The Stereotypic Response of the Pulmonary Vasculature to Respiratory Viral Infections: Findings in Mouse Models of SARS-CoV-2, Influenza A and Gammaherpesvirus Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viruses
2.2. Animals and Virus Infections
2.3. Tissue Collection, Preparation and Processing
2.4. Histology, Immunohistology
2.5. RNA Sequencing and Bioinformatic Analysis
2.6. Morphometric Analysis
2.7. Statistical Analysis
3. Results
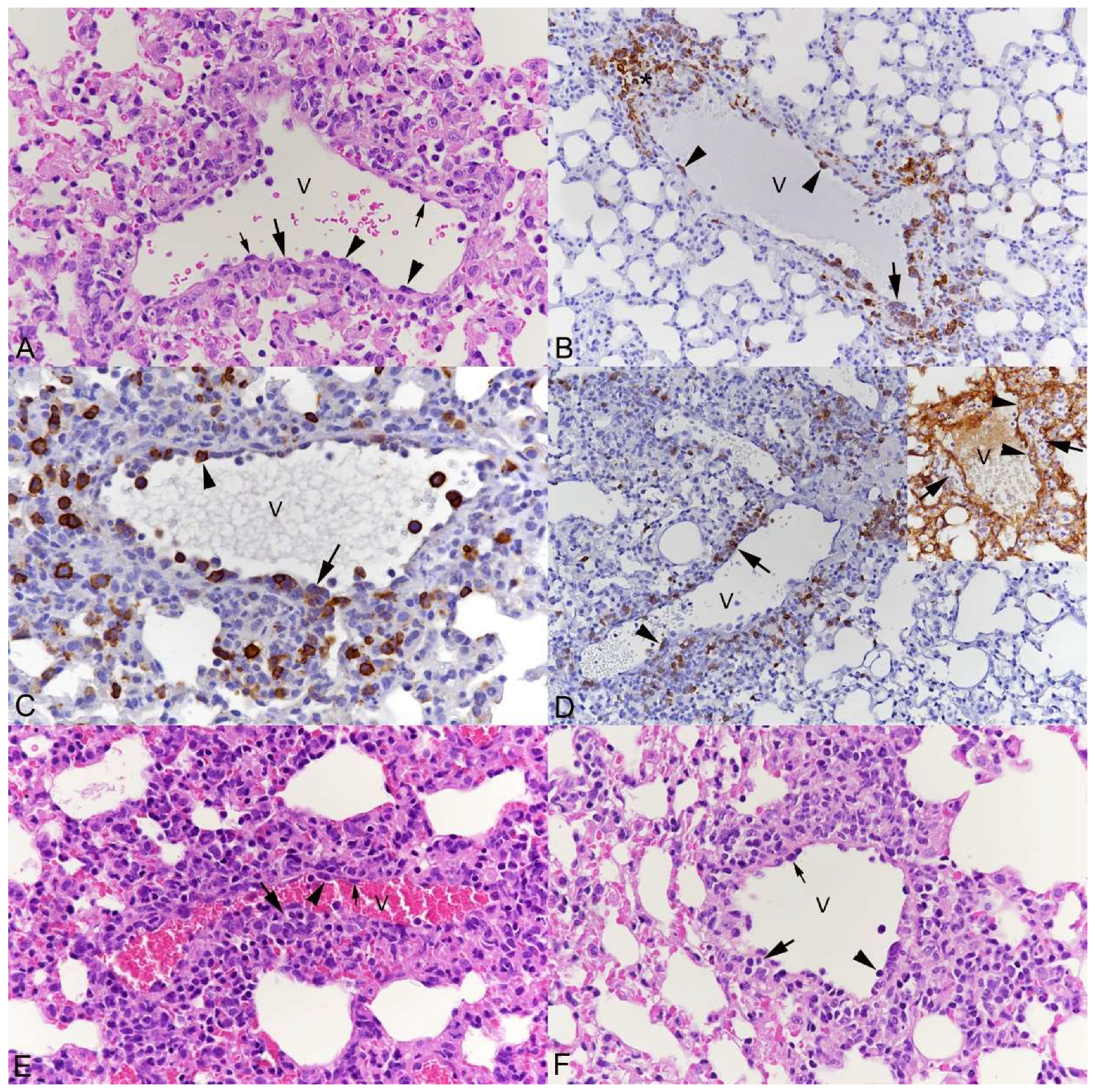
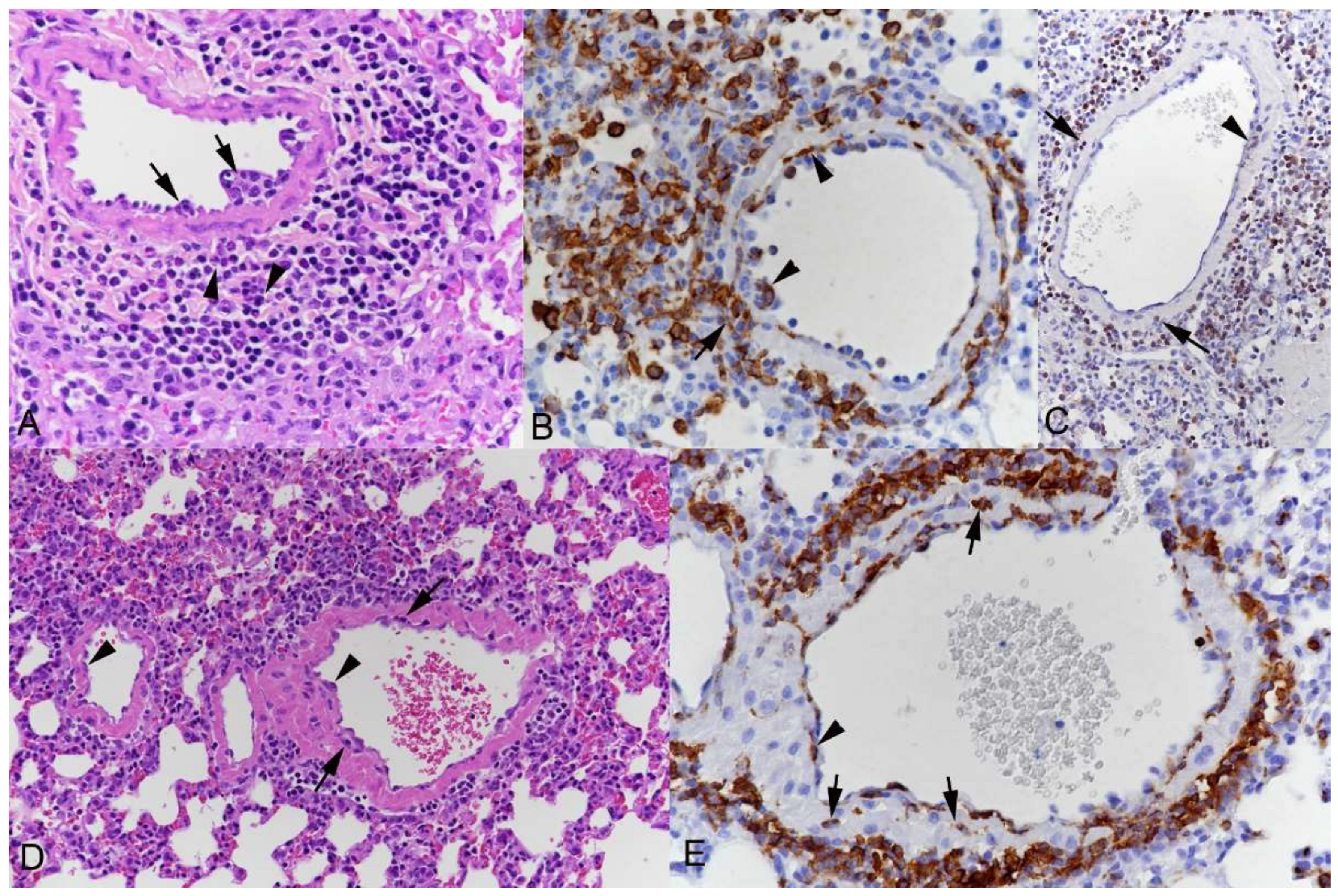
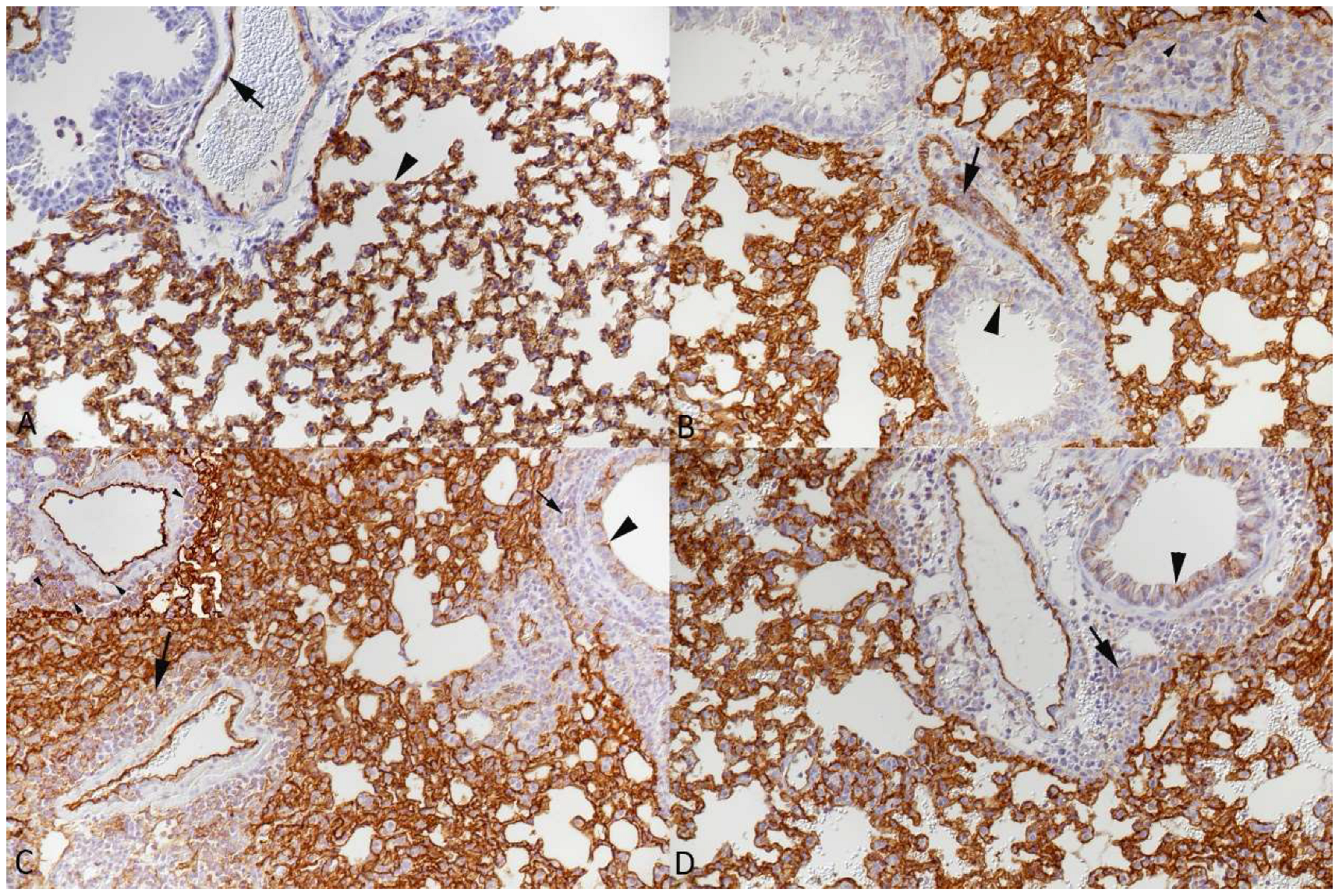
3.1. Pulmonary Vasculitis Is a Consistent Feature of SARS-CoV-2 Infection in Mouse Models
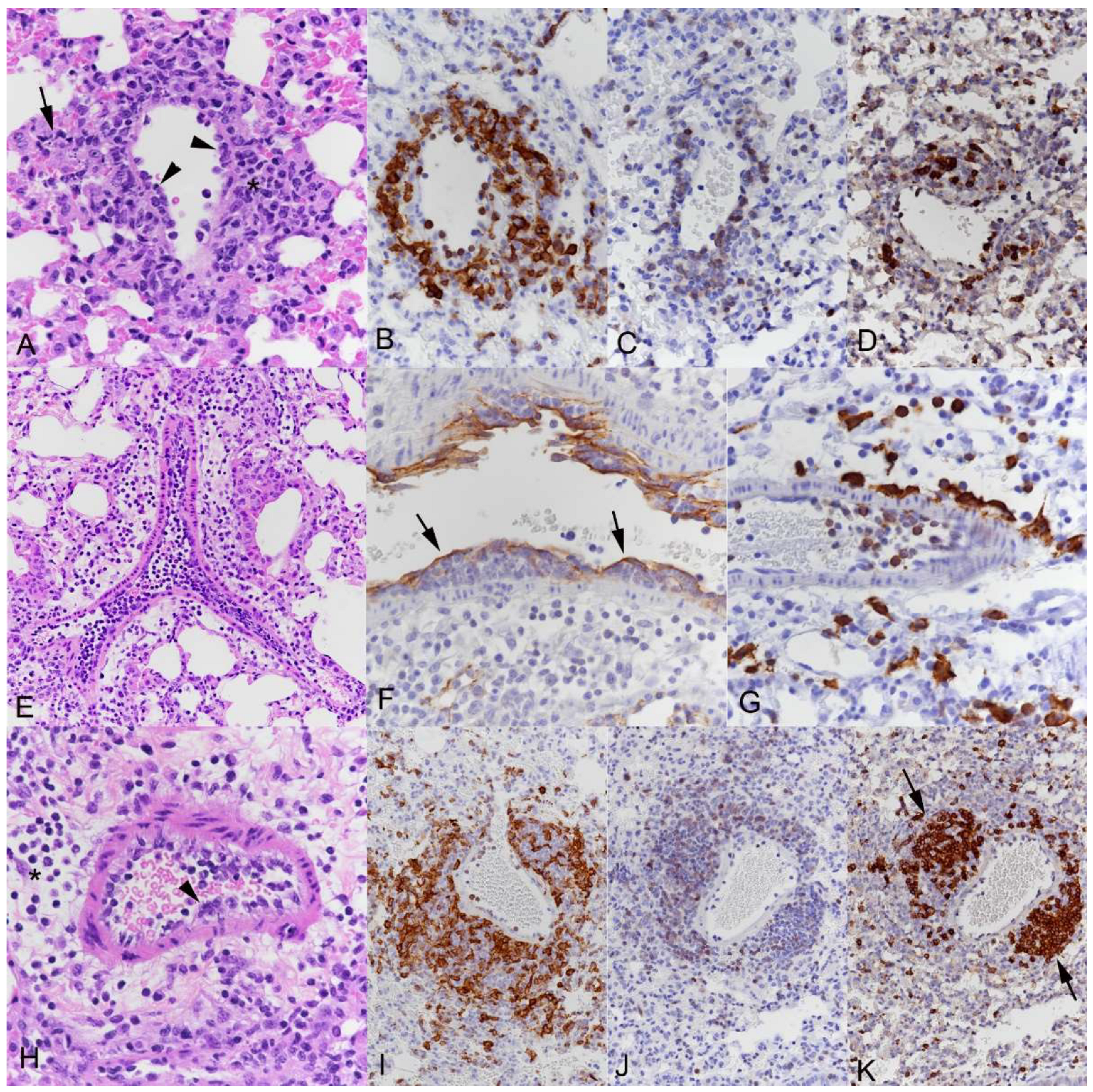
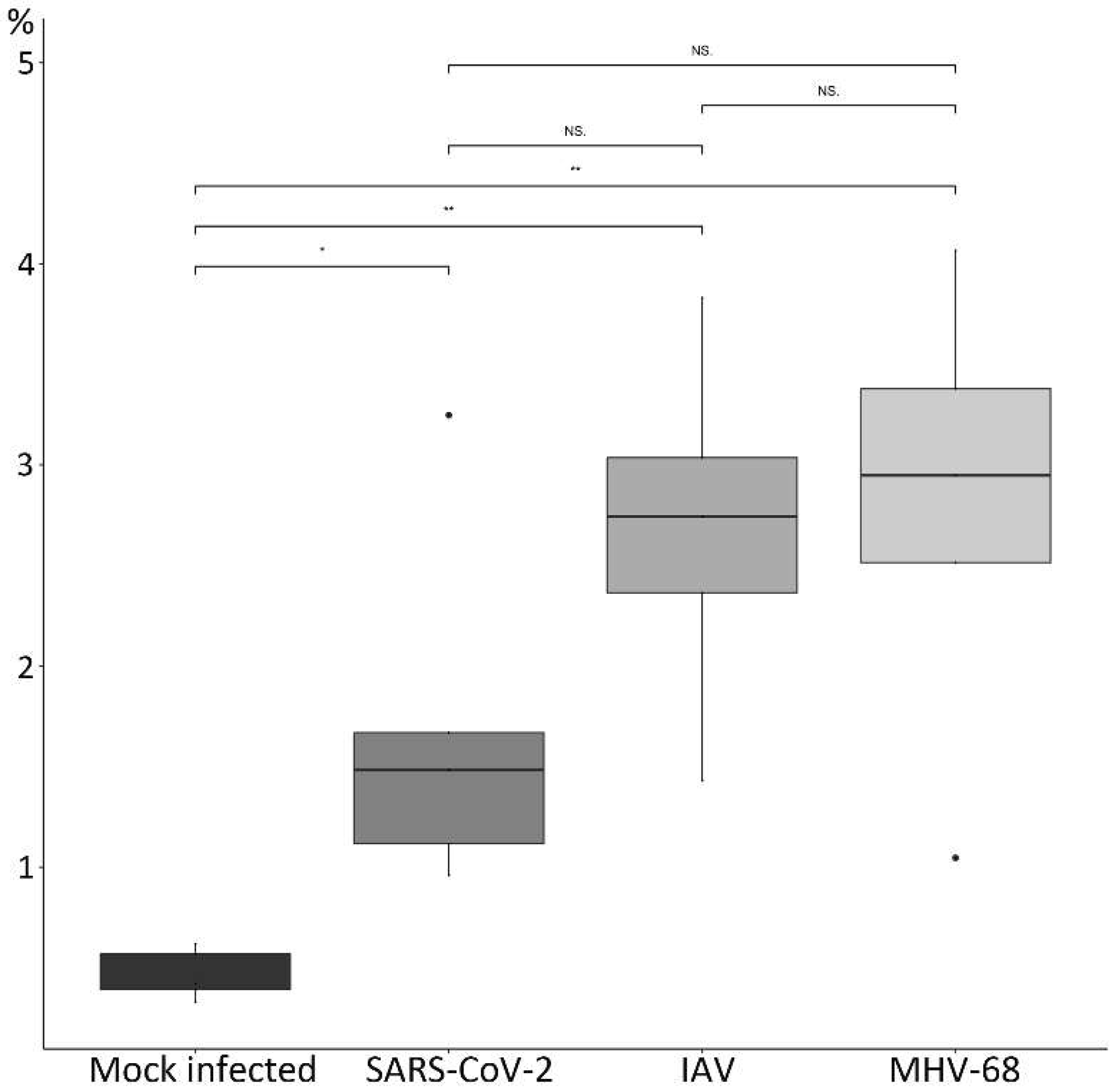
3.2. Respiratory Virus Infections, with Variable Degree of Alveolar Damage, and with or without Damage to Respiratory Epithelium, Elicit a Stereotypic Vascular Response
3.3. The Transcriptomic Analysis Reflects the Differences in Viral Pathogenicity but Also Confirms the Stereotypic Vascular Response in SARS-CoV-2 and IAV Infection
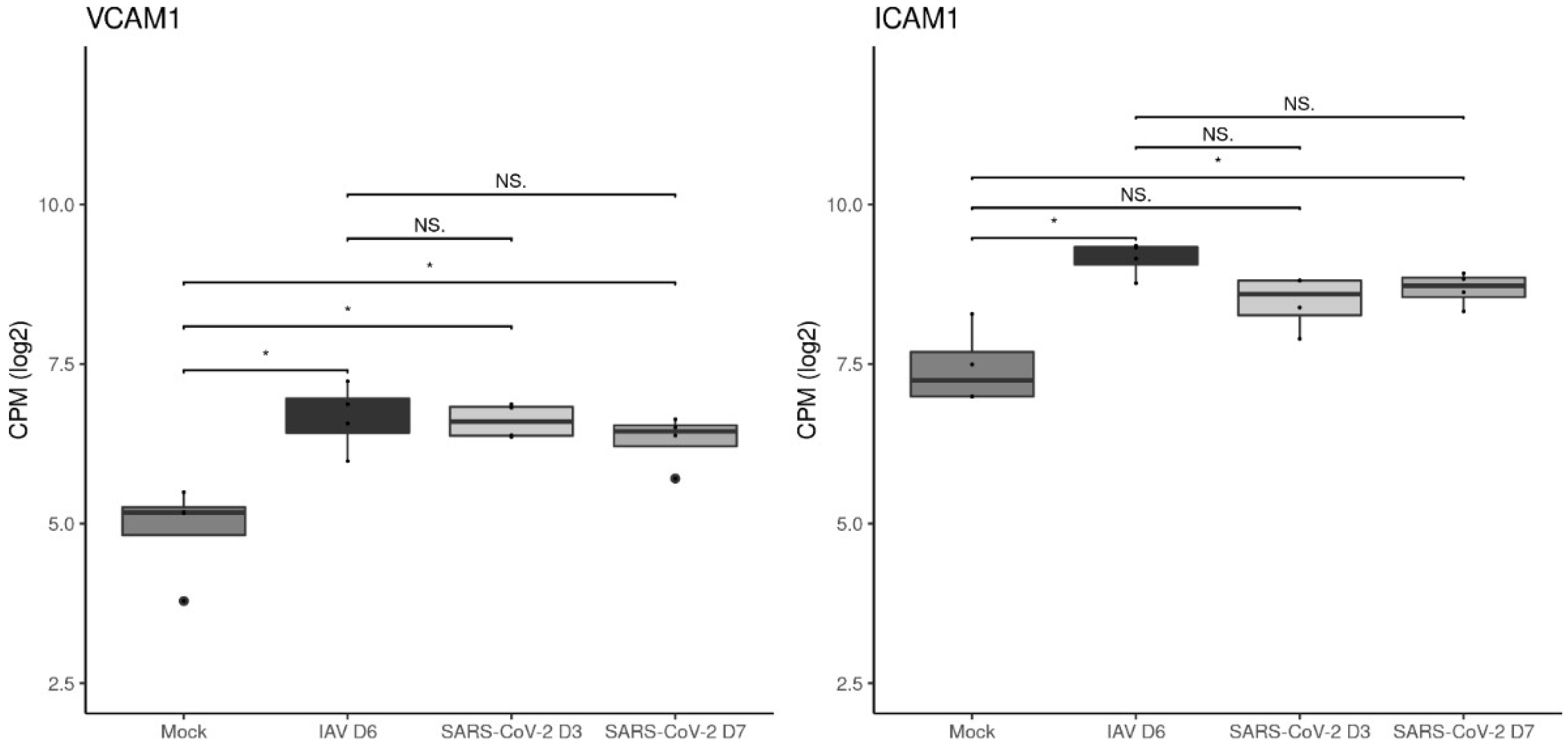
3.4. The Vascular Response after Respiratory Virus Infections Is Associated with Increased Expression of Adhesion Molecules in the Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Osuchowski, M.F.; Winkler, M.S.; Skirecki, T.; Cajander, S.; Shankar-Hari, M.; Lachmann, G.; Monneret, G.; Venet, F.; Bauer, M.; Brunkhorst, F.M.; et al. The COVID-19 puzzle: Deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir. Med. 2021, 9, 622–642. [Google Scholar] [CrossRef] [PubMed]
- Bösmüller, H.; Matter, M.; Fend, F.; Tzankov, A. The pulmonary pathology of COVID-19. Virchows Arch. 2021, 478, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Milross, L.; Majo, J.; Cooper, N.; Kaye, P.M.; Bayraktar, O.; Filby, A.; Fisher, A.J. Post-mortem lung tissue: The fossil record of the pathophysiology and immunopathology of severe COVID-19. Lancet Respir. Med. 2022, 10, 95–106. [Google Scholar] [CrossRef] [PubMed]
- Jonigk, D.; Werlein, C.; Acker, T.; Aepfelbacher, M.; Amann, K.U.; Baretton, G.; Barth, P.; Bohle, R.M.; Büttner, A.; Büttner, R.; et al. Organ manifestations of COVID-19: What have we learned so far (not only) from autopsies? Virchows Arch. 2022, 481, 139–159. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Dorward, D.A.; Russell, C.D.; Um, I.H.; Elshani, M.; Armstrong, S.D.; Penrice-Randal, R.; Millar, T.; Lerpiniere, C.E.B.; Tagliavini, G.; Hartley, C.S.; et al. Tissue-specific immunopathology in fatal COVID-19. Am. J. Respir. Crit. Care Med. 2021, 203, 192–201. [Google Scholar] [CrossRef]
- Bussani, R.; Schneider, E.; Zentilin, L.; Collesi, C.; Ali, H.; Braga, L.; Volpe, M.C.; Colliva, A.; Zanconati, F.; Berlot, G.; et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine 2020, 61, 103104. [Google Scholar] [CrossRef]
- Haberecker, M.; Schwarz, E.I.; Steiger, P.; Frontzek, K.; Scholkmann, F.; Zeng, X.; Höller, S.; Moch, H.; Varga, Z. Autopsy-based pulmonary and vascular pathology: Pulmonary endotheliitis and multi-organ involvement in COVID-19 associated deaths. Respiration 2022, 101, 155–165. [Google Scholar] [CrossRef]
- Bradley, B.T.; Maioli, H.; Johnston, R.; Chaudhry, I.; Fink, S.L.; Xu, H.; Najafian, B.; Deutsch, G.; Lacy, J.M.; Williams, T.; et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet 2020, 396, 320–332. [Google Scholar] [CrossRef]
- Aid, M.; Busman-Sahay, K.; Vidal, S.J.; Maliga, Z.; Bondoc, S.; Starke, C.; Terry, M.; Jacobson, C.A.; Wrijil, L.; Ducat, S.; et al. Vascular disease and thrombosis in SARS-CoV-2-infected rhesus macaques. Cell 2020, 183, 1354–1366. [Google Scholar] [CrossRef]
- Bösmüller, H.; Traxler, S.; Bitzer, M.; Häberle, H.; Raiser, W.; Nann, D.; Frauenfeld, L.; Vogelsberg, A.; Klingel, K.; Fend, F. The evolution of pulmonary pathology in fatal COVID-19 disease: An autopsy study with clinical correlation. Virchows Arch. 2020, 477, 349–357. [Google Scholar] [CrossRef]
- Ciurkiewicz, M.; Armando, F.; Schreiner, T.; de Buhr, N.; Pilchová, V.; Krupp-Buzimikic, V.; Gabriel, G.; von Köckritz-Blickwede, M.; Baumgärtner, W.; Schulz, C.; et al. Ferrets are valuable models for SARS-CoV-2 research. Vet. Pathol. 2022, 59, 661–672. [Google Scholar] [CrossRef]
- Allnoch, L.; Beythien, G.; Leitzen, E.; Becker, K.; Kaup, F.-J.; Stanelle-Bertram, S.; Schaumburg, B.; Mounogou Kouassi, N.; Beck, S.; Zickler, M.; et al. Vascular inflammation is associated with loss of aquaporin 1 expression on endothelial cells and increased fluid leakage in SARS-CoV-2 infected golden Syrian hamsters. Viruses 2021, 13, 639. [Google Scholar] [CrossRef]
- Osterrieder, N.; Bertzbach, L.D.; Dietert, K.; Abdelgawad, A.; Vladimirova, D.; Kunec, D.; Hoffmann, D.; Beer, M.; Gruber, A.D.; Trimpert, J. Age-dependent progression of SARS-CoV-2 infection in Syrian hamsters. Viruses 2020, 12, 779. [Google Scholar] [CrossRef]
- Xue, Y.; Yang, D.; Vogel, P.; Stabenow, J.; Zalduondo, L.; Kong, Y.; Ravi, Y.; Sai-Sudhakar, C.B.; Parvathareddy, J.; Hayes, E.; et al. Cardiopulmonary injury in the Syrian hamster model of COVID-19. Viruses 2022, 14, 1403. [Google Scholar] [CrossRef]
- Becker, K.; Beythien, G.; de Buhr, N.; Stanelle-Bertram, S.; Tuku, B.; Kouassi, N.M.; Beck, S.; Zickler, M.; Allnoch, L.; Gabriel, G.; et al. Vasculitis and neutrophil extracellular traps in lungs of golden Syrian hamsters with SARS-CoV-2. Front. Immunol. 2021, 12, 640842. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, F.; Blair, R.; Wang, C.; Yang, H.; Mudd, J.; Currey, J.M.; Iwanaga, N.; He, J.; Mi, R.; et al. Endothelial cell infection and dysfunction, immune activation in severe COVID-19. Theranostics 2021, 11, 8076–8091. [Google Scholar] [CrossRef]
- Kirschenbaum, D.; Imbach, L.L.; Rushing, E.J.; Frauenknecht, K.B.M.; Gascho, D.; Ineichen, B.V.; Keller, E.; Kohler, S.; Lichtblau, M.; Reimann, R.R.; et al. Intracerebral endotheliitis and microbleeds are neuropathological features of COVID-19. Neuropathol. Appl. Neurobiol. 2021, 47, 454–459. [Google Scholar] [CrossRef]
- Kantonen, J.; Mahzabin, S.; Mäyränpää, M.I.; Tynninen, O.; Paetau, A.; Andersson, N.; Sajantila, A.; Vapalahti, O.; Carpén, O.; Kekäläinen, E.; et al. Neuropathologic features of four autopsied COVID-19 patients. Brain Pathol. 2020, 30, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Maccio, U.; Zinkernagel, A.S.; Shambat, S.M.; Zeng, X.; Cathomas, G.; Ruschitzka, F.; Schuepbach, R.A.; Moch, H.; Varga, Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine 2021, 63, 103182. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; López-Figueroa, C.; Rodon, J.; Pérez, M.; Brustolin, M.; Cantero, G.; Guallar, V.; Izquierdo-Useros, N.; Carrillo, J.; Blanco, J.; et al. Chronological brain lesions after SARS-CoV-2 infection in hACE2-transgenic mice. Vet. Pathol. 2022, 59, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Seehusen, F.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Subramaniam, K.; Wunderlin-Giuliani, S.; Hughes, G.L.; Patterson, E.I.; Michael, B.D.; et al. Neuroinvasion and neurotropism by SARS-CoV-2 variants in the K18-hACE2 mouse. Viruses 2022, 14, 1020. [Google Scholar] [CrossRef]
- Carossino, M.; Kenney, D.; O’Connell, A.K.; Montanaro, P.; Tseng, A.E.; Gertje, H.P.; Grosz, K.A.; Ericsson, M.; Huber, B.R.; Kurnick, S.A.; et al. Fatal neurodissemination and SARS-CoV-2 tropism in K18-hACE2 mice is only partially dependent on hACE2 expression. Viruses 2022, 14, 535. [Google Scholar] [CrossRef]
- Solomon, I.H.; Normandin, E.; Bhattacharyya, S.; Mukerji, S.S.; Keller, K.; Ali, A.S.; Adams, G.; Hornick, J.L.; Padera, R.F.; Sabeti, P. Neuropathological features of COVID-19. N. Engl. J. Med. 2020, 383, 989–992. [Google Scholar] [CrossRef]
- Goldsmith, C.S.; Miller, S.E.; Martines, R.B.; Bullock, H.A.; Zaki, S.R. Electron microscopy of SARS-CoV-2: A challenging task. Lancet 2020, 395, e99. [Google Scholar] [CrossRef]
- Dittmayer, C.; Meinhardt, J.; Radbruch, H.; Radke, J.; Heppner, B.I.; Heppner, F.L.; Stenzel, W.; Holland, G.; Laue, M. Why misinterpretation of electron micrographs in SARS-CoV-2-infected tissue goes viral. Lancet 2020, 396, e64–e65. [Google Scholar] [CrossRef]
- Nicosia, R.F.; Ligresti, G.; Caporarello, N.; Akilesh, S.; Ribatti, D. COVID-19 vasculopathy: Mounting evidence for an indirect mechanism of endothelial injury. Am. J. Pathol. 2021, 191, 1374–1384. [Google Scholar] [CrossRef]
- Johnson, J.E.; Gonzales, R.A.; Olson, S.J.; Wright, P.F.; Graham, B.S. The histopathology of fatal untreated human respiratory syncytial virus infection. Mod. Pathol. 2007, 20, 108–119. [Google Scholar] [CrossRef]
- Pritt, B.S.; Aubry, M.C. Histopathology of viral infections of the lung. Semin. Diagn. Pathol. 2017, 34, 510–517. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Shen, H.; Li, Z.; Geng, J.; Han, H.; Cai, J.; Li, X.; Kang, W.; Weng, D.; et al. The clinical pathology of severe acute respiratory syndrome (SARS): A report from China. J. Pathol. 2003, 200, 282–289. [Google Scholar] [CrossRef]
- Gu, J.; Gong, E.; Zhang, B.; Zheng, J.; Gao, Z.; Zhong, Y.; Zou, W.; Zhan, J.; Wang, S.; Xie, Z.; et al. Multiple organ infection and the pathogenesis of SARS. J. Exp. Med. 2005, 202, 415–424. [Google Scholar] [CrossRef]
- Hwang, D.M.; Chamberlain, D.W.; Poutanen, S.M.; Low, D.E.; Asa, S.L.; Butany, J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005, 18, 1–10. [Google Scholar] [CrossRef]
- Hughes, D.J.; Kipar, A.; Sample, J.T.; Stewart, J.P. Pathogenesis of a model gammaherpesvirus in a natural host. J. Virol. 2010, 84, 3949–3961. [Google Scholar] [CrossRef]
- Akram, K.M.; Moyo, N.A.; Leeming, G.H.; Bingle, L.; Jasim, S.; Hussain, S.; Schorlemmer, A.; Kipar, A.; Digard, P.; Tripp, R.A.; et al. An innate defense peptide BPIFA1/SPLUNC1 restricts influenza A virus infection. Mucosal Immunol. 2018, 11, 71–81. [Google Scholar] [CrossRef]
- Patterson, E.I.; Prince, T.; Anderson, E.R.; Casas-Sanchez, A.; Smith, S.L.; Cansado-Utrilla, C.; Solomon, T.; Griffiths, M.J.; Acosta-Serrano, Á.; Turtle, L.; et al. Methods of inactivation of SARS-CoV-2 for downstream biological assays. J. Infect. Dis. 2020, 222, 1462–1467. [Google Scholar] [CrossRef]
- Clark, J.J.; Penrice-Randal, R.; Sharma, P.; Kipar, A.; Dong, X.; Pennington, S.H.; Marriott, A.E.; Colombo, S.; Davidson, A.; Williamson, M.K.; et al. Sequential infection with influenza A virus followed by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leads to more severe disease and encephalitis in a mouse model of COVID-19. bioRxiv 2023. [Google Scholar] [CrossRef]
- Cele, S.; Gazy, I.; Jackson, L.; Hwa, S.-H.; Tegally, H.; Lustig, G.; Giandhari, J.; Pillay, S.; Wilkinson, E.; Naidoo, Y.; et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature 2021, 593, 142–146. [Google Scholar] [CrossRef]
- Kant, R.; Kareinen, L.; Smura, T.; Freitag, T.L.; Jha, S.K.; Alitalo, K.; Meri, S.; Sironen, T.; Saksela, K.; Strandin, T.; et al. Common laboratory mice are susceptible to infection with the SARS-CoV-2 beta variant. Viruses 2021, 13, 2263. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Shaw, R.H.; Supasa, P.; Liu, C.; Stuart, A.S.; Pollard, A.J.; Liu, X.; Lambe, T.; Crook, D.; Stuart, D.I.; et al. Reduced neutralisation of SARS-CoV-2 omicron B.1.1.529 variant by post-immunisation serum. Lancet 2022, 399, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Bentley, E.G.; Kirby, A.; Sharma, P.; Kipar, A.; Mega, D.F.; Bramwell, C.; Penrice-Randal, R.; Prince, T.; Brown, J.C.; Zhou, J.; et al. SARS-CoV-2 omicron-B.1.1.529 variant leads to less severe disease than Pango B and Delta variants strains in a mouse model of severe COVID-19. bioRxiv 2021. [Google Scholar] [CrossRef]
- Salzer, R.; Clark, J.J.; Vaysburd, M.; Chang, V.T.; Albecka, A.; Kiss, L.; Sharma, P.; Gonzalez Llamazares, A.; Kipar, A.; Hiscox, J.A.; et al. Single-dose immunisation with a multimerised SARS-CoV-2 receptor binding domain (RBD) induces an enhanced and protective response in mice. FEBS Lett. 2021, 595, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Suarez, C.J.; Dintzis, S.M.; Frevert, C.W. Chapter 9: Respiratory. In Comparative Anatomy and Histology A Mouse and Human Atlas, 1st ed.; Piper, M.T., Dintzis, S.M., Eds.; Academic Press: Cambridge, MA, USA, 2012; p. 132. [Google Scholar] [CrossRef]
- Wang, P.M.; Kachel, D.L.; Cesta, M.F.; Martin, W.J. Direct leukocyte migration across pulmonary arterioles and venules into the perivascular interstitium of murine lungs during bleomycin injury and repair. Am. J. Pathol. 2011, 178, 2560–2572. [Google Scholar] [CrossRef]
- VanLeuven, J.T.; Ridenhour, B.J.; Gonzalez, A.J.; Miller, C.R.; Miura, T.A. Lung epithelial cells have virus-specific and shared gene expression responses to infection by diverse respiratory viruses. PLoS ONE 2017, 12, e0178408. [Google Scholar] [CrossRef]
- Stölting, H.; Baillon, L.; Frise, R.; Bonner, K.; Hewitt, R.J.; Molyneaux, P.L.; Gore, M.L.; Barclay, W.S.; Saglani, S.; Lloyd, C.M. Distinct airway epithelial immune responses after infection with SARS-CoV-2 compared to H1N1. Mucosal Immunol. 2022, 15, 952–963. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Muller, W.A. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22. [Google Scholar] [CrossRef]
- Hickey, M.J.; Westhorpe, C.L.V. Imaging inflammatory leukocyte recruitment in kidney, lung and liver-challenges to the multi-step paradigm. Immunol. Cell Biol. 2013, 91, 281–289. [Google Scholar] [CrossRef]
- Hallmann, R.; Hannocks, M.-J.; Song, J.; Zhang, X.; Di Russo, J.; Luik, A.-L.; Burmeister, M.; Gerwien, H.; Sorokin, L. The role of basement membrane laminins in vascular function. Int. J. Biochem. Cell Biol. 2020, 127, 105823. [Google Scholar] [CrossRef]
- Yousif, L.F.; Di Russo, J.; Sorokin, L. Laminin isoforms in endothelial and perivascular basement membranes. Cell Adh. Migr. 2013, 7, 101–110. [Google Scholar] [CrossRef]
- Chong, D.L.W.; Rebeyrol, C.; José, R.J.; Williams, A.E.; Brown, J.S.; Scotton, C.J.; Porter, J.C. ICAM-1 and ICAM-2 are differentially expressed and up-regulated on inflamed pulmonary epithelium, but neither ICAM-2 nor LFA-1: ICAM-1 are required for neutrophil migration into the airways in vivo. Front. Immunol. 2021, 12, 691957. [Google Scholar] [CrossRef]
- UniProt (Release 2023_02): P13597–ICAM1_MOUSE. Available online: https://www.uniprot.org/uniprotkb/P13597/entry#expression (accessed on 8 June 2023).
- UniProt (Release 2023_02): P29533–VCAM1_MOUSE. Available online: https://www.uniprot.org/uniprotkb/P29533/entry#expression (accessed on 8 June 2023).
- Jiang, H.; Shen, S.-M.; Yin, J.; Zhang, P.-P.; Shi, Y. Sphingosine 1-phosphate receptor 1 (S1PR1) agonist CYM5442 inhibits expression of intracellular adhesion molecule 1 (ICAM1) in endothelial cells infected with influenza A viruses. PLoS ONE 2017, 12, e0175188. [Google Scholar] [CrossRef]
- Hashimoto, R.; Takahashi, J.; Shirakura, K.; Funatsu, R.; Kosugi, K.; Deguchi, S.; Yamamoto, M.; Tsunoda, Y.; Morita, M.; Muraoka, K.; et al. SARS-CoV-2 disrupts respiratory vascular barriers by suppressing Claudin-5 expression. Sci. Adv. 2022, 8, eabo6783. [Google Scholar] [CrossRef]
- Lin, W.-C.; Fessler, M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell. Mol. Life Sci. 2021, 78, 4095–4124. [Google Scholar] [CrossRef]
- Leick, M.; Azcutia, V.; Newton, G.; Luscinskas, F.W. Leukocyte recruitment in inflammation: Basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 2014, 355, 647–656. [Google Scholar] [CrossRef]
- Burns, A.R.; Smith, C.W.; Walker, D.C. Unique structural features that influence neutrophil emigration into the lung. Physiol. Rev. 2003, 83, 309–336. [Google Scholar] [CrossRef]
- Grewal, M.K.; Adams, M.D.; Valentini, R.P. Vasculitis and kidney disease. Pediatr. Clin. N. Am. 2022, 69, 1199–1217. [Google Scholar] [CrossRef]
- Vouters, J. Les endothélites rétiniennes; rétinite septique, rétinite cachectique, rétinite dysorique; pathogénie immunologique; analyse de 78 observations. Arch. Ophtalmol. Rev. Gen. Ophtalmol. 1958, 18, 262–293. [Google Scholar]
- Zech, P.Y.; Colon, S.; Deteix, P.; Blanc-Brunat, N. Distant evolution of kidney arteriolar lesions and hypertension after pregnancy toxemia. Nephron 1980, 26, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Snover, D.C.; Weisdorf, S.A.; Ramsay, N.K.; McGlave, P.; Kersey, J.H. Hepatic graft versus host disease: A study of the predictive value of liver biopsy in diagnosis. Hepatology 1984, 4, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Hattori, K.; Machida, T.; Matsuda, N. Vascular endotheliitis associated with infections: Its pathogenetic role and therapeutic implication. Biochem. Pharmacol. 2022, 197, 114909. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Murri, M.S.; Shah, T.J.; Skanchy, D.F.; Tuckfield, J.Q.; Ronquillo, Y.C.; Birdsong, O.C.; Hofstedt, D.; Hoopes, P.C. A review of corneal endotheliitis and endotheliopathy: Differential diagnosis, evaluation, and treatment. Ophthalmol. Ther. 2019, 8, 195–213. [Google Scholar] [CrossRef]





| Cohort (Mice) | Age | Virus | PFU | DPI | No of Animals |
|---|---|---|---|---|---|
| 1 (hACE2) | 6–8 weeks | Pango B | 104 | 3, 7 | 8, 8 |
| 2 (hACE2) | 6–8 weeks | Pango B | 103 | 6 | 6 |
| 3 (hACE2) | 6–8 weeks | Alpha | 103 | 3, 6/7 | 4, 4 |
| 4 (hACE2) | 6–8 weeks | Beta | 103 | 3, 6/7 | 4, 4 |
| 5 (BALB/c) | 12 weeks | Beta | 2 × 105 | 4 | 3 |
| 6a (hACE2) | 6–8 weeks | Delta | 103 | 3, 5/6, 7 | 4, 4, 5 |
| 6b (hACE2) | 6–8 weeks | Delta | 102 | 6/7 | 8 |
| 6c (hACE2) | 10–11 months | Delta | 103 | 7 | 6 |
| 7a (hACE2) | 8–10 weeks | Omicron BA.1 | 103 | 6 | 6 |
| 7b (hACE2) | 10–11 months | Omicron BA.1 | 103 | 7 | 6 |
| 8 (BALB/c) | 6–8 weeks | IAV X31 | 103 | 2 | 4 |
| 9 (hACE2) | 6–8 weeks | IAV X31 | 103 | 6 | 3 |
| 10 (C57BL/6J) | 6–8 weeks | IAV X31 | 103 | 5, 7 | 5, 5 |
| 11 (C57BL/6J) | 6 weeks | MHV68 | 4 × 105 | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Neck, S.; Penrice-Randal, R.; Clark, J.J.; Sharma, P.; Bentley, E.G.; Kirby, A.; Mega, D.F.; Han, X.; Owen, A.; Hiscox, J.A.; et al. The Stereotypic Response of the Pulmonary Vasculature to Respiratory Viral Infections: Findings in Mouse Models of SARS-CoV-2, Influenza A and Gammaherpesvirus Infections. Viruses 2023, 15, 1637. https://doi.org/10.3390/v15081637
De Neck S, Penrice-Randal R, Clark JJ, Sharma P, Bentley EG, Kirby A, Mega DF, Han X, Owen A, Hiscox JA, et al. The Stereotypic Response of the Pulmonary Vasculature to Respiratory Viral Infections: Findings in Mouse Models of SARS-CoV-2, Influenza A and Gammaherpesvirus Infections. Viruses. 2023; 15(8):1637. https://doi.org/10.3390/v15081637
Chicago/Turabian StyleDe Neck, Simon, Rebekah Penrice-Randal, Jordan J. Clark, Parul Sharma, Eleanor G. Bentley, Adam Kirby, Daniele F. Mega, Ximeng Han, Andrew Owen, Julian A. Hiscox, and et al. 2023. "The Stereotypic Response of the Pulmonary Vasculature to Respiratory Viral Infections: Findings in Mouse Models of SARS-CoV-2, Influenza A and Gammaherpesvirus Infections" Viruses 15, no. 8: 1637. https://doi.org/10.3390/v15081637
APA StyleDe Neck, S., Penrice-Randal, R., Clark, J. J., Sharma, P., Bentley, E. G., Kirby, A., Mega, D. F., Han, X., Owen, A., Hiscox, J. A., Stewart, J. P., & Kipar, A. (2023). The Stereotypic Response of the Pulmonary Vasculature to Respiratory Viral Infections: Findings in Mouse Models of SARS-CoV-2, Influenza A and Gammaherpesvirus Infections. Viruses, 15(8), 1637. https://doi.org/10.3390/v15081637






