The Resistance to Lethal Challenge with Ostreid herpesvirus-1 of Pacific Oysters (Crassostrea gigas) Previously Exposed to This Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Oysters
2.1.1. Previously Exposed Adult Oysters (PEad)
2.1.2. Non-Exposed Adult Oysters (NEad)
2.1.3. Non-Exposed Spat (NEsp)
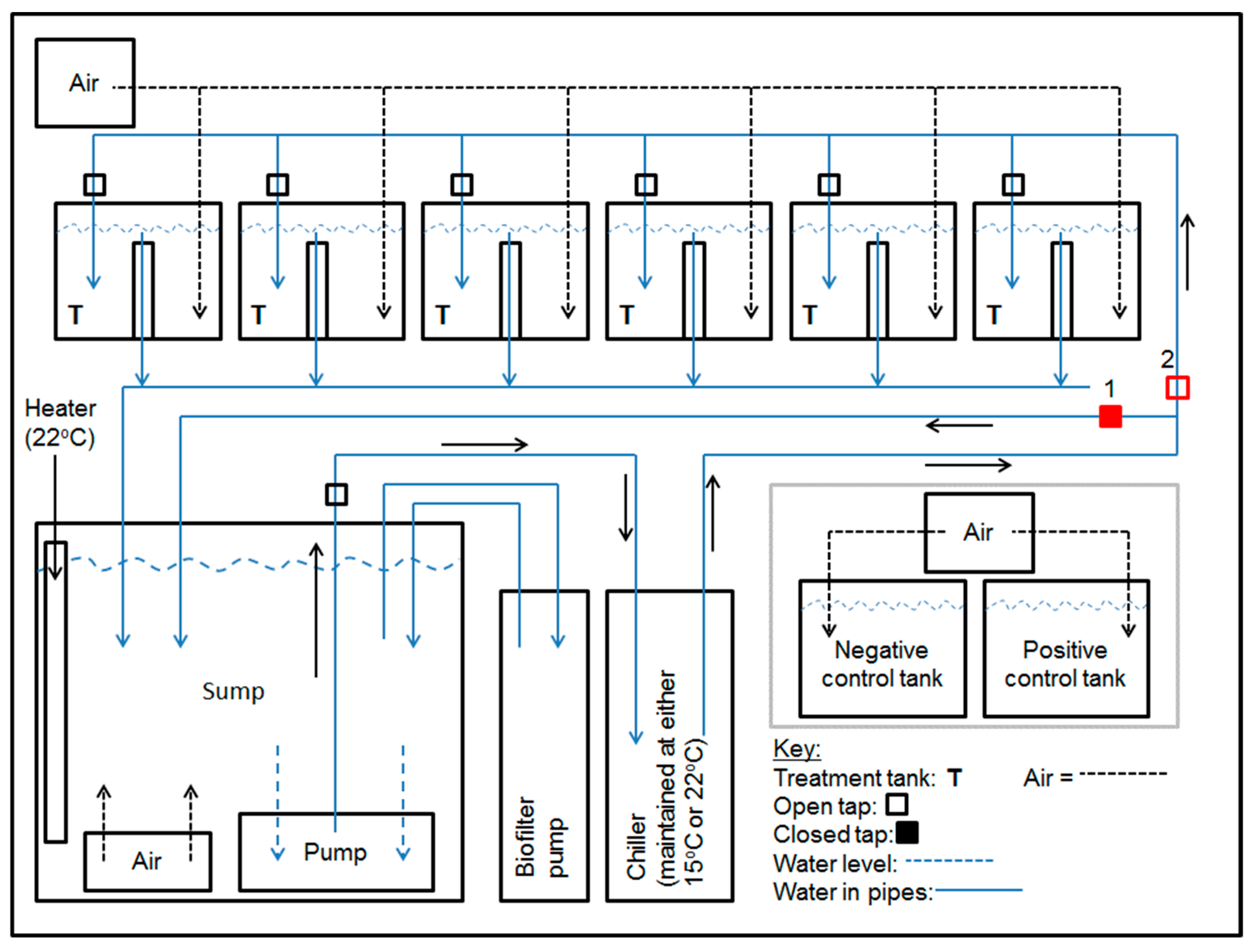
2.2. Aquarium Facility
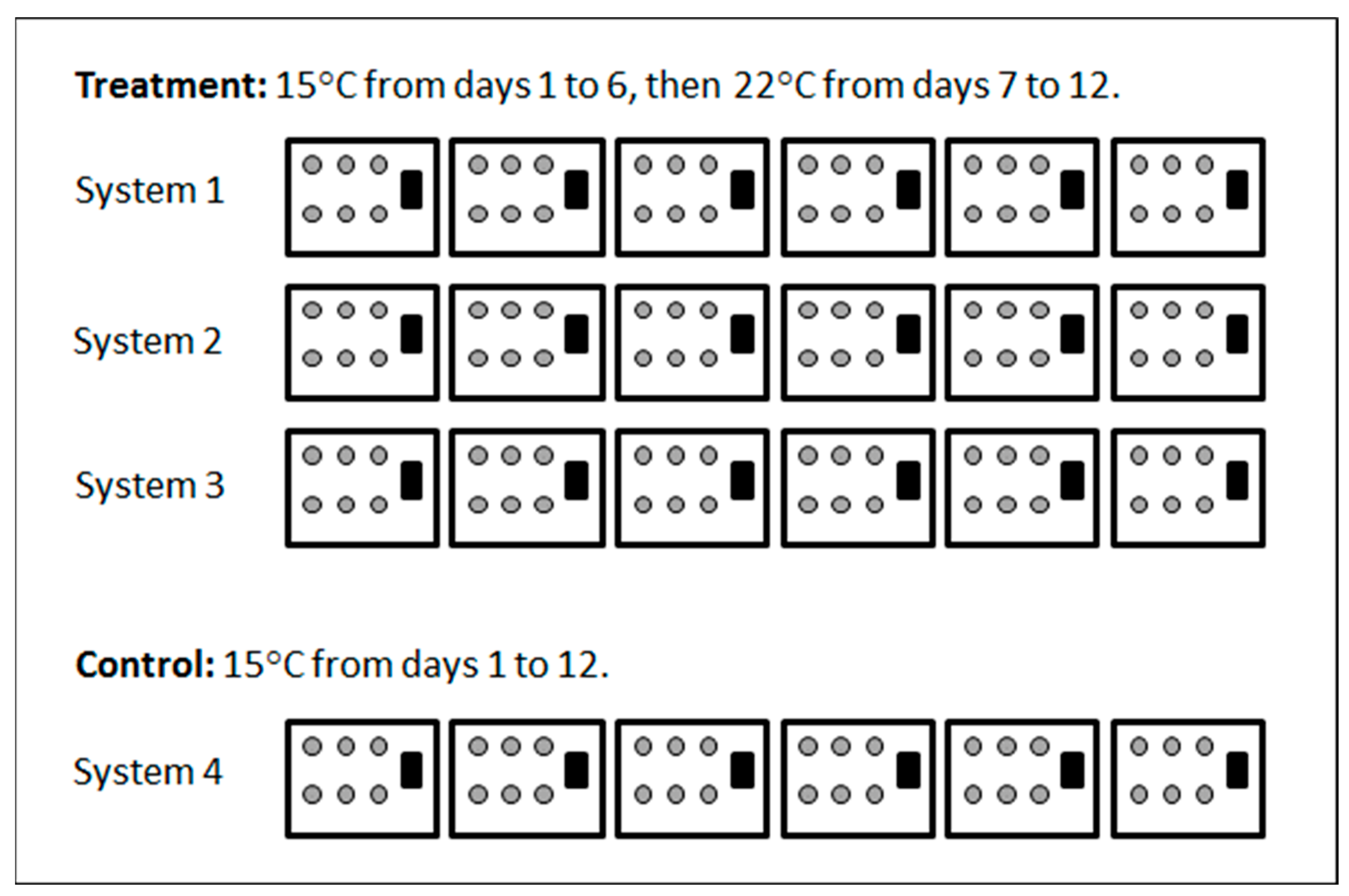
2.3. Experiment 1
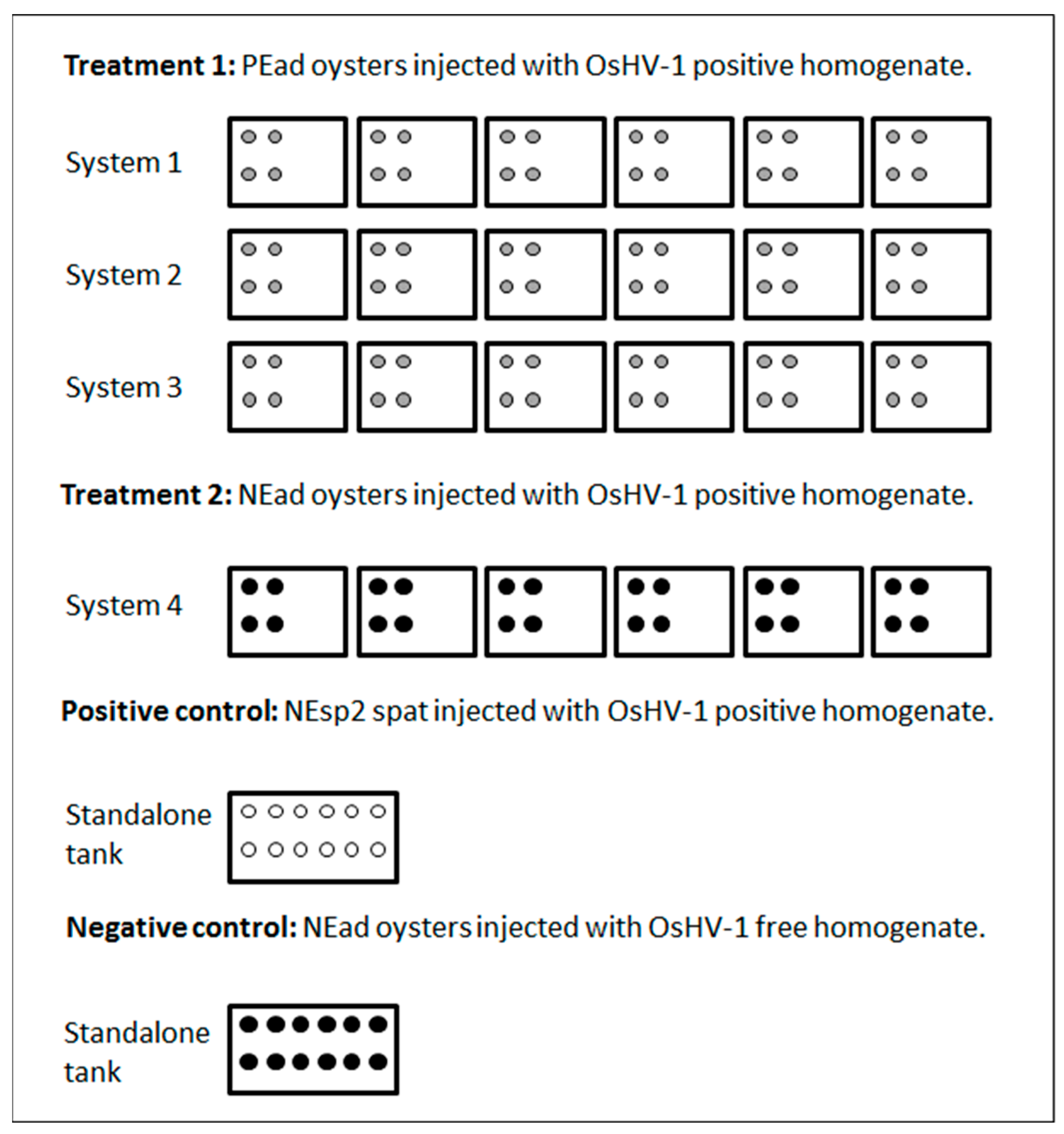
2.4. Experiment 2
2.5. Detection of OsHV-1 DNA
2.6. Statistical Analysis
3. Results
3.1. Experiment 1
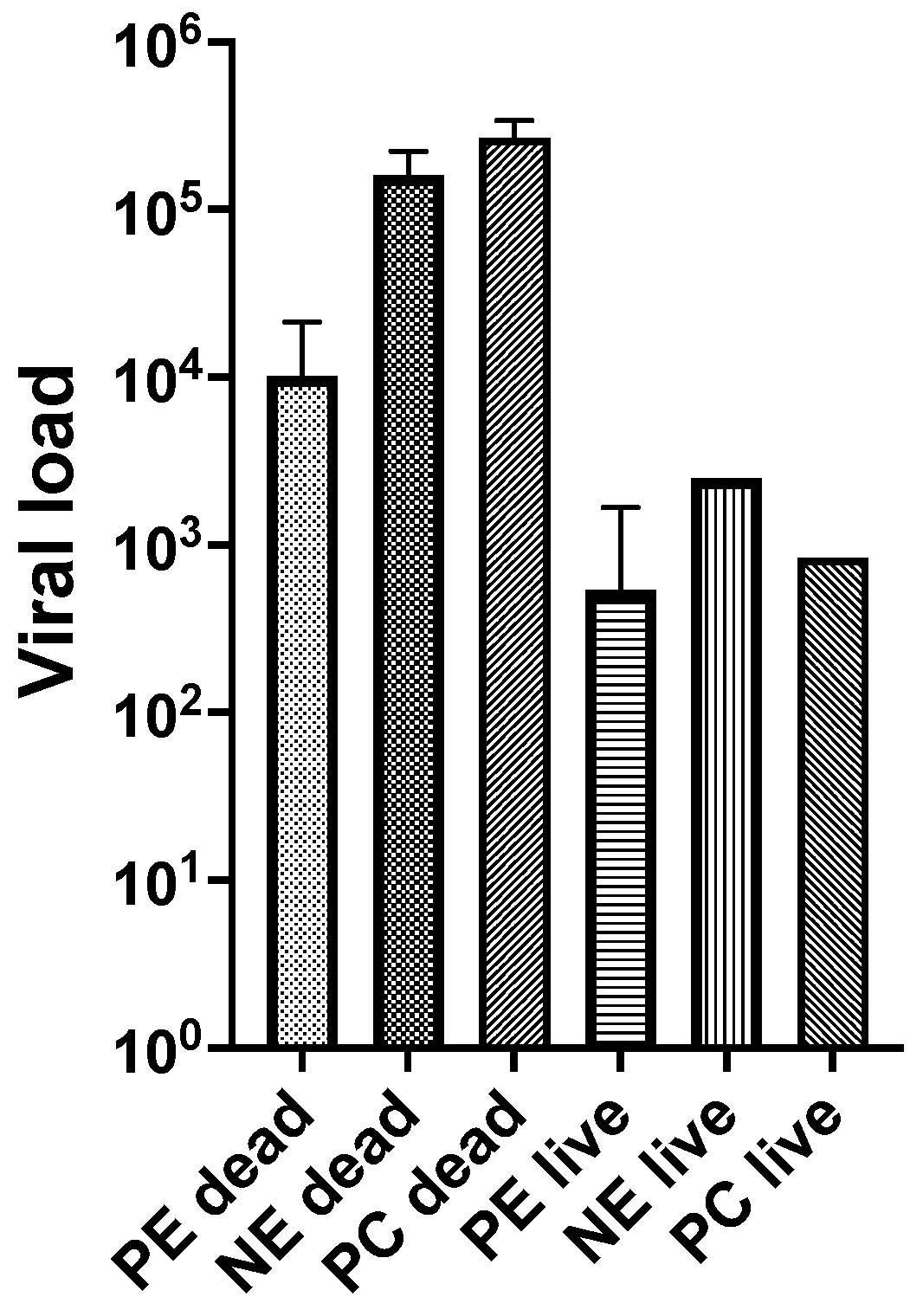
3.2. Experiment 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EFSA. AHAW Panel (EFSA Panel on Animal Health and Welfare): Scientific opinion on oyster mortality. EFSA J. 2015, 13. [Google Scholar]
- Fuhrmann, M.; Castinel, A.; Cheslett, D.; Nozal, D.F.; Whittington, R.J. The impacts of Ostreid herpesvirus 1 microvariants on Pacific oyster aquaculture in the Northern and Southern Hemispheres since 2008. Rev. Sci. Tech.-Off. Int. Epizoot. 2019, 38, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Petton, B.; Destoumieux-Garzón, D.; Pernet, F.; Toulza, E.; de Lorgeril, J.; Degremont, L.; Mitta, G. The Pacific oyster mortality syndrome, a polymicrobial and multifactorial disease: State of knowledge and future directions. Front. Immunol. 2021, 12, 630343. [Google Scholar] [CrossRef] [PubMed]
- Davison, A.J.; Trus, B.L.; Cheng, N.Q.; Steven, A.C.; Watson, M.S.; Cunningham, C.; Le Deuff, R.M.; Renault, T. A novel class of herpesvirus with bivalve hosts. J. Gen. Virol. 2005, 86, 41–53. [Google Scholar] [CrossRef]
- Davison, A.J.; Eberle, R.; Ehlers, B.; Hayward, G.S.; McGeoch, D.J.; Minson, A.C.; Pellett, P.E.; Roizman, B.; Studdert, M.J.; Thiry, E. The order Herpesvirales. Arch. Virol. 2009, 154, 171–177. [Google Scholar] [CrossRef]
- Segarra, A.; Pépin, J.F.; Arzul, I.; Morga, B.; Faury, N.; Renault, T. Detection and description of a particular Ostreid herpesvirus 1 genotype associated with massive mortality outbreaks of Pacific oysters, Crassostrea gigas, in France in 2008. Virus Res. 2010, 153, 92–99. [Google Scholar] [CrossRef] [Green Version]
- OIE, Infection with Ostreid herpesvirus 1 Microvariants. In Manual of Diagnostic Test for Aquatic Animals; World Organisation for Animal Health: Paris, France, 2018; p. 14.
- Jenkins, C.; Hick, P.; Gabor, M.; Spiers, Z.; Fell, S.A.; Gu, X.; Read, A.; Go, J.; Dove, M.; O’Connor, W.; et al. Identification and characterisation of an Ostreid herpesvirus-1 microvariant (OsHV-1 micro-var) in Crassostrea gigas (Pacific oysters) in Australia. Dis. Aquat. Org. 2013, 105, 109–126. [Google Scholar] [CrossRef] [Green Version]
- Trancart, S.; Tweedie, A.; Liu, O.; Paul-Pont, I.; Hick, P.; Houssin, M.; Whittington, R.J. Diversity and molecular epidemiology of Ostreid herpesvirus 1 in farmed Crassostrea gigas in Australia: Geographic clusters and implications for “microvariants” in global mortality events. Virus Res. 2022, 323, 198994. [Google Scholar] [CrossRef]
- Schikorski, D.; Renault, T.; Saulnier, D.; Faury, N.; Moreau, P.; Pepin, J.F. Experimental infection of Pacific oyster Crassostrea gigas spat by ostreid herpesvirus 1: Demonstration of oyster spat susceptibility. Vet. Res. 2011, 42, 27. [Google Scholar] [CrossRef] [Green Version]
- Dégremont, L. Size and genotype affect resistance to mortality caused by OsHV-1 in Crassostrea gigas. Aquaculture 2013, 416–417, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Paul-Pont, I.; Dhand, N.K.; Whittington, R.J. Influence of husbandry practices on OsHV-1 associated mortality of Pacific oysters Crassostrea gigas. Aquaculture 2013, 412, 202–214. [Google Scholar] [CrossRef]
- Paul-Pont, I.; Evans, O.; Dhand, N.K.; Rubio, A.; Coad, P.; Whittington, R.J. Descriptive epidemiology of mass mortality due to Ostreid herpesvirus-1 (OsHV-1) in commercially farmed Pacific oysters (Crassostrea gigas) in the Hawkesbury River estuary, Australia. Aquaculture 2014, 422–423, 146–159. [Google Scholar] [CrossRef] [Green Version]
- Whittington, R.; Dhand, N.; Evans, O.; Paul-Pont, I. Further observations on the influence of husbandry practices on OsHV-1 μVar mortality in Pacific oysters Crassostrea gigas: Age, cultivation structures and growing height. Aquaculture 2015, 438, 82–97. [Google Scholar] [CrossRef]
- Hick, P.M.; Evans, O.; Rubio, A.; Dhand, N.K.; Whittington, R.J. Both age and size influence susceptibility of Pacific oysters (Crassostrea gigas) to disease caused by Ostreid herpesvirus-1 (OsHV-1) in replicated field and laboratory experiments. Aquaculture 2018, 489, 110–120. [Google Scholar] [CrossRef]
- de Kantzow, M.C.; Hick, P.M.; Dhand, N.K.; Whittington, R.J. Risk factors for mortality during the first occurrence of Pacific Oyster Mortality Syndrome due to Ostreid herpesvirus-1 in Tasmania, 2016. Aquaculture 2017, 468, 328–336. [Google Scholar] [CrossRef]
- Peeler, E.J.; Allan Reese, R.; Cheslett, D.L.; Geoghegan, F.; Power, A.; Thrush, M.A. Investigation of mortality in Pacific oysters associated with Ostreid herpesvirus-1 μVar in the Republic of Ireland in 2009. Prev. Vet. Med. 2012, 105, 136–143. [Google Scholar] [CrossRef]
- Pernet, F.; Lagarde, F.; Le Gall, P.; D’Orbcastel, E.R. Associations between farming practices and disease mortality of Pacific oyster Crassostrea gigas in a Mediterranean lagoon. Aquac. Env. Interact 2014, 5, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Schikorski, D.; Faury, N.; Pepin, J.F.; Saulnier, D.; Tourbiez, D.; Renault, T. Experimental ostreid herpesvirus 1 infection of the Pacific oyster Crassostrea gigas: Kinetics of virus DNA detection by q-PCR in seawater and in oyster samples. Virus Res. 2011, 155, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Paul-Pont, I.; Evans, O.; Dhand, N.K.; Whittington, R.J. Experimental infections of Pacific oyster Crassostrea gigas using the Australian Ostreid herpesvirus-1 (OsHV-1) uVar strain. Dis. Aquat. Org. 2015, 113, 137–147. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Jouaux, A.; Ford, S.E.; Lelong, C.; Sourdaine, P.; Mathieu, M.; Guo, X. Transcriptome analysis reveals strong and complex antiviral response in a mollusc. Fish Shellfish Immunol. 2015, 46, 131–144. [Google Scholar] [CrossRef]
- de Kantzow, M.C.; Whittington, R.J.; Hick, P.M. Different in vivo growth of ostreid herpesvirus 1 at 18 degrees C and 22 degrees C alters mortality of Pacific oysters (Crassostrea gigas). Arch. Virol. 2019, 164, 3035–3043. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Baillon, L.; Tourbiez, D.; Benabdelmouna, A.; Faury, N.; Bourgougnon, N.; Renault, T. Ostreid herpesvirus type 1 replication and host response in adult Pacific oysters, Crassostrea gigas. Vet. Res. 2014, 45. [Google Scholar] [CrossRef] [PubMed]
- Evans, O.; Hick, P.; Whittington, R.J. Detection of Ostreid herpesvirus-1 microvariants in healthy Crassostrea gigas following disease events and their possible role as reservoirs of infection. J. Invert. Pathol. 2017, 148, 20–33. [Google Scholar] [CrossRef]
- Evans, O.; Kan, J.Z.F.; Pathirana, E.; Whittington, R.J.; Dhand, N.; Hick, P. Effect of emersion on the mortality of Pacific oysters (Crassostrea gigas) infected with Ostreid herpesvirus-1 (OsHV-1). Aquaculture 2019, 505, 157–166. [Google Scholar] [CrossRef]
- Arzul, I.; Renault, T.; Thébault, A.; Gérard, A. Detection of oyster herpesvirus DNA and proteins in asymptomatic Crassostrea gigas adults. Virus Res. 2002, 84, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Jee, B.Y.; Lee, S.J.; Cho, M.Y.; Lee, S.J.; Kim, J.W.; Choi, S.H.; Jeong, H.D.; Kim, K.H. Detection of Ostreid Herpesvirus 1 from adult Pacific Oysters Crassostrea gigas Cultured in Korea. Fish. Aquat. Sci. 2013, 16, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Shimahara, Y.; Kurita, J.; Kiryu, I.; Nishioka, T.; Yuasa, K.; Kawana, M.; Kamaishi, T.; Oseko, N. Surveillance of Type 1 Ostreid herpesvirus (OsHV-1) variants in Japan. Fish Pathol. 2012, 47, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Oden, E.; Martenot, C.; Berthaux, M.; Travaille, E.; Malas, J.P.; Houssin, M. Quantification of ostreid herpesvirus 1 (OsHV-1) in Crassostrea gigas by real-time PCR: Determination of a viral load threshold to prevent summer mortalities. Aquaculture 2011, 317, 27–31. [Google Scholar] [CrossRef]
- Gray, W.L.; Williams, R.J.; Jordan, R.L.; Griffin, B.R. Detection of channel catfish virus DNA in latently infected catfish. J. Gen. Virol. 1999, 80, 1817–1822. [Google Scholar] [CrossRef]
- Eide, K.E.; Miller-Morgan, T.; Heidel, J.R.; Kent, M.L.; Bildfell, R.J.; LaPatra, S.; Watson, G.; Jin, L. Investigation of Koi Herpesvirus Latency in Koi. J. Virol. 2011, 85, 4954–4962. [Google Scholar] [CrossRef] [Green Version]
- Reed, A.N.; Izume, S.; Dolan, B.P.; LaPatra, S.; Kent, M.; Dong, J.; Jin, L. Identification of B Cells as a Major Site for Cyprinid Herpesvirus 3 Latency. J. Virol. 2014, 88, 9297–9309. [Google Scholar] [CrossRef]
- Uchii, K.; Minamoto, T.; Honjo, M.N.; Kawabata, Z. Seasonal reactivation enables Cyprinid herpesvirus 3 to persist in a wild host population. Fems Microbiol. Ecol. 2014, 87, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Griffin, B.D.; Verweij, M.C.; Wiertz, E.J.H.J. Herpesviruses and immunity: The art of evasion. Vet. Microbiol. 2010, 143, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Petton, B.; Pernet, F.; Robert, R.; Boudry, P. Temperature influence on pathogen transmission and subsequent mortalities in juvenile Pacific oysters Crassostrea gigas. Aquac. Env. Interact 2013, 3, 257–273. [Google Scholar] [CrossRef] [Green Version]
- Renault, T.; Bouquet, A.L.; Maurice, J.-T.; Lupo, C.; Blachier, P. Ostreid herpesvirus 1 infection among Pacific oyster (Crassostrea gigas) spat: Relevance of water temperature to virus replication and circulation prior to the onset of mortality. Appl. Environ. Microbiol. 2014, 80, 5419–5426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martenot, C.; Denechere, L.; Hubert, P.; Metayer, L.; Oden, E.; Trancart, S.; Travaille, E.; Houssin, M. Virulence of Ostreid herpesvirus 1 mu Var in sea water at 16 degrees C and 25 degrees C. Aquaculture 2015, 439, 1–6. [Google Scholar] [CrossRef]
- de Kantzow, M.; Hick, P.; Becker, J.; Whittington, R. Effect of water temperature on mortality of Pacific oysters Crassostrea gigas associated with microvariant Ostreid herpesvirus 1 (OsHV-1 μVar). Aquac. Environ. Interact. 2016, 8, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Pernet, F.; Tamayo, D.; Petton, B. Influence of low temperatures on the survival of the Pacific oyster (Crassostrea gigas) infected with ostreid herpes virus type 1. Aquaculture 2015, 445, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Petton, B.; Boudry, P.; Alunno-Bruscia, M.; Pernet, F. Factors influencing disease-induced mortality of Pacific oysters Crassostrea gigas. Aquac. Environ. Interact. 2015, 6, 205–222. [Google Scholar] [CrossRef] [Green Version]
- Whittington, R.J.; Paul-Pont, I.; Evans, O.; Hick, P.; Dhand, N.K. Counting the dead to determine the source and transmission of the marine herpesvirus OsHV-1 in Crassostrea gigas. Vet Res. 2018, 49, 34. [Google Scholar] [CrossRef] [Green Version]
- Dégremont, L. Evidence of herpesvirus (OsHV-1) resistance in juvenile Crassostrea gigas selected for high resistance to the summer mortality phenomenon. Aquaculture 2011, 317, 94–98. [Google Scholar] [CrossRef] [Green Version]
- Azema, P.; Lamy, J.B.; Boudry, P.; Renault, T.; Travers, M.A.; Degremont, L. Genetic parameters of resistance to Vibrio aestuarianus, and OsHV-1 infections in the Pacific oyster, Crassostrea gigas, at three different life stages. Genet. Sel. Evol. 2017, 49, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Whittington, R.J.; Liu, O.; Hick, P.M.; Dhand, N.; Rubio, A. Long-term temporal and spatial patterns of Ostreid herpesvirus 1 (OsHV-1) infection and mortality in sentinel Pacific oyster spat (Crassostrea gigas) inform farm management. Aquaculture 2019, 513, 734395. [Google Scholar] [CrossRef]
- Evans, O.; Hick, P.; Dhand, N.; Whittington, R.J. Transmission of Ostreid herpesvirus-1 in Crassostrea gigas by cohabitation: Effects of food and number of infected donor oysters. Aquac. Environ. Interact. 2015, 7, 281–295. [Google Scholar] [CrossRef] [Green Version]
- Evans, O.; Paul-Pont, I.; Hick, P.; Whittington, R.J. A simple centrifugation method for improving the detection of Ostreid herpesvirus-1 (OsHV-1) in natural seawater samples with an assessment of the potential for particulate attachment. J. Virol. Methods 2014, 210, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Martenot, C.; Oden, E.; Travaillé, E.; Malas, J.P.; Houssin, M. Comparison of two real-time PCR methods for detection of ostreid herpesvirus 1 in the Pacific oyster Crassostrea gigas. J. Virol. Methods 2010, 170, 86–89. [Google Scholar] [CrossRef] [PubMed]
- de Kantzow, M.C.; Whittington, R.J.; Hick, P. Prior exposure to Ostreid herpesvirus 1 (OsHV-1) at 18 degrees C is associated with improved survival of juvenile Pacific oysters (Crassostrea gigas) following challenge at 22 degrees C. Aquaculture 2019, 507, 443–450. [Google Scholar] [CrossRef]
- Divilov, K.; Wang, X.; Fleener, G.B.; Schoolfield, B.; Langdon, C.; Jin, L. Persistent Infections of Ostreid Herpesvirus 1 in Adult Pacific Oysters, Crassostrea gigas. Prepr. Unpubl. Artic. 2023. [Google Scholar]
- Degremont, L.; Lamy, J.-B.; Pepin, J.-F.; Travers, M.-A.; Renault, T. New insight for the genetic evaluation of resistance to ostreid herpesvirus infection, a worldwide disease, in Crassostrea gigas. PLoS ONE 2015, 10, e0127917. [Google Scholar] [CrossRef] [Green Version]
- Green, T.J.; Rolland, J.-L.; Vergnes, A.; Raftos, D.; Montagnani, C. OsHV-1 countermeasures to the Pacific oyster’s anti-viral response. Fish Shellfish Immunol. 2015, 47, 435–443. [Google Scholar] [CrossRef] [Green Version]
- Martenot, C.; Gervais, O.; Chollet, B.; Houssin, M.; Renault, T. Haemocytes collected from experimentally infected Pacific oysters, Crassostrea gigas: Detection of ostreid herpesvirus 1 DNA, RNA, and proteins in relation with inhibition of apoptosis. PLoS ONE 2017, 12, e0177448. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.; Moreau, K.; Segarra, A.; Tourbiez, D.; Travers, M.-A.; Rubinsztein, D.C.; Renault, T. Autophagy plays an important role in protecting Pacific oysters from OsHV-1 and Vibrio aestuarianus infections. Autophagy 2015, 11, 516–526. [Google Scholar] [CrossRef] [Green Version]
- Pauletto, M.; Segarra, A.; Montagnani, C.; Quillien, V.; Faury, N.; Le Grand, J.; Miner, P.; Petton, B.; Labreuche, Y.; Fleury, E.; et al. Long dsRNAs promote an anti-viral response in Pacific oyster hampering ostreid herpesvirus 1 replication. J. Exp. Biol. 2017, 220, 3671–3685. [Google Scholar] [CrossRef] [Green Version]
- Rowley, A.F.; Pope, E.C. Vaccines and crustacean aquaculture—A mechanistic exploration. Aquaculture 2012, 334–337, 1–11. [Google Scholar] [CrossRef]
- Schulenburg, H.; Boehnisch, C.; Michiels, N.K. How do invertebrates generate a highly specific innate immune response? Mol. Immunol. 2007, 44, 3338–3344. [Google Scholar] [CrossRef]
- Owens, L.; Malham, S. Review of the RNA Interference Pathway in Molluscs Including Some Possibilities for Use in Bivalves in Aquaculture. J. Mar. Sci. Eng. 2015, 3, 87–99. [Google Scholar] [CrossRef] [Green Version]
- Degremont, L.; Nourry, M.; Maurouard, E. Mass selection for survival and resistance to OsHV-1 infection in Crassostrea gigas spat in field conditions: Response to selection after four generations. Aquaculture 2015, 446, 111–121. [Google Scholar] [CrossRef]
- Green, T.J.; Speck, P. Antiviral Defense and Innate Immune Memory in the Oyster. Viruses 2018, 10, 133. [Google Scholar] [CrossRef] [Green Version]
- Green, T.J.; Montagnani, C. Poly I:C induces a protective antiviral immune response in the Pacific oyster (Crassostrea gigas) against subsequent challenge with Ostreid herpesvirus (OsHV-1 mu var). Fish Shellfish Immunol. 2013, 35, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Delisle, L.; Rolton, A.; Vignier, J. Inactivated ostreid herpesvirus-1 induces an innate immune response in the Pacific oyster, Crassostrea gigas, hemocytes. Front. Immunol. 2023, 14, 1161145. [Google Scholar] [CrossRef]
- Degremont, L.; Guyader, T.; Tourbiez, D.; Pepin, J.-F. Is horizontal transmission of the Ostreid herpesvirus OsHV-1 in Crassostrea gigas affected by unselected or selected survival status in adults to juveniles? Aquaculture 2013, 408, 51–57. [Google Scholar]
- Martinez-Garcia, M.F.; Grijalva-Chon, J.M.; Castro-Longoria, R.; de la Re-Vega, E.; Varela-Romero, A.; Chavez-Villalba, J.E. Prevalence and genotypic diversity of ostreid herpesvirus type 1 in Crassostrea gigas cultured in the Gulf of California, Mexico. Dis. Aquat. Org. 2020, 138, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Divilov, K.; Schoolfield, B.; Cortez, D.M.; Wang, X.S.; Fleener, G.B.; Jin, L.; Dumbauld, B.R.; Langdon, C. Genetic improvement of survival in Pacific oysters to the Tomales Bay strain of OsHV-1 over two cycles of selection. Aquaculture 2021, 543, 737020. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Park, J.J.; Yu, H.J.; Hur, Y.B.; Arzul, I.; Couraleau, Y.; Park, M.A. Ostreid herpesvirus 1 infection in farmed Pacific oyster larvae Crassostrea gigas (Thunberg) in Korea. J. Fish Dis. 2013, 36, 969–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mineur, F.; Provan, J.; Arnott, G. Phylogeographical analyses of shellfish viruses: Inferring a geographical origin for ostreid herpesviruses OsHV-1 (Malacoherpesviridae). Mar. Biol. 2015, 162, 181–192. [Google Scholar] [CrossRef]

| System | Type of Oyster | Number of Oysters Sampled on Day 12 | Number Positive for OsHV-1 DNA a | Viral Load b | Water Temperature Days 1 to 6 | Water Temperature Days 7 to 12 |
|---|---|---|---|---|---|---|
| 1 | PEad | 12 | 0 | - | 16.0 °C ± 0.3 °C | 23.1 °C ± 0.9 °C |
| NEsp1 | 100 | 0 | - | |||
| 2 | PEad | 12 | 2 | bloq | 15.3 °C ± 0.3 °C | 22.4 °C ± 0.9 °C |
| NEsp1 | 100 | 0 | - | |||
| 3 | PEad | 12 | 2 | bloq | 15.4 °C ± 0.3 °C | 22.6 °C ± 0.9 °C |
| NEsp1 | 100 | 0 | - | |||
| 4 | PEad | 36 | 1 | bloq | 15.6 °C ± 0.2 °C | 15.7 °C ± 0.3 °C |
| NEsp1 | 100 | 0 | - |
| System/Tank | Oysters | Challenged with OsHV-1 | Water Temperature Days 1 to 10 |
|---|---|---|---|
| 1 | PEad | Yes | 23.4 °C ± 0.4 °C |
| 2 | PEad | Yes | 22.9 °C ± 0.4 °C |
| 3 | PEad | Yes | 22.7 °C ± 0.3 °C |
| 4 | NEad | Yes | 23.0 °C ± 0.4 °C |
| SAT 1 | NEsp2 | Yes | 22.1 °C ± 1.4 °C |
| SAT 2 | NEad | No | 22.0 °C ± 1.0 °C |
| Treatment (Oysters Used) | Day Post Injection | Live or Dead | Aquarium System | No. Sampled | No. Positive OsHV-1 PCR | Viral Load a | ||
|---|---|---|---|---|---|---|---|---|
| Geometric Mean | Lower 95% CI | Upper 95% CI | ||||||
| Previously exposed adult oysters (PEad) challenged with OsHV-1 | 2 | dead | 2 | 1 | 1 | 2.14 × 104 | - | - |
| 7 | dead | 3 | 1 | 1 | 1.74 × 104 | - | - | |
| 9 | dead | 3 | 1 | 1 | 4.41 × 102 | - | - | |
| 10 | dead | 1 | 1 | 1 | 1.56 × 103 | - | - | |
| 4 | live | 1 | 12 | 10 | 4.44 × 102 | 3.30 × 102 | 5.96 × 102 | |
| 4 | live | 2 | 12 | 9 | 1.67 × 103 | 1.08 × 103 | 2.67 × 103 | |
| 4 | live | 3 | 12 | 11 | 8.91 × 102 | 7.01 × 102 | 1.13 × 103 | |
| 10 | live | 1 | 11 | 9 | 6.69 × 101 | 4.46 × 101 | 9.40 × 101 | |
| 10 | live | 2 | 11 | 9 | 9.58 × 101 | 8.76 × 101 | 1.05 × 102 | |
| 10 | live | 3 | 10 | 8 | 6.55 × 101 | 5.21 × 101 | 8.23 × 101 | |
| Non-exposed adult oysters (NEad) challenged with OsHV-1 | 2 | dead | 4 | 13 | 13 | 2.23 × 105 | 6.03 × 104 | 2.54 × 105 |
| 3 | dead | 4 | 6 | 6 | 1.69 × 105 | 3.15 × 103 | 2.52 × 105 | |
| 4 | dead | 4 | 2 | 2 | 8.84 × 104 | 4.60 × 103 | 1.70 × 106 | |
| 4 | live | 4 | 3 | 2 | 2.50 × 103 | 7.14 × 102 | 8.77 × 103 | |
| Positive control (NEsp2) challenged with OsHV-1 | 2 | dead | SAT 1 | 2 | 2 | 3.26 × 105 | 2.05 × 105 | 5.18 × 105 |
| 3 | dead | SAT 1 | 7 | 7 | 1.31 × 105 | 5.12 × 103 | 1.82 × 105 | |
| 4 | dead | SAT 1 | 1 | 1 | 3.37 × 105 | - | - | |
| 4 | live | SAT 1 | 2 | 2 | 8.39 × 102 | 1.16 × 102 | 6.07 × 103 | |
| Negative control (NEad) | 4 | live | SAT 2 | 6 | 0 | - | - | - |
| not exposed to OsHV-1 | 10 | live | SAT 2 | 6 | 0 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, O.M.; Hick, P.M.; Whittington, R.J. The Resistance to Lethal Challenge with Ostreid herpesvirus-1 of Pacific Oysters (Crassostrea gigas) Previously Exposed to This Virus. Viruses 2023, 15, 1706. https://doi.org/10.3390/v15081706
Liu OM, Hick PM, Whittington RJ. The Resistance to Lethal Challenge with Ostreid herpesvirus-1 of Pacific Oysters (Crassostrea gigas) Previously Exposed to This Virus. Viruses. 2023; 15(8):1706. https://doi.org/10.3390/v15081706
Chicago/Turabian StyleLiu, Olivia M., Paul M. Hick, and Richard J. Whittington. 2023. "The Resistance to Lethal Challenge with Ostreid herpesvirus-1 of Pacific Oysters (Crassostrea gigas) Previously Exposed to This Virus" Viruses 15, no. 8: 1706. https://doi.org/10.3390/v15081706
APA StyleLiu, O. M., Hick, P. M., & Whittington, R. J. (2023). The Resistance to Lethal Challenge with Ostreid herpesvirus-1 of Pacific Oysters (Crassostrea gigas) Previously Exposed to This Virus. Viruses, 15(8), 1706. https://doi.org/10.3390/v15081706






