Variant Analysis of the Thymidine Kinase and DNA Polymerase Genes of Herpes Simplex Virus in Korea: Frequency of Acyclovir Resistance Mutations
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Clinical Samples
2.2. UL23 and UL30 Gene PCR and Sequencing of HSV-1 and HSV-2 Using NGS
2.3. Variation Analysis of the UL23 and UL30 Genes in HSV-1 and HSV-2
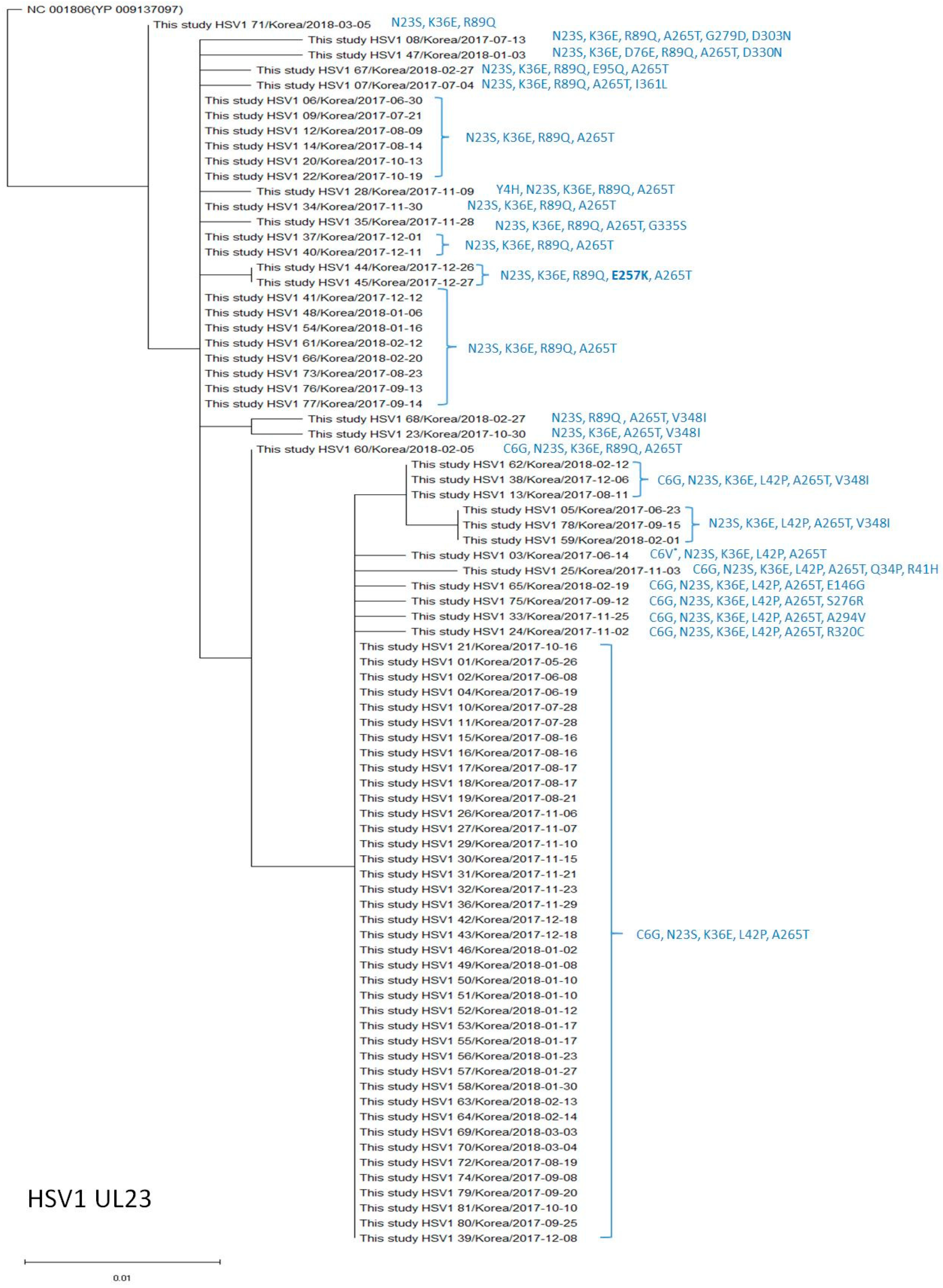
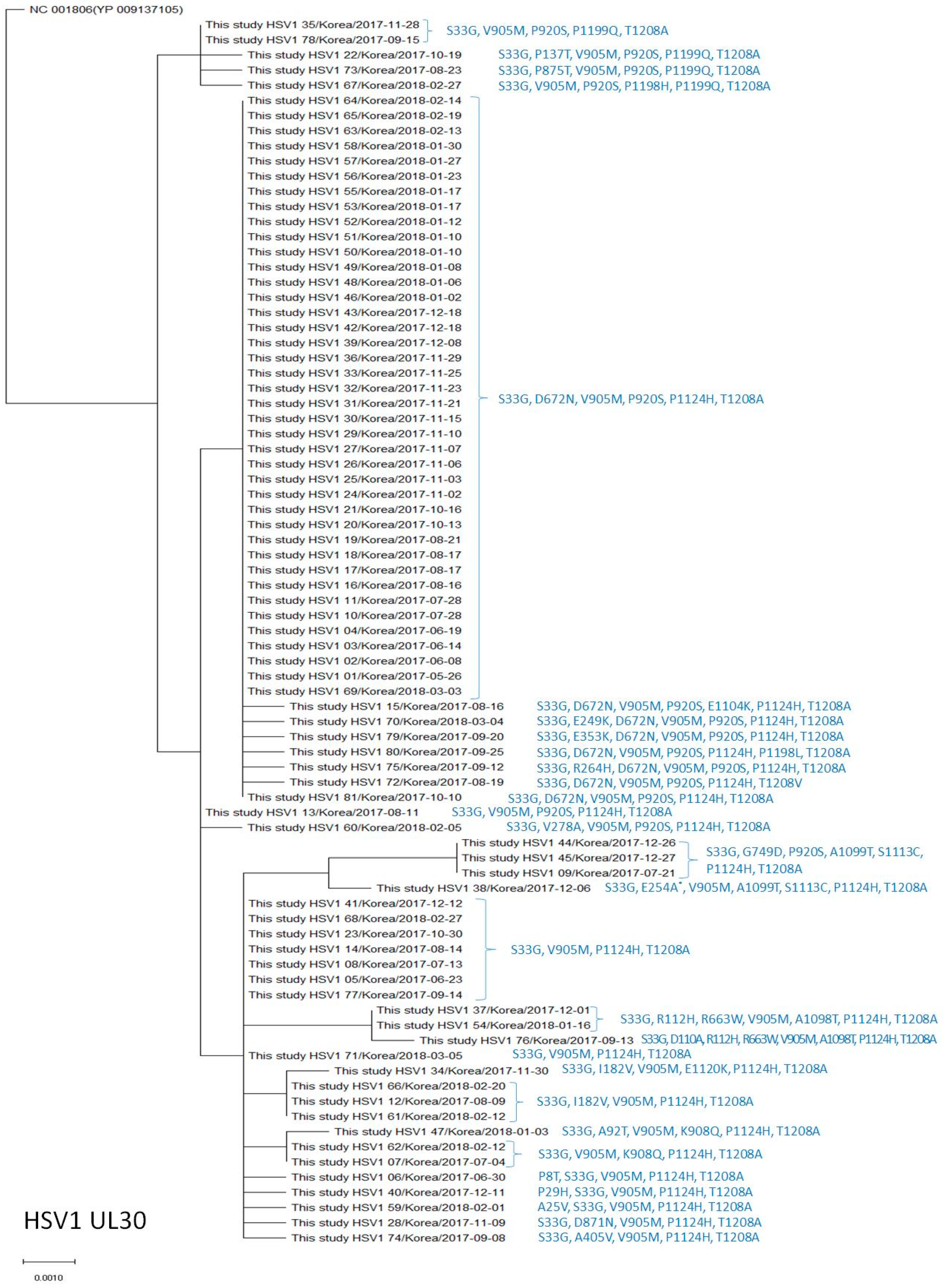
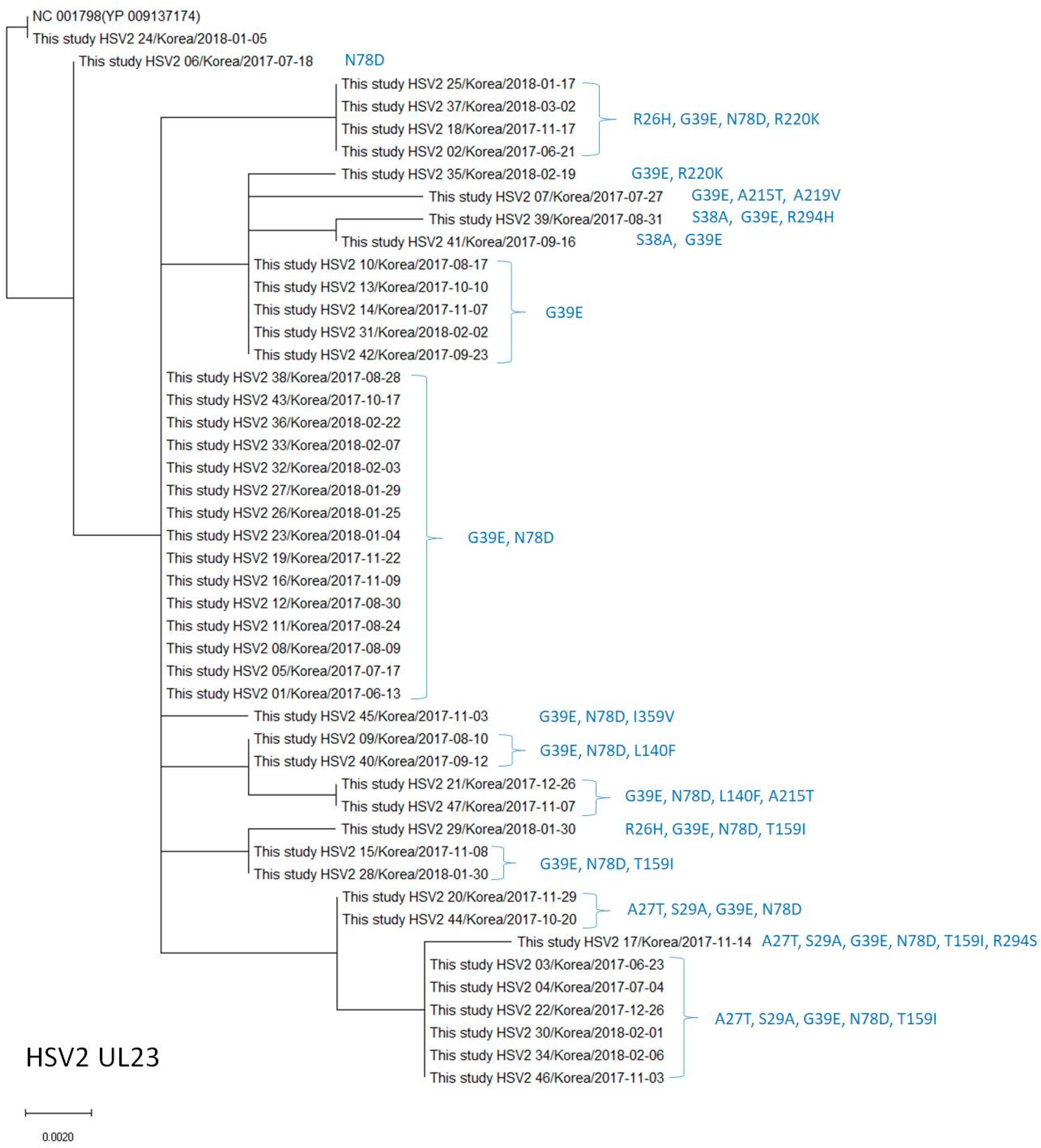
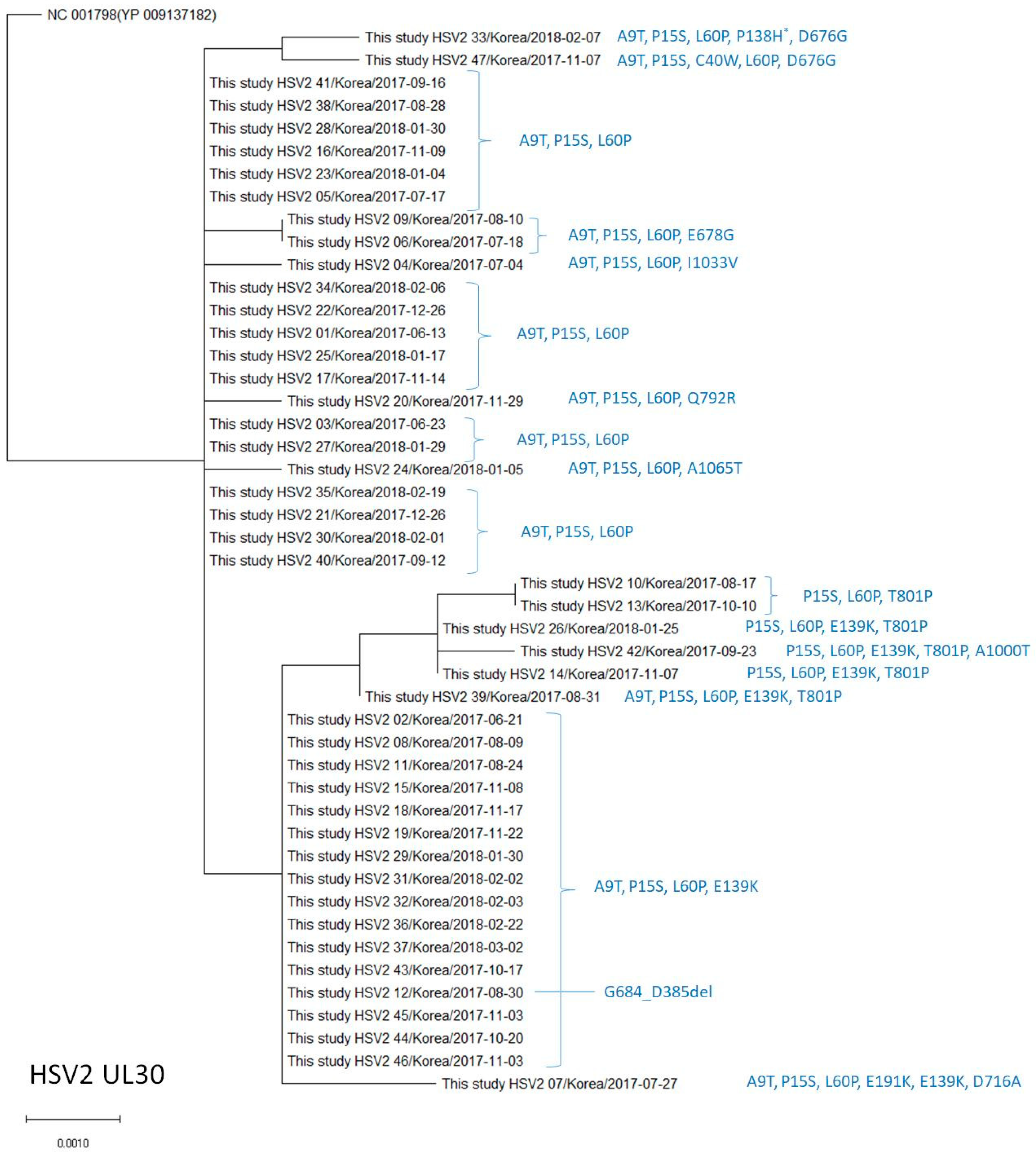
2.4. Phylogenetic Analysis of the UL23 and UL30 Genes in HSV-1 and HSV-2
3. Results
3.1. Polymorphisms of the Thymidine Kinase (UL23) Genes in HSV-1
3.2. Polymorphisms of the DNA Polymerase (UL30) Gene in HSV-1
3.3. Polymorphisms of the Thymidine Kinase (UL23) Gene in HSV-2
3.4. Polymorphisms of the DNA Polymerase (UL30) Gene in HSV-2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piret, J.; Boivin, G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: Diagnosis and management. Curr. Opin. Infect. Dis. 2016, 29, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Kotton, B.D.; Kotton, C.N. Resistant herpes simplex virus infections—Who, when, and what’s new. Curr. Opin. Infect. Dis. 2022, 35, 530–535. [Google Scholar] [CrossRef]
- Sauerbrei, A.; Bohn-Wippert, K.; Kaspar, M.; Krumbholz, A.; Karrasch, M.; Zell, R. Database on natural polymorphisms and resistance-related non-synonymous mutations in thymidine kinase and DNA polymerase genes of herpes simplex virus types 1 and 2. J. Antimicrob. Chemother. 2016, 71, 6–16. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Resistance of Herpes Simplex Viruses to Nucleoside Analogues: Mechanisms, Prevalence, and Management. Antimicrob. Agents Chemother. 2011, 55, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; de Vries, R.D.; Osterhaus, A.D.M.E.; Remeijer, L.; Verjans, G.M.G.M. Acyclovir-Resistant Corneal HSV-1 Isolates from Patients with Herpetic Keratitis. J. Infect. Dis. 2008, 198, 659–663. [Google Scholar] [CrossRef] [PubMed]
- Langston, A.A.; Redei, I.; Caliendo, A.M.; Somani, J.; Hutcherson, D.; Lonial, S.; Bucur, S.; Cherry, J.; Allen, A.; Waller, E.K. Development of drug-resistant herpes simplex virus infection after haploidentical hematopoietic progenitor cell transplantation. Blood 2002, 99, 1085–1088. [Google Scholar] [CrossRef] [PubMed]
- Burrel, S.; Deback, C.; Agut, H.; Boutolleau, D. Genotypic characterization of UL23 thymidine kinase and UL30 DNA polymerase of clinical isolates of herpes simplex virus: Natural polymorphism and mutations associated with resistance to antivirals. Antimicrob. Agents Chemother. 2010, 54, 4833–4842. [Google Scholar] [CrossRef] [PubMed]
- Bohn, K.; Zell, R.; Schacke, M.; Wutzler, P.; Sauerbrei, A. Gene Polymorphism of Thymidine Kinase and Dna Polymerase in Clinical Strains of Herpes Simplex Virus. Antivir. Ther. 2011, 16, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Bohn-Wippert, K.; Schlattmann, P.; Zell, R.; Sauerbrei, A. Sequence Analysis of Herpes Simplex Virus 1 Thymidine Kinase and DNA Polymerase Genes from over 300 Clinical Isolates from 1973 to 2014 Finds Novel Mutations That May Be Relevant for Development of Antiviral Resistance. Antimicrob. Agents Chemother. 2015, 59, 4938–4945. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Choi, S.; Kim, J.-S.; Lee, E.J.; Hyun, J.; Kim, H.S. Whole-genome analysis of rotavirus G4P[6] strains isolated from Korean neonates: Association of Korean neonates and rotavirus P[6] genotypes. Gut Pathog. 2019, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; García-Crespo, C.; Lobo-Vega, R.; Perales, C. Mutation Rates, Mutation Frequencies, and Proofreading-Repair Activities in RNA Virus Genetics. Viruses 2021, 13, 1882. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.K.; Schulz, S.L.; Ebel, C. Education and counselling for genital herpes: Perspectives from patients. Herpes 2002, 9, 78–82. [Google Scholar]
- Morfin, F.; Thouvenot, D. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 2003, 26, 29–37. [Google Scholar] [CrossRef]
- Muller, E.E.; Maseko, D.V.; Kularatne, R.S. Phenotypic and genotypic acyclovir resistance surveillance of genital herpes simplex virus 2 in South Africa. Antivir. Res. 2022, 200, 105277. [Google Scholar] [CrossRef] [PubMed]
- Majewska, A.; Mlynarczyk-Bonikowska, B. 40 Years after the Registration of Acyclovir: Do We Need New Anti-Herpetic Drugs? Int. J. Mol. Sci. 2022, 23, 3431. [Google Scholar] [CrossRef] [PubMed]
- Bestman-Smith, J.; Boivin, G. Drug Resistance Patterns of Recombinant Herpes Simplex Virus DNA Polymerase Mutants Generated with a Set of Overlapping Cosmids and Plasmids. J. Virol. 2003, 77, 7820–7829. [Google Scholar] [CrossRef] [PubMed]
- Frobert, E.; Burrel, S.; Ducastelle-Lepretre, S.; Billaud, G.; Ader, F.; Casalegno, J.-S.; Nave, V.; Boutolleau, D.; Michallet, M.; Lina, B.; et al. Resistance of herpes simplex viruses to acyclovir: An update from a ten-year survey in France. Antivir. Res. 2014, 111, 36–41. [Google Scholar] [CrossRef]
- Piret, J.; Boivin, G. Antiviral drug resistance in herpesviruses other than cytomegalovirus. Rev. Med. Virol. 2014, 24, 186–218. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhu, Q.; Zhou, R.; Liu, J.; Peng, T. Identification and characterization of acyclovir-resistant clinical HSV-1 isolates from children. J. Clin. Virol. 2011, 52, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, H.H.; Snoeck, R.; Andrei, G. Acyclovir resistance in herpes simplex viruses: Prevalence and therapeutic alternatives. Biochem. Pharmacol. 2022, 206, 115322. [Google Scholar] [CrossRef]
| HSV-1 (n = 81) | HSV-2 (n = 47) | |
|---|---|---|
| Median age (range) | 35 (1–88) | 50 (20–82) |
| Sex | ||
| Male | 30 (37.0%) | 17 (36.2%) |
| Female | 51 (63.0%) | 30 (63.8%) |
| Species of samples | ||
| Cutaneous | 58 | 27 |
| Oro and nasopharyngeal | 15 | 0 |
| Ocular | 4 | 0 |
| Genital | 3 | 15 |
| Bronchoalveolar lavage | 1 | 0 |
| CSF | 0 | 4 |
| Pleural fluid | 0 | 1 |
| Immunosuppression | ||
| HIV infection | 0 | 0 |
| Immunosuppressive treatment | 1 | 3 |
| Malignancy | 0 | 2 |
| Transplantation | 0 | 0 |
| Undescribed | 80 | 42 |
| Virus | Region | Primer Name | Binding | 5′→3′ Sequence | * Nucleotide No. of Complete HSV Genome | Amplicon (bp) |
|---|---|---|---|---|---|---|
| HSV-1 | TK | primer1 | Forward | TTTTATTCTGTCCTTTTATTGCCGTCA | 46,608 | 1301 |
| primer8 | Reverse | CGAGCGACCCTGCAGCGACCCGCT | 47,908 | |||
| Pol 1 | primer17 | Forward | ATCCGCCAGACAAACAAGGCCCTT | 62,655 | 2001 | |
| primer23 | Reverse | GGCCGTCGTAGATGGTGCGGGTG | 64,655 | |||
| Pol 2 | primer24 | Forward | GAAGGACCTGAGCTATCGCGACATC | 64,462 | 2233 | |
| primer30 | Reverse | GGCTCATAGACCGGATGCTCAC | 66,694 | |||
| HSV-2 | TK | Primer9 | Forward | TTTTATTCTGTTCTTTTATTGCCGTCA | 46,844 | 1301 |
| Primer16 | Reverse | CGAGCGACCCTGCAGCGACCCGCT | 46,144 | |||
| Pol 1 | primer31 | Forward | CCCGGGCGCGGGTCCGCCGGTCCG | 63,159 | 1046 | |
| primer34 | Reverse | GTGGTGGCGTCGACGCCCCCTCG | 64,204 | |||
| Pol 2 | primer35 | Forward | GTGCGAAGCGGGCGCGCGCTGGCC | 64,119 | 1042 | |
| primer38 | Reverse | GGATCTGCTGGCCGTCGTAGATGG | 65,160 | |||
| Pol 3 | primer39 | Forward | GGATCTGAGCTACCGCGACATC | 64,961 | 2242 | |
| primer44 | Reverse | GGCTCATCGATCGGATGCTGAC | 67,202 |
| HSV-1 (n = 81) | HSV-2 (n = 47) | |||
|---|---|---|---|---|
| TK (UL23) | DNA Polymerase (UL30) | TK (UL23) | DNA Polymerase (UL30) | |
| No. of nucleotides | 1128 | 3705 | 1128 | 3720 |
| Nucleotide identity% | 99.1 (98.9–99.3) | 99.6 (99.5–99.7) | 99.7 (99.5–100) | 99.8 (99.7–99.9) |
| Nucleotide substitution (no.) | 9.8 (8–12) | 13.3 (11–17) | 3.1 (0–6) | 6.0 (3–11) |
| No. of amino acids | 376 | 1235 | 376 | 1240 |
| Amino acid identity (%) | 98.7 (98.1–99.2) | 99.5 (99.4–99.7) | 99.2 (98.4–100) | 99.7 (99.5–99.8) |
| Amino acid changes (no.) | 4.9 (3–7) | 5.8 (4–8) | 2.8 (0–6) | 3.8 (3–6) |
| Silent mutations (%) | 50.19 | 56.44 | 9.52 | 37.01 |
| Thymidine Kinase (UL23) | This Study (n = 81, 2017–2018, Korea) | Burrel (n = 44, 2010, France) [6] | Bone (n = 56, 2011, Germany) [7] | Schmidt (n = 17, 2015, Germany) [8] | p Value |
|---|---|---|---|---|---|
| AA change | No. of isolates (%) | ||||
| Y4H | 1 (1.2) | ||||
| C6G † | 49 (60.5) | 14 (31.8) * | 6 (10.7) * | 4 (23.5) * | <0.0001 |
| C6V | 1 (1.2) | ||||
| N23S † | 80 (98.8) | 44(100) | 54 (96.4) | 15 (88.2) * | 0.0496 |
| Q34P | 1 (1.2) | ||||
| K36E † | 81 (100) | 44 (100) | 33 (58.9) * | 15 (88.2) | <0.0001 |
| R41H † | 1 (1.2) | 1 (2.3) | 1 (1.8) | ||
| L42P † | 52 (64.2) | 15 (34.1) * | 11 (19.6) * | 4 (23.5) * | <0.0001 |
| D76E | 1 (1.2) | ||||
| R89Q † | 28 (34.6) | 26 (59.1) * | 11 (19.6) | 7 (41.2) | 0.0008 |
| E95Q | 1 (1.2) | ||||
| E146G † | 1 (1.2) | 1 (2.3) | |||
| E257K ‡ | 2(2.5) | 1 (5.9) | |||
| A265T † | 80 (98.8) | 43 (97.7) | 56 (100) | 7 (41.2) * | <0.0001 |
| S276R † | 1 (1.2) | 1 (1.8) | 1 (5.9) | ||
| G279D | 1 (1.2) | ||||
| A294V † | 1 (1.2) | ||||
| D303N † | 1 (1.2) | ||||
| R320C | 1 (1.2) | ||||
| D330N | 1 (1.2) | ||||
| G335S | 1 (1.2) | ||||
| V348I † | 8 (9.9) | 9 (20.5) | 7 (12.5) | ||
| I361L | 1 (1.2) | ||||
| DNA Polymerase (UL30) | This Study (n = 81, 2017–2018, Korea) | Burrel (n = 44, 2010, France) [6] | Bone (n = 56, 2011, Germany) [7] | Schmidt (n = 17, 2015, Germany) [8] | p Value |
|---|---|---|---|---|---|
| AA change | No. of isolates (%) | ||||
| P8T | 1 (1.2) | ||||
| A25V † | 1 (1.2) | 1 (1.8) | |||
| P29H † | 1 (1.2) | 1 (1.8) | |||
| S33G † | 81 (100) | 40 (90.9) * | 46 (86.7) * | 16 (94.1) | 0.0059 |
| A92T | 1 (1.2) | ||||
| D110A | 1 (1.2) | ||||
| R112H † | 3 (3.7) | 1 (1.8) | |||
| P137T | 1 (1.2) | ||||
| I182V | 4 (4.9) | ||||
| E249K | 1 (1.2) | ||||
| E254A | 1 (1.2) | ||||
| R264H † | 1 (1.2) | ||||
| V278A | 1 (1.2) | ||||
| E353K | 1 (1.2) | ||||
| A405V | 1 (1.2) | ||||
| R663W | 3 (3.7) | ||||
| D672N † | 47 (58.0) | 3 (6.8) * | 14 (26.4) * | 1 (5.9) * | <0.0001 |
| G749D † | 3 (3.7) | 1 (2.3) | 1 (1.8) | ||
| D871N † | 1 (1.2) | ||||
| P875T | 1 (1.2) | ||||
| V905M † | 78 (96.3) | 33 (75) * | 43 (81.1) * | 15 (88.2) | 0.0019 |
| K908Q | 3 (3.7) | ||||
| P920S † | 57 (70.4) | 4 (9.1) * | 11 (20.8) * | 1 (5.9) * | <0.0001 |
| A1098T | 3 (3.7) | ||||
| A1099T † | 4 (4.9) | 1 (2.3) | |||
| E1104K † | 1 (1.2) | 1 (5.9) | |||
| S1113C † | 4 (4.9) | 1 (2.3) | |||
| E1120K | 1 (1.2) | ||||
| P1124H † | 76 (93.8) | 17 (38.6) * | 25 (47.2) * | 8 (47.1) * | <0.0001 |
| P1198H | 1 (1.2) | ||||
| P1198L | 1 (1.2) | ||||
| P1199Q † | 5 (6.2) | 2 (4.5) | 7 (13.2) | 2 (11.8) | 0.4041 |
| T1208A † | 80 (98.8) | 37 (84.1) * | 38 (71.7) * | 13 (76.5) * | <0.0001 |
| T1208V | 1 (1.2) | ||||
| Thymidine Kinase (UL23) | This Study (n = 47, 2017–2018, Korea) | Burrel (n = 54, 2010, France) [6] | Bone (n = 12, 2011, Germany) [7] | p Value |
|---|---|---|---|---|
| AA change | No. of isolates (%) | |||
| R26H † | 5 (10.6) | |||
| A27T † | 9 (19.1) | 2 (3.7) * | ||
| S29A † | 9 (19.1) | |||
| S38A | 2 (4.3) | |||
| G39E † | 45 (95.7) | 40 (74.1) | 11 (91.7) | 0.1612 |
| N78D † | 37 (78.7) | 19 (35.2) * | 9 (75.0) | <0.0001 |
| L140F † | 4 (8.5) | 16 (29.6) * | 7 (58.3) * | 0.0006 |
| T159I † | 10 (21.3) | |||
| A215T † | 3 (6.4) | 2 (3.7) | 2 (16.7) | 0.2412 |
| A219V | 1 (2.1) | |||
| R220K † | 5 (10.6) | 1 (8.3) | ||
| R294H | 1 (2.1) | |||
| R294S | 1 (2.1) | |||
| I359V | 1 (2.1) |
| DNA Polymerase (UL30) | This Study (n = 47, 2017–2018, Korea) | Burrel (n = 54, 2010, France) [6] | Bone (n = 12, 2011, Germany) [7] | p Value |
|---|---|---|---|---|
| AA change | No. of isolates (%) | |||
| A9T † | 42 (89.4) | 49 (90.7) | 12 (100) | 0.5059 |
| P15S † | 47 (100) | 49 (90.7) | 12 (100) | 0.0574 |
| C40W † | 1 (2.1) | 1 (8.3) | ||
| L60P † | 47 (100) | 49 (90.7) | 12 (100) | 0.0574 |
| P138H | 1 (2.1) | |||
| E139K † | 21 (44.7) | 16 (29.6) | 2 (16.7) | 0.1102 |
| E191K | 1 (2.1) | |||
| D676G † | 2 (4.3) | 4 (7.4) | 2 (16.7) | 0.3197 |
| E678G † | 2 (4.3) | 11 (20.4) | ||
| G684_D685del | 1 (2.1) | |||
| D716A | 1 (2.1) | |||
| Q792R | 1 (2.1) | |||
| T801P † | 6 (12.8) | |||
| A1000T † | 1 (2.1) | 1 (1.9) | ||
| I1033V | 1 (2.1) | |||
| A1065T | 1 (2.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, J.; Lee, S.K.; Kim, J.H.; Cho, E.-J.; Kim, H.-S.; Kim, J.-S.; Song, W.; Kim, H.S. Variant Analysis of the Thymidine Kinase and DNA Polymerase Genes of Herpes Simplex Virus in Korea: Frequency of Acyclovir Resistance Mutations. Viruses 2023, 15, 1709. https://doi.org/10.3390/v15081709
Hyun J, Lee SK, Kim JH, Cho E-J, Kim H-S, Kim J-S, Song W, Kim HS. Variant Analysis of the Thymidine Kinase and DNA Polymerase Genes of Herpes Simplex Virus in Korea: Frequency of Acyclovir Resistance Mutations. Viruses. 2023; 15(8):1709. https://doi.org/10.3390/v15081709
Chicago/Turabian StyleHyun, Jungwon, Su Kyung Lee, Ji Hyun Kim, Eun-Jung Cho, Han-Sung Kim, Jae-Seok Kim, Wonkeun Song, and Hyun Soo Kim. 2023. "Variant Analysis of the Thymidine Kinase and DNA Polymerase Genes of Herpes Simplex Virus in Korea: Frequency of Acyclovir Resistance Mutations" Viruses 15, no. 8: 1709. https://doi.org/10.3390/v15081709
APA StyleHyun, J., Lee, S. K., Kim, J. H., Cho, E.-J., Kim, H.-S., Kim, J.-S., Song, W., & Kim, H. S. (2023). Variant Analysis of the Thymidine Kinase and DNA Polymerase Genes of Herpes Simplex Virus in Korea: Frequency of Acyclovir Resistance Mutations. Viruses, 15(8), 1709. https://doi.org/10.3390/v15081709






