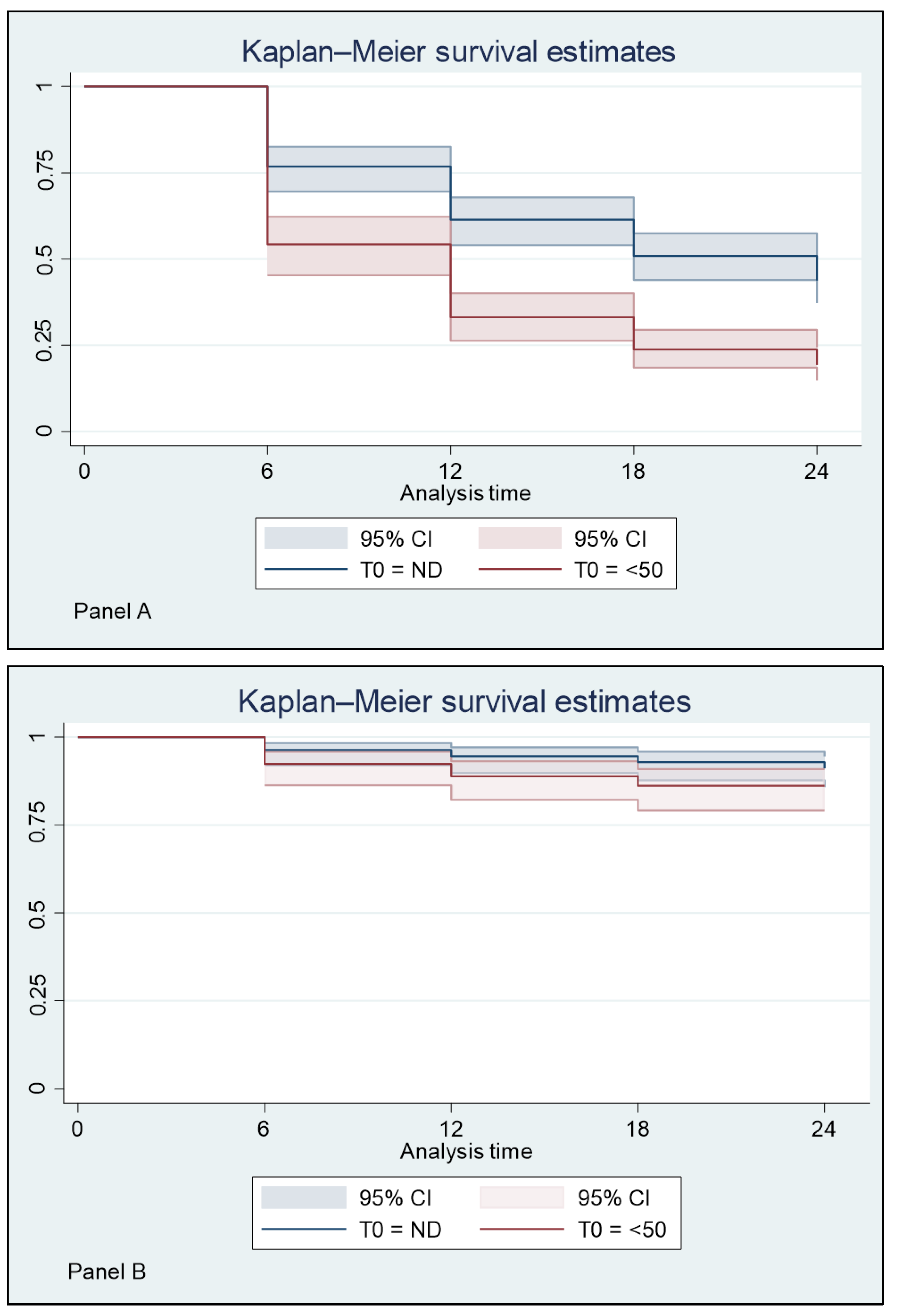
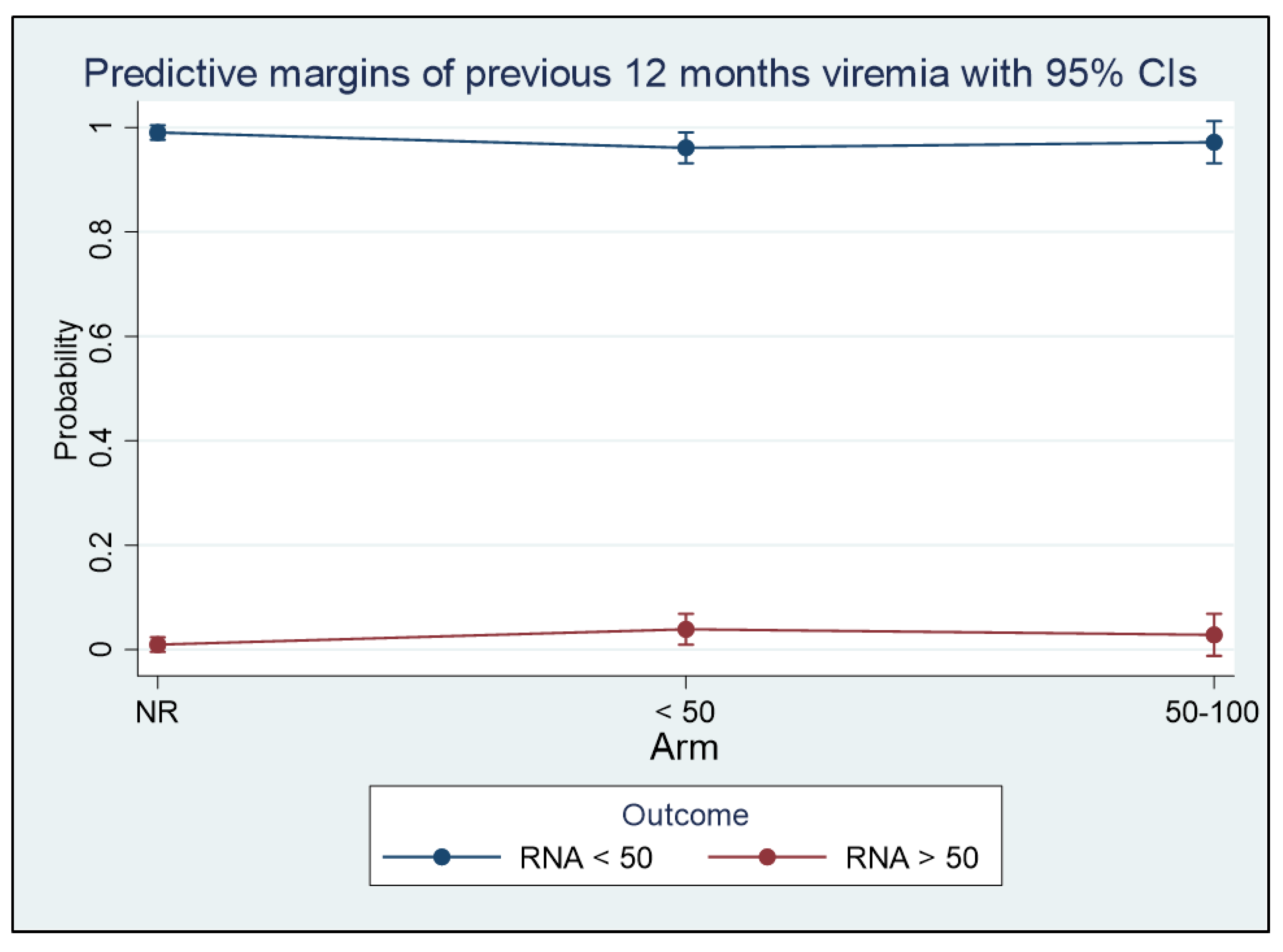
3. Results
3.1. Plasma HIV RNA Control in the 12 Months before the Switch, and Virological Response to BIC/FTC/TAF
3.2. Plasma HIV RNA Control in the 12 Months before the Switch and Virological Response to BIC/FTC/TAF
3.3. Influence of ART before the Switch to BIC/FTC/TAF
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deeks, S.G.; Lewin, S.R.; Havlir, D.V. The end of AIDS: HIV infection as a chronic disease. Lancet 2013, 382, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, F.; May, M.; Phillips, A. Life expectancy living with HIV: Recent estimates and future implications. Curr. Opin. Infect. Dis. 2013, 26, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Baldin, G.; Ciccullo, A.; Lombardi, F.; D’Angelillo, A.; Dusina, A.; Emiliozzi, A.; Farinacci, D.; Moschese, D.; Picarelli, C.; Borghetti, A.; et al. Short Communication: Comparing Lamivudine+Dolutegravir and Bictegravir/Emtricitabine/Tenofovir Alafenamide as Switch Strategies: Preliminary Results from Clinical Practice. AIDS Res. Hum. Retroviruses 2021, 37, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Ward, D.; Brar, I.; Mills, A.; Stellbrink, H.J.; López-Cortés, L.; Ruane, P.; Podzamczer, D.; Brinson, C.; Custodio, J.; et al. Switching to fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide from dolutegravir plus abacavir and lamivudine in virologically suppressed adults with HIV-1: 48 week results of a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet HIV 2018, 5, e357–e365. [Google Scholar]
- Sax, P.E.; Rockstroh, J.K.; Luetkemeyer, A.F.; Yazdanpanah, Y.; Ward, D.; Trottier, B.; Rieger, A.; Liu, H.; Acosta, R.; Collins, S.E.; et al. Switching to Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Virologically Suppressed Adults with Human Immunodeficiency Virus. Clin. Infect. Dis. 2021, 73, e485–e493. [Google Scholar] [CrossRef]
- Pham, H.T.; Mesplède, T. Bictegravir in a fixed-dose tablet with emtricitabine and tenofovir alafenamide for the treatment of HIV infection: Pharmacology and clinical implications. Expert Opin. Pharmacother. 2019, 20, 385–397. [Google Scholar] [CrossRef]
- Chang, H.M.; Chou, P.Y.; Chou, C.H.; Tsai, H.C. Outcomes After Switching to BIC/FTC/TAF in Patients with Virological Failure to Protease Inhibitors or Non-Nucleoside Reverse Transcriptase Inhibitors: A Real-World Cohort Study. Infect. Drug Resist. 2021, 14, 4877–4886. [Google Scholar] [CrossRef]
- Rolle, C.P.; Nguyen, V.; Patel, K.; Cruz, D.; DeJesus, E.; Hinestrosa, F. Real-world efficacy and safety of switching to bictegravir/emtricitabine/tenofovir alafenamide in older people living with HIV. Medicine 2021, 100, e27330. [Google Scholar] [CrossRef]
- Armenia, D.; Forbici, F.; Bertoli, A.; Berno, G.; Malagnino, V.; Gagliardini, R.; Borghi, V.; Gennari, W.; Cicalini, S.; Buonomini, A.; et al. Bictegravir/emtricitabine/tenofovir alafenamide ensures high rates of virological suppression maintenance despite previous resistance in PLWH who optimize treatment in clinical practice. J. Glob. Antimicrob. Resist. 2022, 30, 326–334. [Google Scholar] [CrossRef]
- Chen, L.Y.; Sun, H.Y.; Chuang, Y.C.; Huang, Y.S.; Liu, W.D.; Lin, K.Y.; Chang, H.Y.; Luo, Y.Z.; Wu, P.Y.; Su, Y.C.; et al. Patient-reported outcomes among virally suppressed people living with HIV after switching to Co-formulated bictegravir, emtricitabine and tenofovir alafenamide. J. Microbiol. Immunol. Infect. 2023, 56, 575–585. [Google Scholar] [CrossRef]
- Hayes, E.; Derrick, C.; Smalls, D.; Smith, H.; Kremer, N.; Weissman, S. Short-term Adverse Events With BIC/FTC/TAF: Postmarketing Study. Open Forum Infect. Dis. 2020, 7, ofaa285. [Google Scholar] [CrossRef]
- European AIDS Clinical Society Guidelines Version 11.1, October 2022. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 16 May 2023).
- Gandhi, R.T.; Bedimo, R.; Hoy, J.F.; Landovitz, R.J.; Smith, D.M.; Eaton, E.F.; Lehmann, C.; Springer, S.A.; Sax, P.E.; Thompson, M.A.; et al. Antiretroviral Drugs for Treatment and Prevention of HIV Infection in Adults: 2022 Recommendations of the International Antiviral Society-USA Panel. JAMA 2023, 329, 63–84. [Google Scholar] [CrossRef]
- Ryscavage, P.; Kelly, S.; Li, J.Z.; Harrigan, P.R.; Taiwo, B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob. Agents Chemother. 2014, 58, 3585–3598. [Google Scholar] [CrossRef] [PubMed]
- Sarmati, L.; D’Ettorre, G.; Parisi, S.G.; Andreoni, M. HIV Replication at Low Copy Number and its Correlation with the HIV Reservoir: A Clinical Perspective. Curr. HIV Res. 2015, 13, 250–257. [Google Scholar] [CrossRef]
- Malagnino, V.; Teti, E.; Compagno, M.; Coppola, L.; Salpini, R.; Svicher, V.; Basso, M.; Battagin, G.; Panese, S.; Rossi, M.C.; et al. HBcAb Positivity Is a Risk Factor for an Increased Detectability of HIV RNA after Switching to a Two-Drug Regimen Lamivudine-Based (2DR-3TC-Based) Treatment: Analysis of a Multicenter Italian Cohort. Microorganisms 2021, 9, 396. [Google Scholar] [CrossRef]
- Parisi, S.G.; Sarmati, L.; Andreis, S.; Scaggiante, R.; Cruciani, M.; Ferretto, R.; Manfrin, V.; Basso, M.; Andreoni, M.; Mengoli, C.; et al. Strong and persistent correlation between baseline and follow-up HIV-DNA levels and residual viremia in a population of naïve patients with more than 4 years of effective antiretroviral therapy. Clin. Microbiol. Infect. 2015, 21, 288.e5-7. [Google Scholar] [CrossRef] [PubMed]
- Parisi, S.G.; Andreis, S.; Mengoli, C.; Scaggiante, R.; Cruciani, M.; Ferretto, R.; Manfrin, V.; Panese, S.; Basso, M.; Boldrin, C.; et al. A stable CC-chemokine receptor (CCR)-5 tropic virus is correlated with the persistence of HIV RNA at less than 2.5 copies in successfully treated naïve subjects. BMC Infect. Dis. 2013, 13, 314. [Google Scholar] [CrossRef] [PubMed]
- European AIDS Clinical Society Guidelines Version 9.1, October 2018. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 16 May 2023).
- European AIDS Clinical Society Guidelines Version 10.0, November 2019. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 16 May 2023).
- European AIDS Clinical Society Guidelines Version 10.1, October 2020. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 16 May 2023).
- European AIDS Clinical Society Guidelines Version 11, October 2021. Available online: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed on 16 May 2023).
- Parisi, S.G.; Basso, M.; Scaggiante, R.; Andreis, S.; Mengoli, C.; Cruciani, M.; Del Vecchio, C.; Menegotto, N.; Zago, D.; Sarmati, L.; et al. Oral and anal high-risk human papilloma virus infection in HIV-positive men who have sex with men over a 24-month longitudinal study: Complexity and vaccine implications. BMC Public Health 2019, 19, 645. [Google Scholar] [CrossRef]
- Parisi, S.G.; Andreis, S.; Mengoli, C.; Menegotto, N.; Cavinato, S.; Scaggiante, R.; Andreoni, M.; Palù, G.; Basso, M.; Cattelan, A.M. Soluble CD163 and soluble CD14 plasma levels but not cellular HIV-DNA decrease during successful interferon-free anti-HCV therapy in HIV-1-HCV co-infected patients on effective combined anti-HIV treatment. Med. Microbiol. Immunol. 2018, 207, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Micán, R.; de Gea Grela, A.; Cadiñanos, J.; de Miguel, R.; Busca, C.; Bernardino, J.I.; Valencia, E.; Montes, M.L.; Montejano, R.; Moreno, V.; et al. Impact of preexisting nucleos(t)ide reverse transcriptase inhibitor resistance on the effectiveness of bictegravir/emtricitabine/tenofovir alafenamide in treatment experience patients. AIDS 2022, 36, 1941–1947. [Google Scholar] [CrossRef]
- Parisi, S.G.; Andreis, S.; Scaggiante, R.; Cruciani, M.; Ferretto, R.; Manfrin, V.; Panese, S.; Rossi, M.C.; Francavilla, E.; Boldrin, C.; et al. Decreasing trends of drug resistance and increase of non-B subtypes amongst subjects recently diagnosed as HIV-infected over the period 2004-2012 in the Veneto Region, Italy. J. Glob. Antimicrob. Resist. 2013, 1, 201–206. [Google Scholar] [CrossRef]
- Alejos, B.; Díez, C.; Galindo, M.J.; López, J.C.; Moreno-García, E.; Estrada, V.; Poveda, E.; Omar, M.; Jarrín, I.; Berenguer, J. For CoRIS. Progress in the quality of care for newly diagnosed people with HIV in Spain (2004–2019). Antivir. Ther. 2022, 27, 13596535221112729. [Google Scholar] [CrossRef] [PubMed]
- Andreis, S.; Basso, M.; Scaggiante, R.; Cruciani, M.; Ferretto, R.; Manfrin, V.; Panese, S.; Rossi, M.C.; Francavilla, E.; Boldrin, C.; et al. Drug resistance in B and non-B subtypes amongst subjects recently diagnosed as primary/recent or chronic HIV-infected over the period 2013-2016: Impact on susceptibility to first-line strategies including integrase strand-transfer inhibitors. J. Glob. Antimicrob. Resist. 2017, 10, 106–112. [Google Scholar] [CrossRef]
- Rodger, A.J.; McCabe, L.; Phillips, A.N.; Lampe, F.C.; Burns, F.; Ward, D.; Delpech, V.; Weatherburn, P.; Witzel, T.C.; Pebody, R.; et al. Free HIV self-test for identification and linkage to care of previously undetected HIV infection in men who have sex with men in England and Wales (SELPHI): An open-label, internet-based, randomised controlled trial. Lancet HIV 2022, 9, e838–e847. [Google Scholar] [CrossRef] [PubMed]
- Svicher, V.; Marchetti, G.; Ammassari, A.; Ceccherini-Silberstein, F.; Sarmati, L. Impact Study Group. Novelties in Evaluation and Monitoring of Human Immunodeficiency Virus-1 Infection: Is Standard Virological Suppression Enough for Measuring Antiretroviral Treatment Success? AIDS Rev. 2017, 19, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Falasca, F.; Di Carlo, D.; De Vito, C.; Bon, I.; d’Ettorre, G.; Fantauzzi, A.; Mezzaroma, I.; Fimiani, C.; Re, M.C.; Vullo, V.; et al. Evaluation of HIV-DNA and inflammatory markers in HIV-infected individuals with different viral load patterns. BMC Infect. Dis. 2017, 17, 581. [Google Scholar] [CrossRef]
- Baroncelli, S.; Pirillo, M.F.; Galluzzo, C.M.; Antoni, A.D.; Ladisa, N.; Francisci, D.; d’Ettorre, G.; Segala, D.; Vivarelli, A.; Sozio, F.; et al. Rate and determinants of residual viremia in multidrug-experienced patients successfully treated with raltegravir-based regimens. AIDS Res. Hum. Retroviruses 2015, 31, 71–77. [Google Scholar] [CrossRef]
- Sarmati, L.; Parisi, S.G.; Montano, M.; Andreis, S.; Scaggiante, R.; Galgani, A.; Viscione, M.; Maffongelli, G.; Ricciardi, A.; Andreoni, C.; et al. Nevirapine use, prolonged antiretroviral therapy and high CD4 nadir values are strongly correlated with undetectable HIV-DNA and -RNA levels and CD4 cell gain. J. Antimicrob. Chemother. 2012, 67, 2932–2938. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parisi, S.G.; Andreis, S.; Mengoli, C.; Scaggiante, R.; Ferretto, R.; Manfrin, V.; Cruciani, M.; Giobbia, M.; Boldrin, C.; Basso, M.; et al. Baseline cellular HIV DNA load predicts HIV DNA decline and residual HIV plasma levels during effective antiretroviral therapy. J. Clin. Microbiol. 2012, 50, 258–263. [Google Scholar] [CrossRef]
- Maggiolo, F.; Valenti, D.; Teocchi, R.; Comi, L.; Di Filippo, E.; Rizzi, M. Real World Data on Forgiveness to Uncomplete Adherence to Bictegravir/ Emtricitabine/Tenofovir Alafenamide. J. Int. Assoc. Provid. AIDS Care 2022, 21, 23259582221140208. [Google Scholar] [CrossRef]
- Gelé, T.; Gouget, H.; Furlan, V.; Becker, P.H.; Taburet, A.M.; Lambotte, O.; Barrail-Tran, A. Characteristics of Dolutegravir and Bictegravir Plasma Protein Binding: A First Approach for the Study of Pharmacologic Sanctuaries. Antimicrob. Agents Chemother. 2020, 64, e00895-20. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.; Durant, J.; Fabre, R.; Lotte, L.; Sindt, A.; Puchois, A.; De Monte, A.; Cezar, R.; Corbeau, P.; Pradier, C. Inflammatory Markers after Switching to a Dual Drug Regimen in HIV-Infected Subjects: A Two-Year Follow-Up. Viruses 2022, 14, 927. [Google Scholar] [CrossRef] [PubMed]
- Younas, M.; Psomas, C.; Reynes, C.; Cezar, R.; Kundura, L.; Portalès, P.; Merle, C.; Atoui, N.; Fernandez, C.; Le Moing, V.; et al. Residual Viremia Is Linked to a Specific Immune Activation Profile in HIV-1-Infected Adults Under Efficient Antiretroviral Therapy. Front. Immunol. 2021, 12, 663843. [Google Scholar] [CrossRef] [PubMed]
- Hatano, H.; Jain, V.; Hunt, P.W.; Lee, T.H.; Sinclair, E.; Do, T.D.; Hoh, R.; Martin, J.N.; McCune, J.M.; Hecht, F.; et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J. Infect. Dis. 2013, 208, 50–56. [Google Scholar] [CrossRef]
- Malagnino, V.; Salpini, R.; Teti, E.; Compagno, M.; Ferrari, L.; Mulas, T.; Svicher, V.; Zordan, M.; Basso, M.; Battagin, G.; et al. Role of HBcAb Positivity in Increase of HIV-RNA Detectability after Switching to a Two-Drug Regimen Lamivudine-Based (2DR-3TC-Based) Treatment: Months 48 Results of a Multicenter Italian Cohort. Viruses 2023, 15, 193. [Google Scholar] [CrossRef]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Allain, J.P.; Berg, T.; Bertoletti, A.; Brunetto, M.R.; et al. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, H.Y.; Chang, S.Y.; Chuang, Y.C.; Su, Y.C.; Liu, W.C.; Hung, C.C. Virological response to tenofovir-alafenamide-containing antiretroviral therapy in people living with HIV co-infected with lamivudine-resistant or lamivudine-susceptible hepatitis B virus. Int. J. Antimicrob. Agents 2022, 60, 106682. [Google Scholar] [CrossRef]
- Zerbato, J.M.; Avihingsanon, A.; Singh, K.P.; Zhao, W.; Deleage, C.; Rosen, E.; Cottrell, M.L.; Rhodes, A.; Dantanarayana, A.; Tumpach, C.; et al. HIV DNA persists in hepatocytes in people with HIV-hepatitis B co-infection on antiretroviral therapy. EBioMedicine 2023, 87, 104391. [Google Scholar] [CrossRef]
- Holtkamp, C.; Fiedler, M.; Dittmer, U.; Anastasiou, O.E. The Course of Anti-HBc Antibodies over Time in Immunocompromised Hosts. Vaccines 2022, 10, 137. [Google Scholar] [CrossRef]
- Yuan, D.; Li, M.; Zhou, Y.; Shi, L.; Lu, J.; Fu, G.; Wang, B. Influencing factors and adverse outcomes of virologic rebound states in anti-retroviral-treated individuals with HIV infection. J. Virus Erad. 2023, 9, 100320. [Google Scholar] [CrossRef]
- Lombardi, F.; Bruzzesi, E.; Bouba, Y.R.; Di Carlo, D.; Costabile, V.; Ranzenigo, M.; Maggiolo, F.; Castagna, A.; Callegaro, A.P.; Zoncada, A.; et al. Factors Associated with Low-Level Viremia in People Living with HIV in the Italian Antiviral Response Cohort Analysis Cohort: A Case-Control Study. AIDS Res. Hum. Retroviruses, 2023; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hanners, E.K.; Benitez-Burke, J.; Badowski, M.E. HIV: How to manage low-level viraemia in people living with HIV. Drugs Context 2022, 11, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Elvstam, O.; Malmborn, K.; Elén, S.; Marrone, G.; García, F.; Zazzi, M.; Sönnerborg, A.; Böhm, M.; Seguin-Devaux, C.; Björkman, P. Virologic Failure Following Low-level Viremia and Viral Blips During Antiretroviral Therapy: Results from a European Multicenter Cohort. Clin. Infect. Dis. 2023, 76, 25–31. [Google Scholar] [CrossRef]
- Fleming, J.; Mathews, W.C.; Rutstein, R.M.; Aberg, J.; Somboonwit, C.; Cheever, L.W.; Berry, S.A.; Gebo, K.A.; Moore, R.D. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS 2019, 33, 2005–2012. [Google Scholar] [CrossRef]
- Bai, R.; Lv, S.; Hua, W.; Su, B.; Wang, S.; Shao, Y.; Li, Z.; Liu, A.; Sun, L.; Dai, L. Factors associated with human immunodeficiency virus-1 low-level viremia and its impact on virological and immunological outcomes: A retrospective cohort study in Beijing, China. HIV Med. 2022, 23 (Suppl. S1), 72–83. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Ding, H.; An, M.; Wang, X.; Tian, W.; Zhao, B.; Han, X. Factors associated with high-risk low-level viremia leading to virologic failure: 16-year retrospective study of a Chinese antiretroviral therapy cohort. BMC Infect. Dis. 2020, 20, 147. [Google Scholar] [CrossRef]
- Chen, G.J.; Sun, H.Y.; Chen, L.Y.; Hsieh, S.M.; Sheng, W.H.; Liu, W.D.; Chuang, Y.C.; Huang, Y.S.; Lin, K.Y.; Wu, P.Y.; et al. Low-level viraemia and virologic failure among people living with HIV who received maintenance therapy with co-formulated bictegravir, emtricitabine and tenofovir alafenamide versus dolutegravir-based regimens. Int. J. Antimicrob. Agents 2022, 60, 106631. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, S.; Hofstra, L.M.; Mudrikova, T.; Wensing, A.M.J.; Oomen, P.G.A.; Hoepelman, A.I.M.; van Welzen, B.J. Lower Incidence of HIV-1 Blips Observed During Integrase Inhibitor-Based Combination Antiretroviral Therapy. J. Acquir. Immune Defic. Syndr. 2022, 89, 575–582. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | n (%) |
|---|---|
| Male, n (%) | 140 (71.1) |
| Caucasian origin, n (%) | 162 (82.2) |
| HBsAg negative and HBcAb positive *, n (%) | 58 (33) |
| Positive HCV serology, n (%) | 29 (14.7) |
| Age at HIV diagnosis (years), median (IQR) | 34 (26–43) |
| Age at switching (years), median (IQR) | 53 (41–59) |
| Absolute number of CD4+ T cell count at nadir (cells/mm3), median (IQR) | 235 (73–346) |
| CD4+ T cell count (cells/mm3) at switch, median (IQR) | 677 (490–880) |
| Pre-switch therapy: 2 NRTI+INSTI | 100 (50.8) |
| Pre-switch therapy: 2 NRTI+NNRTI | 37 (18.7) |
| Pre-switch therapy: 2 NRTI+PI | 30 (15.2) |
| Pre-switch therapy: other regimen including INSTI | 22 (11.2) |
| Pre-switch therapy: other regimen not including INSTI | 8 (4.1) |
| Virologic Control at T0 | Always Undetectable HIV RNA in the 12 Months before Switch (n = 67) | At Least One Test < 50 Copies/mL in the 12 Months before Switch (n = 99) | At Least One Test ≥ 50 Copies/mL in the 12 Months before Switch (n = 31) |
|---|---|---|---|
| Undetectable HIV RNA at T0, n = 108 | 49 (45.4) | 42 (38.9) | 17 (15.7) |
| Detectable HIV RNA < 50 copies/mL at T0, n = 89 | 18 (20.2) | 57 (64) | 14 (15.8) |
| T6 (158 Patients) | |||||
|---|---|---|---|---|---|
| Plasma HIV RNA Control at T0 | ND | <50 Copies/mL | 50–100 Copies/mL | 101–1000 Copies/mL | >1000 Copies/mL |
| HIV RNA NR at T0 (n = 87) | 61 (70.1) | 21 (24.1) | 2 (2.3) | 3 (3.5) | 0 |
| HIV RNA < 50 copies/mL at T0 (n = 71) | 25 (35.2) | 39 (54.9) | 5 (7.1) | 2 (2.8) | 0 |
| T12 (143 patients) | |||||
| Plasma HIV RNA control at T0 | ND | <50 copies/mL | 50–100 copies/mL | 101–1000 copies/mL | >1000 copies/mL |
| HIV RNA NR at T0 (n = 78) | 53 (68) | 23 (29.5) | 2 (2.5) | 0 | 0 |
| HIV RNA < 50 copies/mL at T0 (n = 65) | 30 (46.2) | 31 (47.7) | 2 (3.1) | 1 (1.5) | 1 (1.5) |
| T18 (115 patients) | |||||
| Plasma HIV RNA control at T0 | ND | <50 copies/mL | 50–100 copies/mL | 101–1000 copies/mL | >1000 copies/mL |
| HIV RNA NR at T0 (n = 64) | 44 (68.8) | 17 (26.6) | 2 (3.1) | 0 | 1(1.6) |
| HIV RNA < 50 copies/mL at T0 (n = 51) | 25 (49) | 24 (47) | 1(2) | 0 | 1(2) |
| T 24 (91 patients) | |||||
| Plasma HIV RNA control at T0 | ND | <50 copies/mL | 50–100 copies/mL | 101–1000 copies/mL | >1000 copies/mL |
| HIV RNA NR at T0 (n = 54) | 34 (63) | 17 (31.5) | 2 (3.7) | 1 (1.8) | 0 |
| HIV RNA < 50 copies/mL at T0 (n = 37) | 19 (51.4) | 18 (48.6) | 0 | 0 | 0 |
| T6 (158 Patients) | |||||
|---|---|---|---|---|---|
| Plasma HIV RNA Control in the 12 Months before Switch | ND | <50 Copies/mL | 50–100 Copies/mL | 101–1000 Copies/mL | >1000 Copies/mL |
| HIV RNA ND (n = 56) | 46 (82.1) | 9 (16.1) | 0 | 1 (1.8) | 0 |
| HIV RNA < 50 copies/mL (n = 81) | 31 (38.3) | 42 (51.9) | 4 (4.9) | 4 (4.9) | 0 |
| HIV RNA ≥ 50 copies/mL (n = 21) | 9 (42.9) | 9 (42.9) | 3 (14.2) | 0 | 0 |
| T12 (143 patients) | |||||
| Plasma HIV RNA control in the 12 months before switch | ND | <50 copies/mL | 50–100 copies/mL | 101–1000 copies/mL | >1000 copies/mL |
| HIV RNA ND (n = 52) | 38 (73.1) | 14 (26.9) | 0 | 0 | 0 |
| HIV RNA < 50 copies/mL (n = 71) | 38 (53.5) | 28 (39.4) | 4 (5.6) | 1 (1.4) | 0 |
| HIV RNA ≥ 50 copies/mL (n = 20) | 7 (35) | 12 (60) | 0 | 0 | 1 (5) |
| T18 (115 patients) | |||||
| Plasma HIV RNA control in the 12 months before switch | ND | <50 copies/mL | 50–100 copies/mL | 101–1000 copies/mL | >1000 copies/mL |
| HIV RNA ND (n = 45) | 37 (82.2) | 6 (13.3) | 1 (2.2) | 0 | 1 (2.2) |
| HIV RNA < 50 copies/mL (n = 56) | 25 (44.6) | 30 (53.6) | 0 | 0 | 1 (1.8) |
| HIV RNA ≥ 50 copies/mL (n = 14) | 7 (50) | 5 (25.7) | 2 (14.3) | 0 | 0 |
| T24 (91 patients) | |||||
| Plasma HIV RNA control in the 12 months before switch | ND | <50 copies/mL | 50–100 copies/mL | 101–1000 copies/mL | >1000 copies/mL |
| HIV RNA ND (n = 34) | 26 (76.5) | 8 (23.5) | 0 | 0 | 0 |
| HIV RNA < 50 copies/mL (n = 44) | 20 (45.5) | 21 (47.7) | 2 (4.5) | 1 (2.3) | 0 |
| HIV RNA ≥ 50 copies/mL (n = 13) | 7 (53.8) | 6 (46.2) | 0 | 0 | 0 |
| PLWH | Caucasian | Last Follow-Up Point Available | Reason |
|---|---|---|---|
| M, 66 years | Yes | T6: HIV RNA ND | Stopped BIC/FTC/TAF (reason not reported), restarted previous treatment (RPV/TAF/FTC) |
| M, 55 years | Yes | T18: HIV RNA < 50 copies/mL | Stopped BIC/FTC/TAF due to side effects, started DTG/3TC |
| M, 52 years | No | T12: HIV RNA ND | Stopped BIC/FTC/TAF due to side effects, started DTG/3TC |
| M, 44 years | Yes | T6: HIV-RNA 50–100 copies/mL | Stopped BIC/FTC/TAF due to side effects, started DOR, 3TC, TDF |
| M, 49 years | Yes | T6: HIV RNA 101–1000 copies/mL | Stopped BIC/FTC/TAF due to therapeutic failure, started DOR, 3TC, TDF |
| F, 58 years | Yes | T6: HIV RNA ND | Stopped BIC/FTC/TAF due to side effect (weight increase), restarted TAF/FTC/RAL |
| PLWH | Caucasian | First Detection of HIV-RNA > 101 Copies/mL | Reason | Follow-Up |
|---|---|---|---|---|
| M, 63 years | Yes | T6: 101–1000 copies/mL | Not adherent to therapy | No further data available |
| F, 33 years | Yes | T6: 101–1000 copies/mL | Blip a | T12: HIV RNA < 50 copies/mL; T18: HIV RNA ND; T24:HIV RNA ND |
| M, 63 years | Yes | T6: 101–1000 copies/mL | Blip a | T12 due on May 2023 |
| M, 49 years | Yes | T6: 101–1000 copies/mL | Therapeutic failure | Started DOR, 3TC, TDF |
| F, 73 years | Yes | T6: 101–1000 copies/mL | Possible low adherence | T12: HIV RNA 101–1000 copies/mL, T18:HIV RNA < 50 copies/mL, T24 due on April 2023 |
| F, 44 years | Yes | T12: >1000 copies/mL | Treatment stopped due to patient’s decision | Not available |
| M, 55 years | Yes | T18: >1000 copies/mL | Treatment stopped due to patient’s decision and then restarted | T24: HIV RNA < 50 copies/mL |
| M, 27 years | Yes | T18: >1000 copies/mL | Treatment stopped due to patient’s decision and then restarted | T24: HIV RNA ND |
| M, 35 years | Yes | T24: 101–1000 copies/mL | Blip a | Not applicable |
| (Panel A) | |||
| Plasma HIV RNA in the 12 Months before the Switch | Time at Risk | Rate | Patients |
| All tests with undetectable plasma HIV RNA | 1.260 | 0.0301587 | 67 |
| At least one test with plasma HIV RNA < 50 copies/mL | 1.722 | 0.0720093 | 99 |
| At least one test with plasma HIV RNA ≥ 50 copies/mL | 486 | 0.0720165 | 31 |
| Total | 3.468 | 0.0568051 | 197 |
| (Panel B) | |||
| Plasma HIV RNA in the 12 Months before the Switch | Time at risk | Rate | Patients |
| All tests with undetectable plasma HIV RNA | 1.260 | 0.0015873 | 67 |
| At least one test with plasma HIV RNA < 50 copies/mL | 1.722 | 0.0087108 | 99 |
| At least one test with plasma HIV RNA ≥ 50 copies/mL | 486 | 0.0102881 | 31 |
| Total | 3.468 | 0.0063437 | 197 |
| (Panel A) | |||
| Plasma HIV RNA at T0 | Time at Risk | Rate | Patients |
| Plasma HIV RNA undetectable | 2592 | 0.35 | 108 |
| Plasma HIV RNA < 50 copies/mL | 2136 | 0.59 | 89 |
| Total | 4728 | 0.46 | 197 |
| (Panel B) | |||
| Plasma HIV RNA at T0 | Time at risk | Rate | Patients |
| Plasma HIV RNA undetectable | 2592 | <0.01 | 108 |
| Plasma HIV RNA < 50 copies/mL | 2136 | <0.01 | 89 |
| Total | 4728 | <0.01 | 197 |
| Predictor Variables | Coefficient | 95% CI | p-Value |
|---|---|---|---|
| Viremia in previous 12 months (ref.: always ND) | - | - | - |
| <50 copies/mL | 123.25 | 67.29–179.20 | <0.001 |
| 50–100 copies/mL | 101.46 | 12.96–189.96 | 0.025 |
| Nadir cell count | 1.06 | 0.92–1.20 | <0.001 |
| HCV status (ref.: negative) | - | - | - |
| positive | −62.90 | −154.85–29.04 | 0.18 |
| HBV status (ref.: negative) | - | - | - |
| Viremia in Previous 12 Months | T0 | T6 | T12 | T18 | T24 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Δ | Mean | SE | Δ | Mean | SE | Δ | Mean | SE | Δ | Mean | SE | Δ | Δ (T24-T0) | |
| HIV RNA ND | 577 | 54 | - | 657 | 63 | 80 | 672 | 75 | 14 | 719 | 85 | 46 | 705 | 75 | −14 | 127 |
| At least one plasma HIV RNA < 50 copies/mL | 764 | 90 | - | 804 | 85 | 40 | 778 | 83 | −26 | 809 | 90 | 30 | 826 | 95 | 17 | 62 |
| At least one plasma HIV RNA ≥ 50 copies/mL | 822 | 103 | - | 792 | 86 | −30 | 843 | 121 | 51 | 867 | 120 | 24 | 828 | 101 | −39 | 5 |
| Viremia in Previous 12 Months | Time at Risk | Incidence Rate | N |
|---|---|---|---|
| ND | 1260 | 0.030 | 67 |
| <50 copies/mL | 1722 | 0.072 | 99 |
| ≥50 copies/mL | 486 | 0.072 | 31 |
| Control variables | Rate Ratio | 95% CI | p-value |
| HBV positive | 0.9 | 0.6–1.2 | 0.254 |
| HCV positive | 1.2 | 0.8–1.8 | 0.525 |
| Male gender | 1.2 | 0.9–1.6 | 0.568 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso, M.; Battagin, G.; Nicolè, S.; Rossi, M.C.; Colombo, F.; Pirola, N.; Baratti, S.; Storato, S.; Giovagnorio, F.; Malagnino, V.; et al. Predicting Factors of Plasma HIV RNA Undetectability after Switching to Co-Formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Experienced HIV-1 Patients: A Multicenter Study. Viruses 2023, 15, 1727. https://doi.org/10.3390/v15081727
Basso M, Battagin G, Nicolè S, Rossi MC, Colombo F, Pirola N, Baratti S, Storato S, Giovagnorio F, Malagnino V, et al. Predicting Factors of Plasma HIV RNA Undetectability after Switching to Co-Formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Experienced HIV-1 Patients: A Multicenter Study. Viruses. 2023; 15(8):1727. https://doi.org/10.3390/v15081727
Chicago/Turabian StyleBasso, Monica, Giuliana Battagin, Stefano Nicolè, Maria Cristina Rossi, Francesco Colombo, Nicole Pirola, Stefano Baratti, Silvia Storato, Federico Giovagnorio, Vincenzo Malagnino, and et al. 2023. "Predicting Factors of Plasma HIV RNA Undetectability after Switching to Co-Formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Experienced HIV-1 Patients: A Multicenter Study" Viruses 15, no. 8: 1727. https://doi.org/10.3390/v15081727
APA StyleBasso, M., Battagin, G., Nicolè, S., Rossi, M. C., Colombo, F., Pirola, N., Baratti, S., Storato, S., Giovagnorio, F., Malagnino, V., Alessio, G., Vinci, A., Maurici, M., Sarmati, L., & Parisi, S. G. (2023). Predicting Factors of Plasma HIV RNA Undetectability after Switching to Co-Formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide in Experienced HIV-1 Patients: A Multicenter Study. Viruses, 15(8), 1727. https://doi.org/10.3390/v15081727







