Prevalence of Human Norovirus GII.4 Sydney 2012 [P31] between 2019 and 2021 among Young Children from Rural Communities in South Africa
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Study Population and Sample Collection
2.3. RNA Extraction and Human Norovirus Detection
2.4. Norovirus RT-PCR and Genotyping, ClustalW Alignment, and Phylogenetic Analysis
2.5. Statistical Analysis
3. Results
3.1. Human Norovirus Prevalence and Sample Characteristics
3.1.1. HNoV Genogroup and Genotype Distribution
3.1.2. Comparison of Nucleotide Sequences among HNoV Strains Circulating in Vhembe District, South Africa
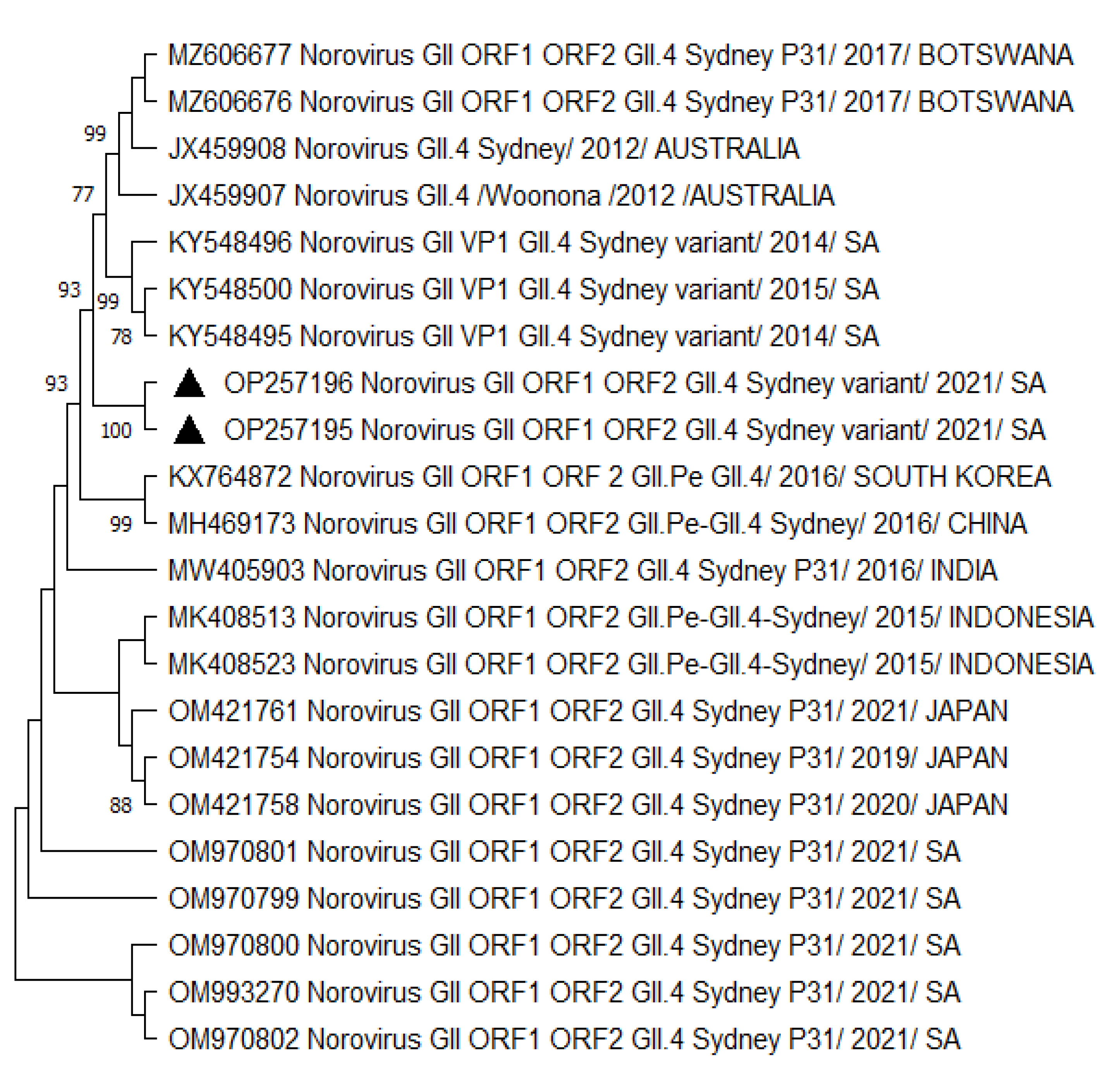
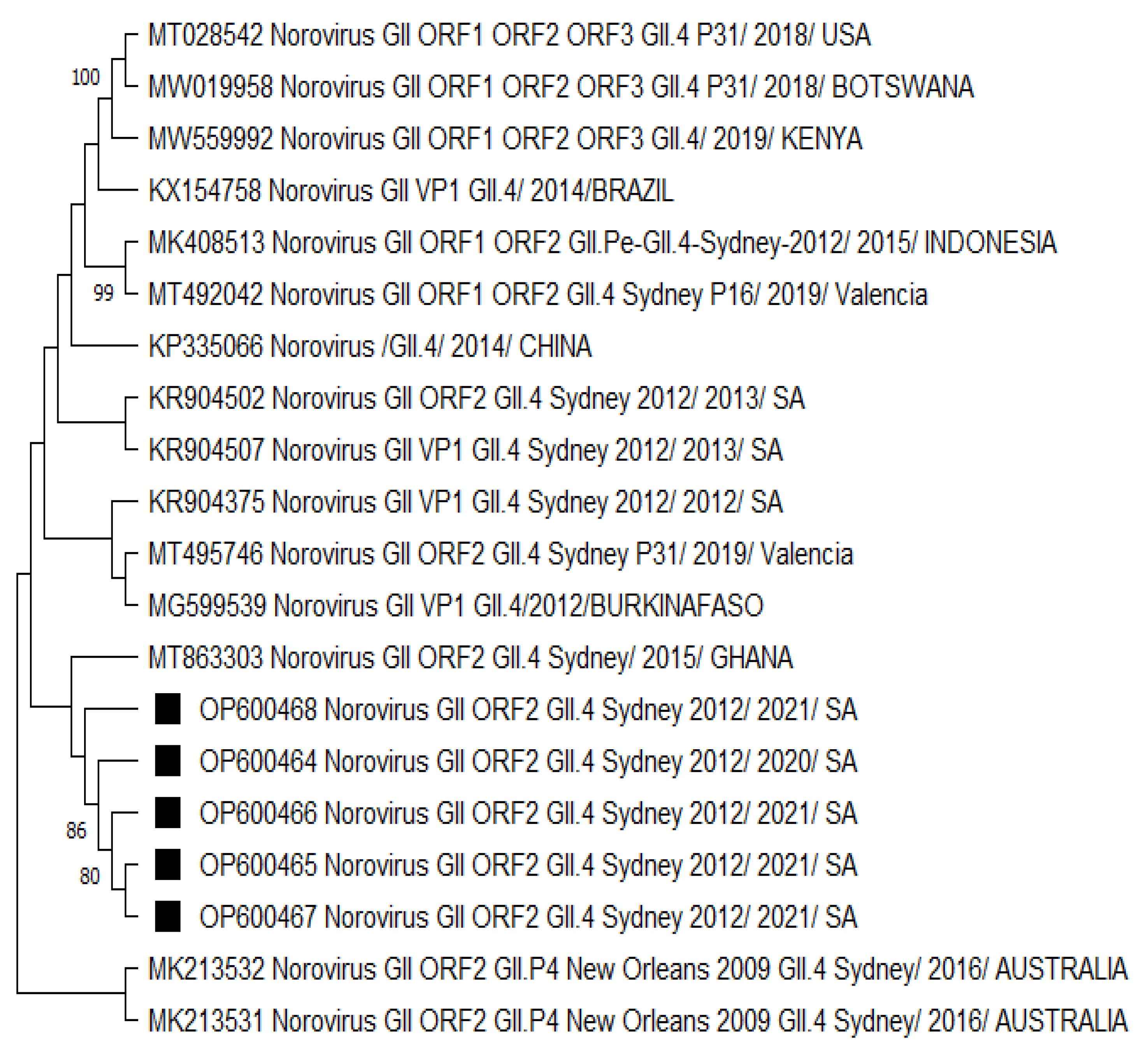
3.2. Phylogenetic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bányai, K.; Estes, M.K.; Martella, V.; Parashar, U.D. Viral gastroenteritis. Lancet 2018, 392, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.-W.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses—The State of the Art, Nearly Fifty Years after Their Initial Discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Lopman, B.A.; Steele, D.; Kirkwood, C.D.; Parashar, U.D. The Vast and Varied Global Burden of Norovirus: Prospects for Prevention and Control. PLoS Med. 2016, 13, e1001999. [Google Scholar] [CrossRef]
- Mans, J. Norovirus infections and disease in lower-middle-and low-income countries, 1997–2018. Viruses 2019, 11, 341. [Google Scholar] [CrossRef]
- Nguyen, G.T.; Phan, K.; Teng, I.; Pu, J.; Watanabe, T. A systematic review and meta-analysis of the prevalence of no-rovirus in cases of gastroenteritis in developing countries. Medicine 2017, 96, e8139. [Google Scholar] [CrossRef]
- Afework, D.T.; Shumie, M.K.; Endalew, G.F.; Adugna, A.G.; Tarekegn, B.G. Pooled prevalence and genetic diversity of norovirus in Africa: A systematic review and meta-analysis. Virol. J. 2022, 19, 115. [Google Scholar] [CrossRef]
- Farahmand, M.; Moghoofei, M.; Dorost, A.; Shoja, Z.; Ghorbani, S.; Kiani, S.J.; Khales, P.; Esteghamati, A.; Sayyahfar, S.; Jafarzadeh, M.; et al. Global prevalence and genotype distribution of norovirus infection in children with gas-troenteritis: A meta-analysis on 6 years of research from 2015 to 2020. Rev. Med. Virol. 2022, 32, e2237. [Google Scholar] [CrossRef]
- Ahmed, S.M.; Hall, A.J.; Robinson, A.E.; Verhoef, L.; Premkumar, P.; Parashar, U.D.; Koopmans, M.; Lopman, B.A. Global prevalence of norovirus in cases of gastroenteritis: A systematic review and meta-analysis. Lancet Infect. Dis. 2014, 14, 725–730. [Google Scholar] [CrossRef]
- Lartey, B.L.; Quaye, O.; Damanka, S.A.; Agbemabiese, C.A.; Armachie, J.; Dennis, F.E.; Enweronu-Laryea, C.; Armah, G.E. Understanding Pediatric Norovirus Epidemiology: A Decade of Study among Ghanaian Children. Viruses 2020, 12, 1321. [Google Scholar] [CrossRef]
- Japhet, M.O.; Adesina, O.A.; Famurewa, O.; Svensson, L.; Nordgren, J. Molecular epidemiology of rotavirus and no-rovirus in Ile-Ife, Nigeria: High prevalence of G12P [8] rotavirus strains and detection of a rare norovirus genotype. J. Med. Virol. 2012, 84, 1489–1496. [Google Scholar] [CrossRef]
- Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Estes, M.K.; Crawford, S.E.; Neill, F.H.; Graham, D.Y. Norwalk Virus Shedding after Experimental Human Infection. Emerg. Infect. Dis. 2008, 14, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Thorne, L.G.; Goodfellow, I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014, 95, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, M.; Wang, K.; Estes, M.K. Sequence and Genomic Organization of Norwalk Virus. Virology 1993, 195, 51–61. [Google Scholar] [CrossRef]
- Robilotti, E.; Deresinski, S.; Pinsky, B.A. Norovirus. Clin. Microbiol. Rev. 2015, 28, 134–164. [Google Scholar] [CrossRef]
- Tan, M. Norovirus Vaccines: Current Clinical Development and Challenges. Pathogens 2021, 10, 1641. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; De Graaf, M.; Parra, G.I.; Chan, M.C.-W.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef]
- Bucardo, F.; Reyes, Y.; Becker-Dreps, S.; Bowman, N.; Gruber, J.F.; Vinjé, J.; Espinoza, F.; Paniagua, M.; Balmaseda, A.; Svensson, L.; et al. Pediatric norovirus GII. 4 infections in Nicaragua, 1999–2015. Infect. Genet. Evol. 2017, 55, 305–312. [Google Scholar] [CrossRef]
- Winder, N.; Gohar, S.; Muthana, M. Norovirus: An Overview of Virology and Preventative Measures. Viruses 2022, 14, 2811. [Google Scholar] [CrossRef]
- Ai, J.; Zhang, M.; Jin, F.; Xie, Z. Recombinant GII. 4 [P31] Was Predominant Norovirus Circulating in Beijing Area, China, 2018–2020. Virol. Sin. 2021, 36, 1245–1247. [Google Scholar] [CrossRef]
- Duan, L.; Yang, X.; Xie, J.; Zhan, W.; Zhang, C.; Liu, H.; Wei, M.; Tang, Y.; Zhao, H.; Luo, M. Prevalence of GII. 4 Sydney Norovirus Strains and Associated Factors of Acute Gastroenteritis in Children: 2019/2020 Season in Guangzhou, China. Food Environ. Virol. 2021, 13, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; He, Y.; Wei, X.; Kong, X.; Zhang, Q.; Li, J.; Jin, M.; Duan, Z. Molecular Epidemiological Characteristics of Gas-troenteritis Outbreaks Caused by Norovirus GII. 4 Sydney [P31] Strains—China, October 2016–December 2020. China CDC Wkly. 2021, 3, 1127. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; de Ávila, A.I.; Gallego, I.; Sheldon, J.; Perales, C. Viral fitness: History and relevance for viral pathogenesis and antiviral interventions. Pathog. Dis. 2019, 77, ftz021. [Google Scholar] [CrossRef] [PubMed]
- Wargo, A.R.; Kurath, G. Viral fitness: Definitions, measurement, and current insights. Curr. Opin. Virol. 2012, 2, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E. Mechanisms of viral emergence. Veter. Res. 2010, 41, 38. [Google Scholar] [CrossRef]
- Rossouw, E.; Brauer, M.; Meyer, P.; Du Plessis, N.M.; Avenant, T.; Mans, J. Virus etiology, diversity and clinical charac-teristics in South African children hospitalised with gastroenteritis. Viruses 2021, 13, 215. [Google Scholar] [CrossRef]
- Kabue, J.P.; Meader, E.; Hunter, P.R.; Potgieter, N. Norovirus prevalence and estimated viral load in symptomatic and asymptomatic children from rural communities of Vhembe district, South Africa. J. Clin. Virol. 2016, 84, 12–18. [Google Scholar] [CrossRef]
- Potgieter, N.; Banda, N.T.; Becker, P.J.; Traore-Hoffman, A.N. WASH infrastructure and practices in primary health care clinics in the rural Vhembe District municipality in South Africa. BMC Fam. Pract. 2021, 22, 8. [Google Scholar] [CrossRef]
- World Health Organization. Treatment of Diarrhea: A Manual for Physicians and Senior Health Workers; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Boom, R.; Sol, C.J.; Salimans, M.M.; Jansen, C.L.; Dillen, P.M.W.-V.; van der Noordaa, J. Rapid and simple method for purification of nucleic acids. J. Clin. Microbiol. 1990, 28, 495–503. [Google Scholar] [CrossRef]
- Dunbar, N.L.; Bruggink, L.D.; Marshall, J.A. Evaluation of the RIDAGENE real-time PCR assay for the detection of GI and GII norovirus. Diagn. Microbiol. Infect. Dis. 2014, 79, 317–321. [Google Scholar] [CrossRef]
- Chhabra, P.; Browne, H.; Huynh, T.; Diez-Valcarce, M.; Barclay, L.; Kosek, M.N.; Ahmed, T.; Lopez, M.R.; Pan, C.-Y.; Vinjé, J. Single-step RT-PCR assay for dual genotyping of GI and GII norovirus strains. J. Clin. Virol. 2020, 134, 104689. [Google Scholar] [CrossRef]
- Cantelli, C.P.; Fumian, T.M.; Malta, F.C.; da Cunha, D.C.; Brasil, P.; Nordgren, J.; Svensson, L.; Miagostovich, M.P.; de Moraes, M.T.B.; Leite, J.P.G. Norovirus infection and HBGA host genetic susceptibility in a birth community-cohort, Rio de Janeiro, Brazil. Infect. Genet. Evol. 2020, 82, 104280. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Kageyama, T.; Fukushi, S.; Hoshino, F.B.; Shinohara, M.; Uchida, K.; Natori, K.; Takeda, N.; Katayama, K. Genogroup-specific PCR primers for detection of Norwalk-like viruses. J. Virol. Methods 2001, 100, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.D.; Garrett, V.D.; Sobel, J.; Monroe, S.S.; Fankhauser, R.L.; Schwab, K.J.; Bresee, J.S.; Mead, P.S.; Higgins, C.; Campana, J.; et al. Multistate Outbreak of Norwalk-like Virus Gastroenteritis Associated with a Common Caterer. Am. J. Epidemiol. 2001, 154, 1013–1019. [Google Scholar] [CrossRef]
- Kageyama, T.; Kojima, S.; Shinohara, M.; Uchida, K.; Fukushi, S.; Hoshino, F.B.; Takeda, N.; Katayama, K. Broadly Reactive and Highly Sensitive Assay for Norwalk-Like Viruses Based on Real-Time Quantitative Reverse Transcription-PCR. J. Clin. Microbiol. 2003, 41, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Kabue, J.P.; Meader, E.; Hunter, P.R.; Potgieter, N. Genetic characterisation of Norovirus strains in outpatient children from rural communities of Vhembe district/South Africa, 2014–2015. J. Clin. Virol. 2017, 94, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, O.; Zhang, W.; Amponsem-Boateng, C.; Oppong, T.B.; Zhao, Q.; Li, D. Evidence of rotavirus vaccine impact in sub-Saharan Africa: Systematic review and meta-analysis. PLoS ONE 2020, 15, e0232113. [Google Scholar] [CrossRef]
- Omore, R.; Powell, H.; Sow, S.O.; Hossain, M.J.; Ogwel, B.; Doh, S.; Ochieng, J.B.; Jones, J.C.M.; Zaman, S.M.A.; Awuor, A.O.; et al. Norovirus Disease Among Children <5 Years in 3 Sub-Saharan African Countries: Findings from the Vaccine Impact on Diarrhea in Africa (VIDA) Study, 2015–2018. Clin. Infect. Dis. 2023, 76, S114–S122. [Google Scholar] [CrossRef]
- Cohen, A.L.; Platts-Mills, J.A.; Nakamura, T.; Operario, D.J.; Antoni, S.; Mwenda, J.M.; Weldegebriel, G.; Rey-Benito, G.; de Oliveira, L.H.; Ortiz, C.; et al. Aetiology and incidence of diarrhoea requiring hospitalisation in children under 5 years of age in 28 low-income and middle-income countries: Findings from the Global Pediatric Diarrhea Surveillance network. BMJ Glob. Health 2022, 7, e009548. [Google Scholar] [CrossRef]
- Mathew, S.; Alansari, K.; Smatti, M.K.; Zaraket, H.; Al Thani, A.A.; Yassine, H.M. Epidemiological, molecular, and clinical features of norovirus infections among pediatric patients in Qatar. Viruses 2019, 11, 400. [Google Scholar] [CrossRef]
- Wang, P.-L.; Chen, S.-Y.; Tsai, C.-N.; Chao, H.-C.; Lai, M.-W.; Chang, Y.-J.; Chen, C.-L.; Chiu, C.-H. Complicated norovirus infection and assessment of severity by a modified Vesikari disease score system in hospitalized children. BMC Pediatr. 2016, 16, 162. [Google Scholar] [CrossRef]
- Rouhani, S.; Peñataro Yori, P.; Paredes Olortegui, M.; Siguas Salas, M.; Rengifo Trigoso, D.; Mondal, D.; Bodhidatta, L.; Platts-Mills, J.; Samie, A.; Kabir, F.; et al. Norovirus infection and acquired immunity in 8 countries: Results from the MAL-ED study. Clin. Infect. Dis. 2016, 62, 1210–1217. [Google Scholar] [CrossRef]
- Papaventsis, D.C.; Dove, W.; Cunliffe, N.A.; Nakagomi, O.; Combe, P.; Grosjean, P.; Hart, C.A. Norovirus Infection in Children with Acute Gastroenteritis, Madagascar, 2004–2005. Emerg. Infect. Dis. 2007, 13, 908–911. [Google Scholar] [CrossRef]
- McCormick, B.J.; Richard, S.A.; Murray-Kolb, L.E.; Kang, G.; Lima, A.A.; Mduma, E.; Kosek, M.N.; McQuade, E.T.R.; Houpt, E.R.; Bessong, P.; et al. Full breastfeeding protection against common enteric bacteria and viruses: Results from the MAL-ED cohort study. Am. J. Clin. Nutr. 2022, 115, 759–769. [Google Scholar] [CrossRef]
- North, K.; Gao, M.; Allen, G.; Lee, A.C. Breastfeeding in a Global Context: Epidemiology, Impact, and Future Directions. Clin. Ther. 2021, 44, 228–244. [Google Scholar] [CrossRef] [PubMed]
- Labayo, H.K.M.; Pajuelo, M.J.; Tohma, K.; Ford-Siltz, L.A.; Gilman, R.H.; Cabrera, L.; Mayta, H.; Sanchez, G.J.; Cornejo, A.T.; Bern, C.; et al. Norovirus-specific immunoglobulin A in breast milk for protection against norovirus-associated di-arrhea among infants. EClinicalMedicine 2020, 27, 100561. [Google Scholar] [CrossRef]
- Vielot, N.A.; François, R.; Huseynova, E.; González, F.; Reyes, Y.; Gutierrez, L.; Nordgren, J.; Toval-Ruiz, C.; Vilchez, S.; Vinjé, J.; et al. Association between breastfeeding, host genetic factors, and calicivirus gastroenteritis in a Nicaraguan birth cohort. PLoS ONE 2022, 17, e0267689. [Google Scholar] [CrossRef] [PubMed]
- Haddadin, Z.; Batarseh, E.; Hamdan, L.; Stewart, L.S.; Piya, B.; Rahman, H.; Spieker, A.J.; Chappell, J.E.; Wikswo, M.; Dunn, J.R.; et al. Characteristics of GII.4 Norovirus Versus Other Genotypes in Sporadic Pediatric Infections in Davidson County, Tennessee, USA. Clin. Infect. Dis. 2020, 73, e1525–e1531. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infant and Young Child Feeding. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/infant-and-young-child-feeding (accessed on 18 February 2023).
- Ghosh, S.; Kumar, M.; Santiana, M.; Mishra, A.; Zhang, M.; Labayo, H.; Chibly, A.M.; Nakamura, H.; Tanaka, T.; Henderson, W.; et al. Enteric viruses replicate in salivary glands and infect through saliva. Nature 2022, 607, 345–350. [Google Scholar] [CrossRef]
- Jaillon, S.; Berthenet, K.; Garlanda, C. Sexual dimorphism in innate immunity. Clin. Rev. Allergy Immunol. 2019, 56, 308–321. [Google Scholar] [CrossRef]
- Luvhimbi, N.; Tshitangano, T.G.; Mabunda, J.T.; Olaniyi, F.C.; Edokpayi, J.N. Water quality assessment and evaluation of human health risk of drinking water from source to point of use at Thulamela municipality, Limpopo Province. Sci. Rep. 2022, 12, 6059. [Google Scholar] [CrossRef]
- Ayukekbong, J.A.; Andersson, M.E.; Vansarla, G.; Tah, F.; Nkuo-Akenji, T.; Lindh, M.; Bergström, T. Monitoring of seasonality of norovirus and other enteric viruses in Cameroon by real-time PCR: An exploratory study. Epidemiol. Infect. 2014, 142, 1393–1402. [Google Scholar] [CrossRef]
- Duizer, E.; Bijkerk, P.; Rockx, B.; De Groot, A.; Twisk, F.; Koopmans, M. Inactivation of caliciviruses. Appl. Environ. Microbiol. 2004, 70, 4538–4543. [Google Scholar] [CrossRef] [PubMed]
- O’reilly, K.M.; Sandman, F.; Allen, D.; Jarvis, C.I.; Gimma, A.; Douglas, A.; Larkin, L.; Wong, K.L.M.; Baguelin, M.; Baric, R.S.; et al. Predicted norovirus resurgence in 2021–2022 due to the relaxation of nonpharmaceutical interventions associated with COVID-19 restrictions in England: A mathematical modeling study. BMC Med. 2021, 19, 299. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, Z.; Xie, H.; Su, W.; Wang, H.; Wang, D.; Lu, J. The Rise in Norovirus-Related Acute Gastroenteritis During the Fight Against the COVID-19 Pandemic in Southern China. Front. Public Health 2022, 9, 785373. [Google Scholar] [CrossRef]
- Lennon, R.P.; Griffin, C.; Miller, E.L.; Dong, H.; Rabago, D.; Zgierska, A.E. Norovirus Infections Drop 49% in the United States with Strict COVID-19 Public Health Interventions. Acta Medica Acad. 2021, 49, 278–280. [Google Scholar] [CrossRef] [PubMed]
- Pham, N.T.K.; Nishimura, S.; Shimizu-Onda, Y.; Trinh, Q.D.; Komine-Aizawa, S.; Khamrin, P.; Okitsu, S.; Sato, S.; Kobayashi, T.; Maneekarn, N.; et al. Emerging norovirus GII. 4 Sydney [P31] causing acute gastroenteritis outbreak in children in Japan, during COVID-19, 2021. J. Infect. Chemother. 2022, 28, 1347–1351. [Google Scholar] [CrossRef]
- Kreidieh, K.; Charide, R.; Dbaibo, G.; Melhem, N.M. The epidemiology of Norovirus in the Middle East and North Africa (MENA) region: A systematic review. Virol. J. 2017, 14, 220. [Google Scholar] [CrossRef]
- Page, N.A.; Groome, M.J.; Nadan, S.; Netshikweta, R.; Keddy, K.H.; Poonsamy, B.; Moyes, J.; Walaza, S.; Kahn, K.; Madhi, S.A.; et al. Norovirus epidemiology in South African children <5 years hospitalised for diarrhoeal illness between 2009 and 2013. Epidemiol. Infect. 2017, 145, 1942–1952. [Google Scholar] [CrossRef]
- Cornejo-Sánchez, T.; Soldevila, N.; Coronas, L.; Alsedà, M.; Godoy, P.; Razquín, E.; Sabaté, S.; Guix, S.; Garrido, V.R.; Bartolomé, R.; et al. Epidemiology of GII.4 and GII.2 norovirus outbreaks in closed and semi-closed institutions in 2017 and 2018. Sci. Rep. 2023, 13, 1659. [Google Scholar] [CrossRef]
- Kitajima, M.; Haramoto, E.; Phanuwan, C.; Katayama, H.; Furumai, H. Molecular detection and genotyping of human noroviruses in influent and effluent water at a wastewater treatment plant in Japan. J. Appl. Microbiol. 2012, 112, 605–613. [Google Scholar] [CrossRef]
- van Beek, J.; de Graaf, M.; Al-Hello, H.; Allen, D.J.; Ambert-Balay, K.; Botteldoorn, N.; Brytting, M.; Buesa, J.; Cabrerizo, M.; Chan, M.; et al. Molecular surveillance of norovirus, 2005–2016: An epidemiological analysis of data collected from the NoroNet network. Lancet Infect. Dis. 2018, 18, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Noel, J.S.; Fankhauser, R.L.; Ando, T.; Monroe, S.S.; Glass, R.I. Identification of a distinct common strain of “Norwalk-like viruses” having a global distribution. J. Infect. Dis. 1999, 179, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Widdowson, M.-A.; Cramer, E.H.; Hadley, L.; Bresee, J.S.; Beard, R.S.; Bulens, S.N.; Charles, M.; Chege, W.; Isakbaeva, E.; Wright, J.G.; et al. Outbreaks of Acute Gastroenteritis on Cruise Ships and on Land: Identification of a Predominant Circulating Strain of Norovirus—United States, 2002. J. Infect. Dis. 2004, 190, 27–36. [Google Scholar] [CrossRef]
- Fankhauser, R.L.; Monroe, S.S.; Noel, J.S.; Humphrey, C.D.; Bresee, J.S.; Parashar, U.D.; Ando, T.; Glass, R.I. Epidemi-ologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J. Infect. Dis. 2002, 186, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Parra, G.I.; Tohma, K.; Ford-Siltz, L.A.; Eguino, P.; Kendra, J.A.; Pilewski, K.A.; Gao, Y. Minimal Antigenic Evolution after a Decade of Norovirus GII.4 Sydney_2012 Circulation in Humans. J. Virol. 2023, 97, e0171622. [Google Scholar] [CrossRef]
- Desai, R.; Hembree, C.D.; Handel, A.; Matthews, J.E.; Dickey, B.W.; McDonald, S.; Hall, A.J.; Parashar, U.D.; Leon, J.S.; Lopman, B. Severe Outcomes Are Associated with Genogroup 2 Genotype 4 Norovirus Outbreaks: A Systematic Literature Review. Clin. Infect. Dis. 2012, 55, 189–193. [Google Scholar] [CrossRef]
- Bull, R.A.; Eden, J.-S.; Luciani, F.; McElroy, K.; Rawlinson, W.D.; White, P.A. Contribution of Intra- and Interhost Dynamics to Norovirus Evolution. J. Virol. 2012, 86, 3219–3229. [Google Scholar] [CrossRef]
- Bull, R.A.; Eden, J.S.; Rawlinson, W.D.; White, A. Rapid evolution of pandemic noroviruses of the GII. 4 lineage. PLoS Pathog. 2010, 6, e1000831. [Google Scholar] [CrossRef]
- McInerney, P.; Adams, P.; Hadi, M.Z. Error Rate Comparison during Polymerase Chain Reaction by DNA Polymerase. Mol. Biol. Int. 2014, 2014, 287430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-L.; Chen, L.-N.; Wang, S.-M.; Tan, M.; Qiu, C.; Qiu, T.-Y.; Wang, X.-Y. Prevalence and Evolution of Noroviruses between 1966 and 2019, Implications for Vaccine Design. Pathogens 2021, 10, 1012. [Google Scholar] [CrossRef] [PubMed]
- Makhaola, K.; Moyo, S.; Kebaabetswe, L.P. Next generation sequencing of near-full length genome of norovirus GII.4 from Botswana. Virus Res. 2021, 302, 198491. [Google Scholar] [CrossRef] [PubMed]

| Symptomatic | Asymptomatic | |||
|---|---|---|---|---|
| Total (%) | HNoV+ (%) | Total (%) | HNoV+ (%) | |
| Detection rate (%) | n = 200 | n = 74 (37) | n = 100 | n = 14 (14) |
| Setting | ||||
| Outpatients | 104 (52) | 30 (41) | 100 (100) | 14 (14) |
| Hospitalised | 96 (48) | 44 (59) | 0 | 0 |
| Gender | ||||
| Males | 116 (58) | 49 (66) | 53 (53) | 8 (57) |
| Females | 84 (42) | 25 (34) | 47 (47) | 6 (43) |
| Age in months | ||||
| 0–5 | 49 (25) | 15 (20) | 21 (21) | 2 (14) |
| 6–23 | 124 (62) | 48 (65) | 59 (59) | 10 (71) |
| 24–60 | 27 (14) | 11 (15) | 20 (20) | 2 (14) |
| Symptoms | ||||
| Diarrhoea only | 48 (24) | 8 (11) | ||
| Diarrhoea and other symptoms | 152 (76) | 66 (89) | ||
| Other symptoms seen together with diarrhoea | ||||
| Dehydration | 132 (66) | 61 (82) | ||
| Vomiting | 119 (60) | 54 (73) | ||
| Fever | 79 (40) | 36 (49) | ||
| Abdominal pain | 29 (15) | 15 (20) | ||
| Stool appearance | ||||
| Watery | 103 (52) | 46 (62) | ||
| Formed | 97 (49) | 28 (38) | 100 (100) | 14 (14) |
| Duration of diarrhoea | ||||
| 3 days | 163 (82) | 62 (84) | ||
| >3 days | 36 (18) | 12 (16) | ||
| Interval | ||||
| 1–3 days | 161 (81) | 61 (82) | ||
| 3 days + | 39 (20) | 13 (18) | ||
| Living conditions | ||||
| Treated water | 131 (66) | 48 (65) | 73 (73) | 10 (71) |
| Untreated water | 37 (19) | 11 (15) | 13 (13) | 4 (29) |
| Mixed | 32 (16) | 15 (20) | 14 (14) | |
| Pit toilets | 111 (56) | 41 (55) | ||
| Flush toilets | 89 (46) | 33 (45) | ||
| Breastfeeding | 130 (65) | 51 (69) | 63 (63) | 10 (71) |
| Not breastfed | 70 (35) | 23 (31) | 37 (37) | 4 (29) |
| Livestock | 67 (34) | 29 (39) | 32 (32) | 3 (21) |
| No Livestock | 21 (11) | 9 (12) | 7 (7) | 1 (7) |
| Symptomatic | Asymptomatic | |||
|---|---|---|---|---|
| Outpatients | Inpatients | |||
| n = 200 (%) | n = 104 (%) | n = 96 (%) | n = 100 (%) | |
| Total HNoV+ | 74 (37) | 30 (41) | 44 (60) | 14 (14) |
| Genogroups | ||||
| GI | 9 (12) | 4 (12) | 5 (11) | 9 (64) |
| GII | 59 (80) | 21 (70) | 38 (86) | 5 (36) |
| Mixed | 5 (7) | 2 (7) | 3 (7) | |
| GII Genotypes | 32 | |||
| GII.4 Sydney 2012 [P31] | 19 (59) | 5 (26) | 14 (74) | 2 (40) |
| GII.4 Sydney 2012 | 13 (41) | 6 (46) | 7 (54) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khumela, R.; Kabue, J.-P.; Moraes, M.T.B.d.; Traore, A.N.; Potgieter, N. Prevalence of Human Norovirus GII.4 Sydney 2012 [P31] between 2019 and 2021 among Young Children from Rural Communities in South Africa. Viruses 2023, 15, 1682. https://doi.org/10.3390/v15081682
Khumela R, Kabue J-P, Moraes MTBd, Traore AN, Potgieter N. Prevalence of Human Norovirus GII.4 Sydney 2012 [P31] between 2019 and 2021 among Young Children from Rural Communities in South Africa. Viruses. 2023; 15(8):1682. https://doi.org/10.3390/v15081682
Chicago/Turabian StyleKhumela, Ronewa, Jean-Pierre Kabue, Marcia Terezinha Baroni de Moraes, Afsatou Ndama Traore, and Natasha Potgieter. 2023. "Prevalence of Human Norovirus GII.4 Sydney 2012 [P31] between 2019 and 2021 among Young Children from Rural Communities in South Africa" Viruses 15, no. 8: 1682. https://doi.org/10.3390/v15081682
APA StyleKhumela, R., Kabue, J.-P., Moraes, M. T. B. d., Traore, A. N., & Potgieter, N. (2023). Prevalence of Human Norovirus GII.4 Sydney 2012 [P31] between 2019 and 2021 among Young Children from Rural Communities in South Africa. Viruses, 15(8), 1682. https://doi.org/10.3390/v15081682






