Clinical Utility of SARS-CoV-2 Antibody Titer Multiplied by Binding Avidity of Receptor-Binding Domain (RBD) in Monitoring Protective Immunity and Clinical Severity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Plasma Samples
2.2. Ethics Statement
2.3. Antigens and Reagents
2.4. Anti-Spike S1 IgG Quantification
2.5. RBD Binding Avidity Measurement of Anti-Spike S1 IgG Antibodies
2.6. Neutralizing Activity of Antibodies against Pseudovirus
2.7. Statistical Analysis
3. Results
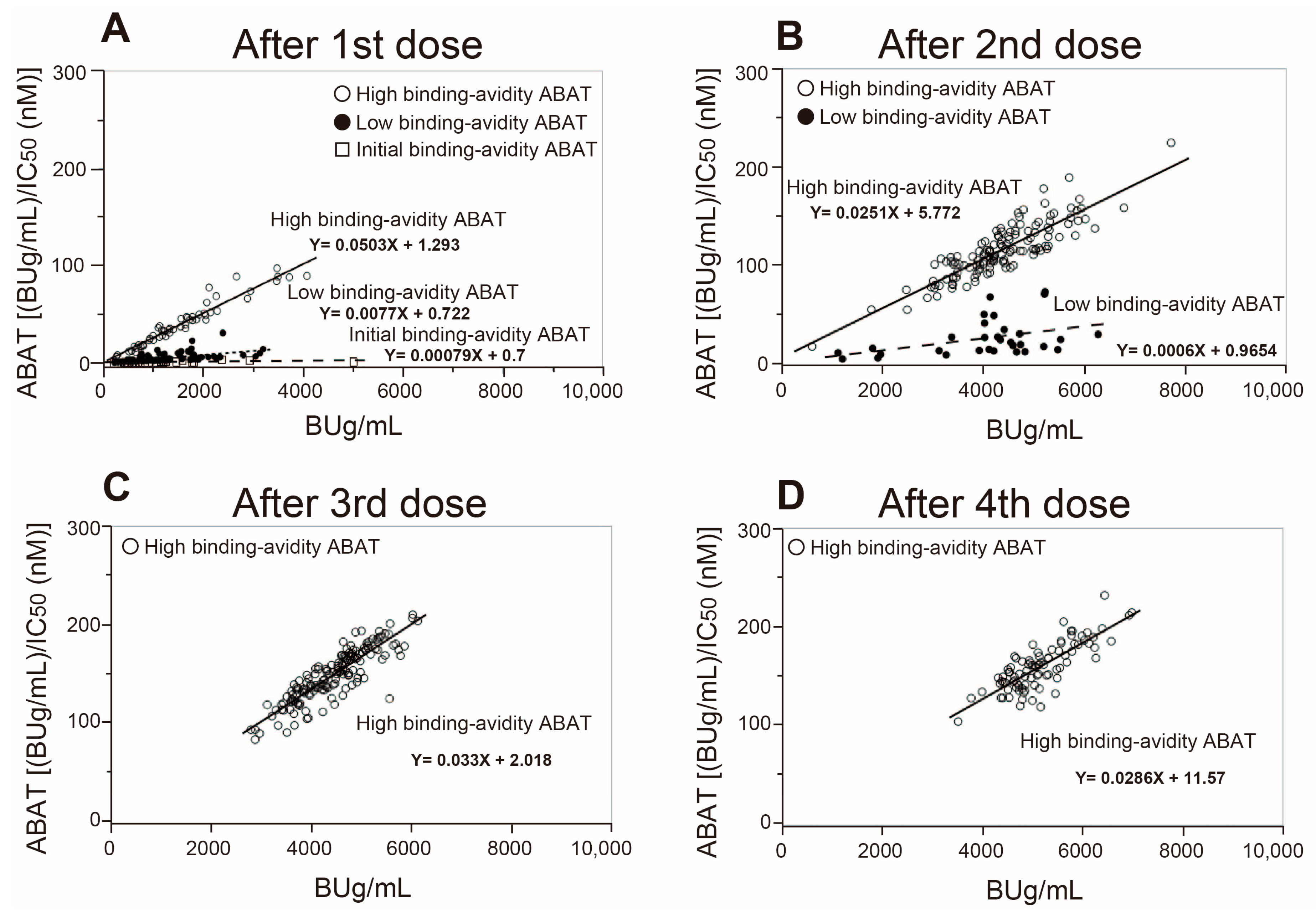
3.1. Changes in Anti-SARS-CoV-2 Spike S1 Specific IgG Antibody Titer and Its RBD Binding Avidity in Vaccinated Subjects and SARS-CoV-2-Infected Patients
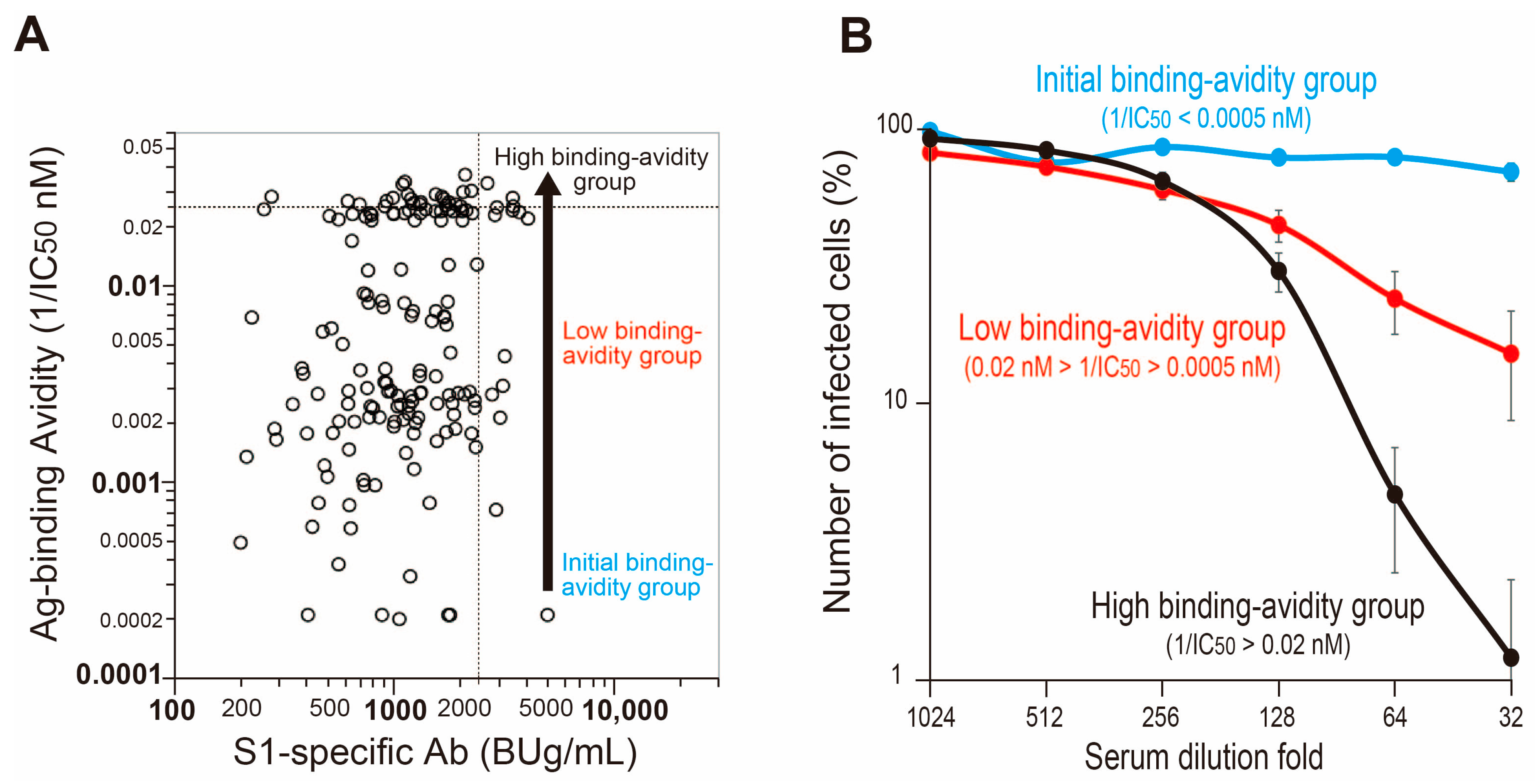
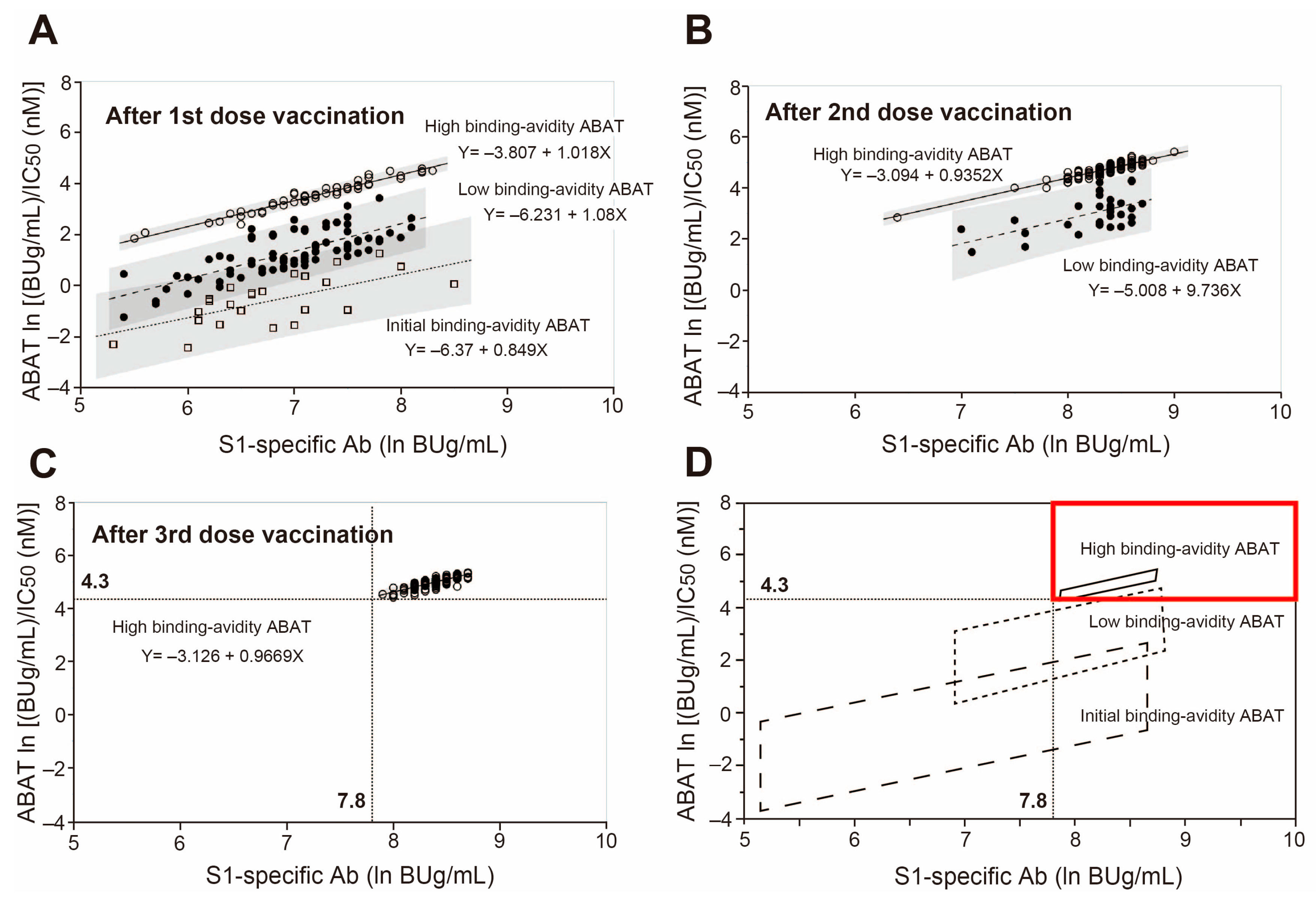
3.2. Three-Stage Maturation of RBD Binding Avidity of Anti-SARS-CoV-2 Antibodies Provides Protection against Infection
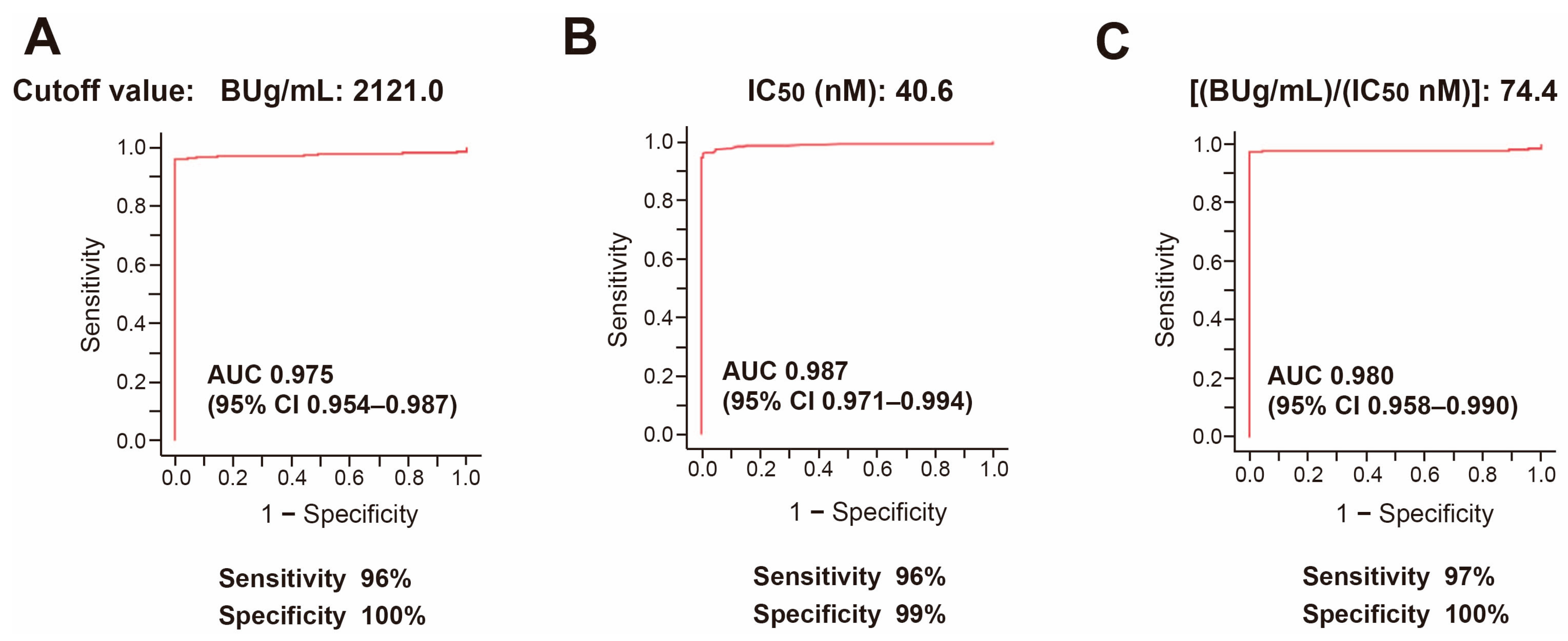
3.3. Receiver Operating Characteristic (ROC) Analysis to Determine Cutoff Values of Anti-SARS-CoV-2 Antibodies of Vaccinated and Nonhospitalized Subjects from Nonvaccinated and Hospitalized COVID-19 Patients
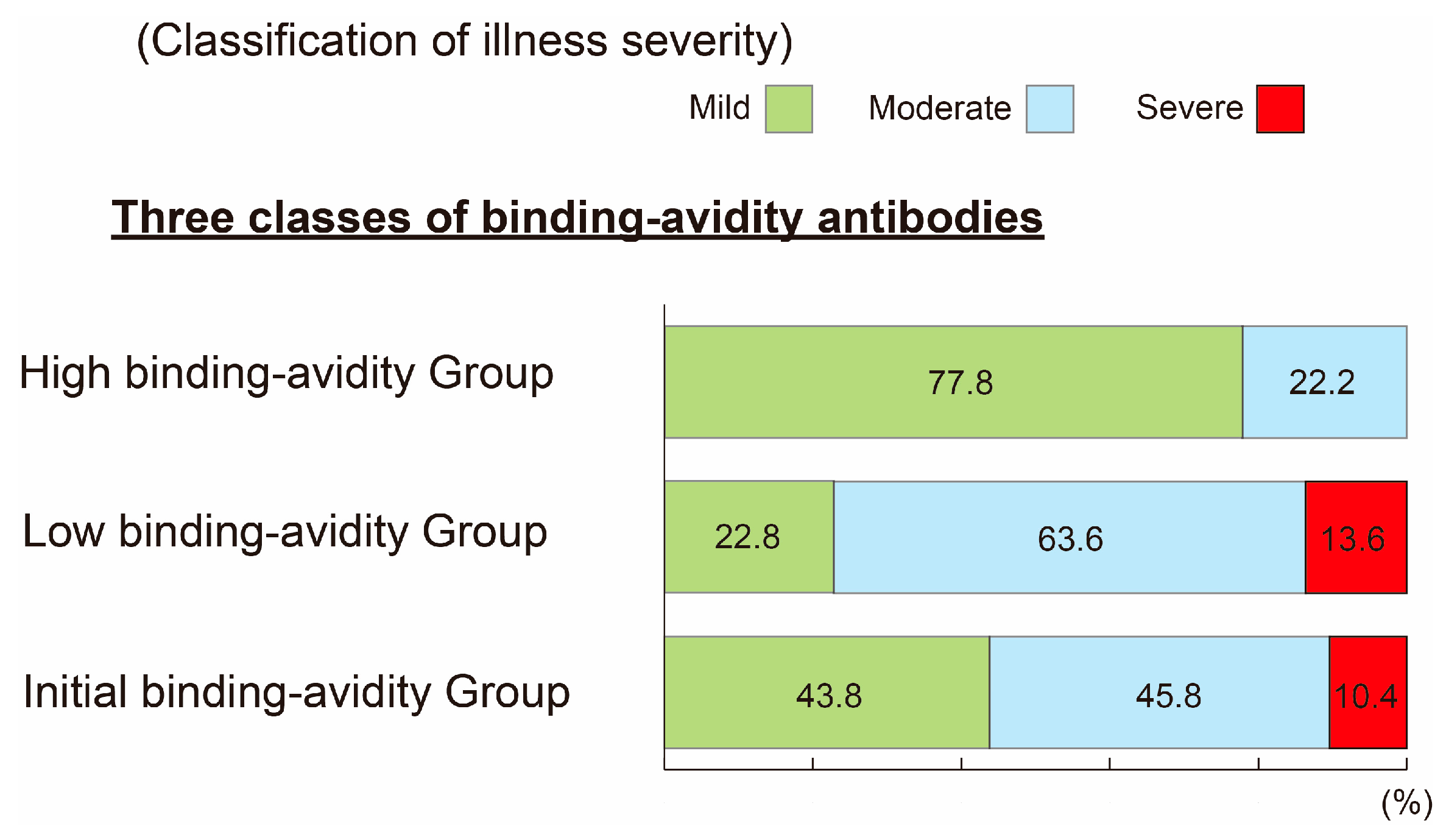
3.4. Analysis of Relationship between ABAT Levels on Admission and Severity of SARS-CoV-2 Infection Outcome after 2–3 Weeks of Hospitalization
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ou, X.; Liu, Y.; Lei, X.; Li, P.; Mi, D.; Ren, L.; Guo, L.; Guo, R.; Chen, T.; Hu, J.; et al. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Khatri, I.; Staal, F.J.T.; van Dongen, J.I.M. Blocking of the high-affinity interaction synapse between SARS-CoV-2 spike and human ACE2 proteins likely requires multiple high-affinity antibodies: An immune perspective. Front. Immunol. 2020, 11, 570018. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Kalinke, U.; Althage, A.; Freer, G.; Burkhart, C.; Roost, H.-P.; Agurt, M.; Hengartner, H.; Zinkernagel, R.M. The role of antibody concentration and avidity in antiviral protection. Science 1997, 276, 2024–2027. [Google Scholar] [CrossRef]
- Victora, G.D.; Nussenzweig, M.C. Germinal centers. Annu. Rev. Immunol. 2012, 30, 429–457. [Google Scholar] [CrossRef]
- Struck, F.; Schreiner, P.; Staschik, E.; Wochinz-Richter, K.; Schulz, S.; Soutschek, E.; Motz, M.; Bauer, G. Vaccination versus infection with SARS-CoV-2: Establishment of a high avidity IgG response versus incomplete avidity maturation. J. Med. Virol. 2021, 93, 6765–6777. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G. High avidity of vaccine-induced immunoglobulin G against SARS-CoV-2: Potential relevance for protective humoral immunity. Explor. Immunol. 2022, 2, 133–156. [Google Scholar] [CrossRef]
- Tauzin, A.; Gendron-Lepage, G.; Nayrac, M.; Anand, S.P.; Bourassa, C.; Medjahed, H.; Goyette, G.; Dubé, M.; Bazin, R.; Kaufmann, D.E.; et al. Evolution of anti-RBD IgG avidity following SARS-CoV-2 infection. Viruses 2022, 14, 532. [Google Scholar] [CrossRef]
- Bauer, G.; Struck, F.; Sicherer, P.; Staschik, E.; Soutschek, E.; Motz, M. The challenge of avidity determination in SARS-CoV-2 serology. J. Med. Virol. 2021, 93, 3092–3104. [Google Scholar] [CrossRef] [PubMed]
- Nakagama, Y.; Candray, K.; Kaku, N.; Komase, Y.; Rodriguez-Funes, M.-V.; Dominguez, R.; Tsuchida, T.; Kunishima, H.; Nagai, E.; Adachi, E.; et al. Antibody avidity maturation following recovery from infection or the booster vaccination grants breadth of SARS-CoV-2 neutralizing capacity. J. Infect. Dis. 2023, 227, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Struck, F.; Staschik, E.; Maile, J.; Wochinz-Richter, K.; Motz, M.; Soutschek, E. Differential avidity determination of IgG directed towards the receptor-binding domain (RBD) of SARS-CoV-2 wild-type and its variants in one assay: Rational tool for the assessment of protective immunity. J. Med. Virol. 2022, 94, 5294–5303. [Google Scholar] [CrossRef]
- Sato, M.; Yamamoto-Hanada, K.; Tada, H.; Irahara, M.; Saito-Abe, M.; Matsumoto, K.; Pak, K.; Kido, H.; Ohya, Y. Diagnostic performance of IgE avidity for hen’s egg allergy in young infants. J. Allergy Clin. Immunol. Pract. 2020, 8, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- Kamemura, N.; Tada, H.; Shimojo, N.; Morita, Y.; Kohno, Y.; Ichioka, T.; Suzuki, K.; Kubota, K.; Hiyoshi, M.; Kido, H. Intrauterine sensitization of allergen-specific IgE analyzed by a highly sensitive new allergen microarray. J. Allergy Clin. Immunol. 2012, 130, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Irahara, M.; Shinahara, W.; Sigimoto, M.; Ogawa, Y.; Shitsukawa, K.; Kubota, K.; Yang, L.; Ohya, Y.; Saito, H.; Kagami, S.; et al. Trajectories of class-switching-related egg and cow’s milk allergen-specific immunoglobulin isotype formation and its modification by eczema with low- and high-affinity immunoglobulin E during early infancy. Immun. Inflamm. Dis. 2019, 7, 74–85. [Google Scholar] [CrossRef]
- Taniuchi, S.; Sakai, R.; Nishida, T.; Goma, M.; Mitomori, M.; Imaide, A.; Enomoto, M.; Nishino, M.; Okizuka, Y.; Kido, H. The combination of binding-avidity of ovomucoid-specific IgE antibody and specific IgG4 antibody can predict positive outcome of oral food challenge during stepwise slow oral immunotherapy in children with hen’s egg allergy. Nutrients 2023, 15, 2770. [Google Scholar] [CrossRef]
- Suzuki, K.; Hiyoshi, M.; Tada, H.; Bando, M.; Ichioka, T.; Kamemura, N.; Kido, H. Allergen diagnosis microarray with high-density immobilization capacity using diamond-like carbon-coated chips for profiling allergen-specific Ige and other immunoglobulins. Anal. Chim. Acta 2011, 706, 321–327. [Google Scholar] [CrossRef]
- Available online: https://www.niid.go.jp/niid/ja/2019-ncov/2551-cepr/10876-sars-cov-2-b-1-1-529.html (accessed on 5 September 2022).
- Lee, H.; Chubachi, S.; Namkoong, H.; Asakura, T.; Tanaka, H.; Otake, S.; Nakagawara, K.; Morita, A.; Fukushima, T.; Watase, M.; et al. Characteristics of hospitalized patients with COVID-19 during the first to fifth waves of infection: A report from the Japan COVID-19 Task Force. BMC Infect. Dis. 2022, 22, 935. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental treatment with favipiravir for COVID-19: An open-label control study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef]
- Clinical Management of Patients with COVID-19. A Guide for Front-Line Healthcare Workers Version 2.1. 2020; The Ministry of Health, Labour and Welfare and the National Institute of Infectious Diseases. Available online: https://www.niph.go.jp/h-crisis/wp-content/uploads/2020/07/20200706103735_content_000646531.pdf (accessed on 30 July 2020).
- Mizuno, D.; Ide-Kurihara, M.; Ichinomiya, T.; Kubo, I.; Kido, H. Modified pulmonary surfactant is a potent adjuvant that stimulates the mucosal IgA productyion in response to the influenza virus antigen. J. Immunol. 2006, 176, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Arashiro, T.; Ueno, A.; Kanno, T.; Saito, S.; Katano, H.; Iida, S.; Ainai, A.; Ozono, S.; Hemmi, T.; et al. Non-Omicron breakthrough infection with higher viral load and longer vaccination-infection interval improves SARS-CoV-2 BA.4/5 neutralization. iScience 2023, 26, 105969. [Google Scholar] [CrossRef] [PubMed]


| Gender | Time of Serum Collection | ||||
|---|---|---|---|---|---|
| n | M (n) | F (n) | Age (Years) | (Weeks after Initial Vaccination) | |
| Healthy subjects | 163 | 39 | 124 | 41 (21–71) | |
| First vaccination | March 2021 (3 weeks) | ||||
| Second vaccination | April 2021 (6–7 weeks) | ||||
| Third vaccination | December 2021 (41–44 weeks) | ||||
| Fourth vaccination | September 2022 (74–77 weeks) | ||||
| COVID-19 patients | 339 | 204 | 135 | 51 (14–98) | on admission (April 2020 to August 2021) |
| 339 | 204 | 135 | 51 (14–98) | on 2–3 weeks after admission or at discharge (May 2020 to September 2021) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, E.; Sawabuchi, T.; Homma, T.; Fukuda, Y.; Sagara, H.; Kinjo, T.; Fujita, K.; Suga, S.; Kimoto, T.; Sakai, S.; et al. Clinical Utility of SARS-CoV-2 Antibody Titer Multiplied by Binding Avidity of Receptor-Binding Domain (RBD) in Monitoring Protective Immunity and Clinical Severity. Viruses 2023, 15, 1662. https://doi.org/10.3390/v15081662
Takahashi E, Sawabuchi T, Homma T, Fukuda Y, Sagara H, Kinjo T, Fujita K, Suga S, Kimoto T, Sakai S, et al. Clinical Utility of SARS-CoV-2 Antibody Titer Multiplied by Binding Avidity of Receptor-Binding Domain (RBD) in Monitoring Protective Immunity and Clinical Severity. Viruses. 2023; 15(8):1662. https://doi.org/10.3390/v15081662
Chicago/Turabian StyleTakahashi, Etsuhisa, Takako Sawabuchi, Tetsuya Homma, Yosuke Fukuda, Hironori Sagara, Takeshi Kinjo, Kaori Fujita, Shigeru Suga, Takashi Kimoto, Satoko Sakai, and et al. 2023. "Clinical Utility of SARS-CoV-2 Antibody Titer Multiplied by Binding Avidity of Receptor-Binding Domain (RBD) in Monitoring Protective Immunity and Clinical Severity" Viruses 15, no. 8: 1662. https://doi.org/10.3390/v15081662
APA StyleTakahashi, E., Sawabuchi, T., Homma, T., Fukuda, Y., Sagara, H., Kinjo, T., Fujita, K., Suga, S., Kimoto, T., Sakai, S., Kameda, K., & Kido, H. (2023). Clinical Utility of SARS-CoV-2 Antibody Titer Multiplied by Binding Avidity of Receptor-Binding Domain (RBD) in Monitoring Protective Immunity and Clinical Severity. Viruses, 15(8), 1662. https://doi.org/10.3390/v15081662






