SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023
Abstract
:1. Background
2. Aims
3. Methods
3.1. Ethical Aspects
3.2. Study Population
3.3. Data Collection
3.4. Study Endpoint
3.5. Statistical Analysis
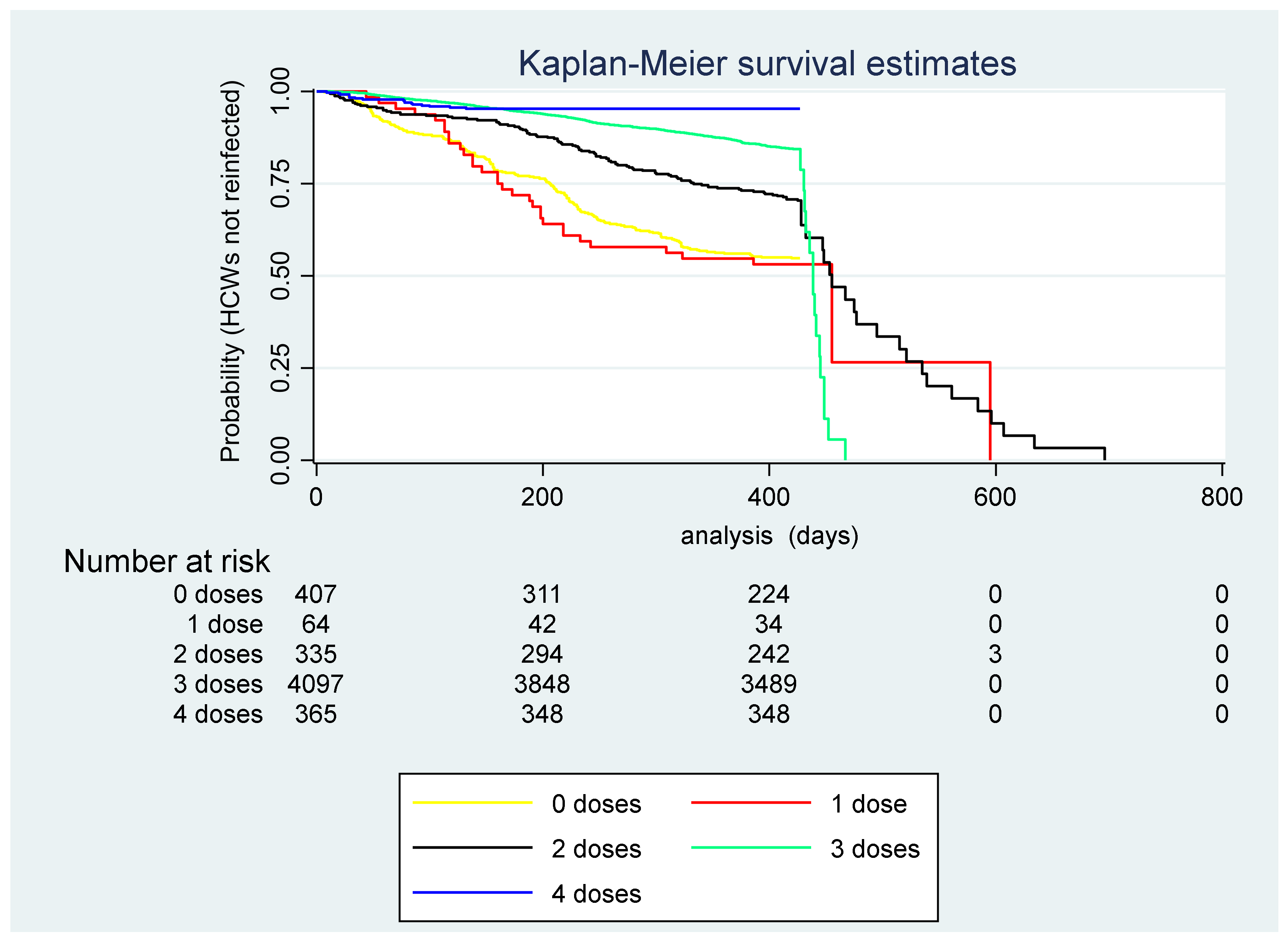
4. Results
5. Discussion
5.1. Main Findings
5.2. Interpretation of Findings
5.3. Strengths and Weaknesses
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Center for Disease Prevention and Control. Overview of Testing for SARS-CoV-2, the Virus That Causes COVID-19. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html (accessed on 14 June 2023).
- Cegolon, L.; Pichierri, J.; Mastrangelo, G.; Cinquetti, S.; Sotgiu, G.; Bellizzi, S.; Pichierri, G. Hypothesis to explain the severe form of COVID-19 in Northern Italy. BMJ Glob. Health 2020, 5, e002564. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Ronchese, F.; Ricci, F.; Negro, C.; Laese-Filon, F. SARS-CoV-2 Infection in Health Care Workers of Trieste (North-Eastern Italy), 1 October 2020–7 February 2022: Occupational Risk and the Impact of the Omicron Variant. Viruses 2022, 14, 1663. [Google Scholar] [CrossRef] [PubMed]
- Tillett, R.L.; Sevinsky, J.R.; Hartley, P.D.; Kerwin, H.; Crawford, N.; Gorzalski, A.; Laverdure, C.; Verma, S.; Rossetto, C.; Jackson, D.; et al. Genomic evidence for reinfection with SARS-CoV-2: A case study. Lancet Infect. Dis. 2021, 21, 52–58. [Google Scholar] [CrossRef]
- Wang, J.; Kaperak, C.; Sato, T.; Sakuraba, A. Covid-19 Reinfection: A Rapid Systematic Review of Case Reports and Case Series. J. Investig. Med. 2021, 69, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, R.A.; Qamar, M.A.; Gilani, J.A.; Irfan, O.; Waqar, U.; Sajid, M.I.; Mahmood, S.F. The mystery of COVID-19 reinfections: A global systematic review and meta-analysis. Ann. Med. Surg. 2021, 72, 103130. [Google Scholar] [CrossRef] [PubMed]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg. Health Eur. 2022, 20, 100453. [Google Scholar] [CrossRef]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P. SARS-CoV-2 reinfections: Overview of efficacy and duration of natural and hybrid immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Hansen, C.H.; Michlmayr, D.; Gubbels, S.M.; Mølbak, K.; Ethelberg, S. Assessment of protection against reinfection with SARS-CoV-2 among 4 million PCR-tested individuals in Denmark in 2020: A population-level observational study. Lancet 2021, 397, 1204–1212. [Google Scholar] [CrossRef]
- OMurchu, E.; Byrne, P.; Carty, P.G.; De Gascun, C.; Keogan, M.; O’Neill, M.; Harrington, P.; Ryan, M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev. Med. Virol. 2022, 32, e2260. [Google Scholar]
- Murillo-Zamora, E.; Trujillo, X.; Huerta, M.; Ríos-Silva, M.; Aguilar-Sollano, F.; Mendoza-Cano, O. Symptomatic SARS-COV-2 reinfection: Healthcare workers and immunosuppressed individuals at high risk. BMC Infect. Dis. 2021, 21, 923. [Google Scholar] [CrossRef]
- Powell, A.A.; Kirsebom, F.; Stowe, J.; Ramsay, M.E.; Lopez-Bernal, J.; Andrews, N.; Ladhani, S.N. Protection against symptomatic infection with delta (B.1.617.2) and omicron (B.1.1.529) BA.1 and BA.2 SARS-CoV-2 variants after previous infection and vaccination in adolescents in England, August 2021–March 2022: A national, observational, test-negative, case-control study. Lancet Infect. Dis. 2023, 23, 435–444. [Google Scholar] [PubMed]
- Altarawneh, H.N.; Chemaitelly, H.; Hasan, M.R.; Ayoub, H.H.; Qassim, S.; AlMukdad, S. Protection against the Omicron variant from previous SARS-CoV-2 infection. N. Engl. J. Med. 2022, 386, 1288–1290. [Google Scholar] [CrossRef]
- Willett, B.J.; Grove, J.; MacLean, O.A.; Wilkie, C.; De Lorenzo, G.; Furnon, W.; Cantoni, D.; Scott, S.; Logan, N.; Ashraf, S.; et al. SARS-CoV-2 Omicron is an immune escape variant with an altered cell entry pathway. Nat. Microbiol. 2022, 7, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Basso, P.; Negro, C.; Cegolon, L.; Larese Filon, F. Risk of Vaccine Breakthrough SARS-CoV-2 Infection and Associated Factors in Healthcare Workers of Trieste Teaching Hospitals (North-Eastern Italy). Viruses 2022, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, M.; Puopolo, M.; Morciano, C.; Spuri, M.; Spila Alegiani, S.; Filia, A.; D’Ancona, F.; Del Manso, M.; Riccardo, F.; Tallon, M.; et al. Effectiveness of mRNA vaccines and waning of protection against SARS-CoV-2 infection and severe covid-19 during predominant circulation of the delta variant in Italy: Retrospective cohort study 2022. BMJ 2022, 376, e069052. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Clinical Characteristics of COVID-19. Available online: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/clinical (accessed on 10 June 2023).
- Guedes, A.R.; Oliveira, M.S.; Tavares, B.M.; Luna-Muschi, A.; Lazari, C.d.S.; Montal, A.C.; de Faria, E.; Maia, F.L.; Barboza, A.d.S.; Leme, M.D.; et al. Reinfection rate in a cohort of healthcare workers over 2 years of the COVID-19 pandemic. Sci. Rep. 2023, 13, 712. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef]
- Cohen, D.; Izak, M.; Stoyanov, E.; Mandelboim, M.; Perlman, S.; Amir, Y.; Goren, S.; Bialik, A.; Kliker, L.; Atari, N.; et al. Predictors of reinfection with pre-Omicron and Omicron variants of concern among individuals who recovered from COVID-19 in the first year of the pandemic. Int. J. Infect. Dis. 2023, 132, 72–79. [Google Scholar] [CrossRef]
- Gazit, S.; Saciuk, Y.; Perez, G.; Peretz, A.; Ben-Tov, A.; Stuart, E.; Patalon, T. Hybrid immunity against reinfection with SARS-CoV-2 following a previous SARS-CoV-2 infection and single dose of the BNT162b2 vaccine in children and adolescents: A target trial emulation. Lancet Microbe 2023, 4, e495–e505. [Google Scholar] [CrossRef]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790. [Google Scholar] [CrossRef]
- Hall, V.; Foulkes, S.; Insalata, F.; Kirwan, P.; Saei, A.; Atti, A.; Wellington, E.; Khawam, J.; Munro, K.; Cole, M.; et al. Protection against SARS-CoV-2 after Covid-19 Vaccination and Previous Infection. N. Engl. J. Med. 2022, 386, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Kang, L.; Cao, G.; Wang, Y.; Gao, P.; Liu, J.; Liu, M. Percentage of Asymptomatic Infections among SARS-CoV-2 Omicron Variant-Positive Individuals: A Systematic Review and Meta-Analysis. Vaccines 2022, 10, 1049. [Google Scholar] [CrossRef]
- Cegolon, L.; Negro, C.; Pesce, M.; Filon, F.L. COVID-19 Incidence and Vaccine Effectiveness in University Staff, 1 March 2020–2 April 2022. Vaccines 2023, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Yahav, D.; Yelin, D.; Eckerle, I.; Eberhardt, C.S.; Wang, J.; Cao, B.; Kaiser, L. Definitions for coronavirus disease 2019 reinfection, relapse and PCR re-positivity. Clin. Microbiol. Infect. 2021, 27, 315–318. [Google Scholar] [CrossRef]
- Araf, Y.; Akter, F.; Tang, Y.D.; Fatemi, R.; Parvez, M.S.A.; Zheng, C.; Hossain, M.G. Omicron variant of SARS-CoV-2: Genomics, transmissibility, and responses to current COVID-19 vaccines. J. Med. Virol. 2022, 94, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Sheward, D.J.; Kim, C.; Ehling, R.A.; Pankow, A.; Dopico, X.C.; Dyrdak, R.; Martin, D.; Reddy, S.; Dillner, J.; Hedestam, G.K.; et al. Neutralisation sensitivity of the SARS-CoV-2 omicron (B.1.1.529) variant: A cross-sectional study. Lancet Infect. Dis. 2022, 22, 813–820. [Google Scholar] [CrossRef]
- Montes-González, J.A.; Zaragoza-Jiménez, C.A.; Antonio-Villa, N.E.; Fermín-Martínez, C.A.; Ramírez-García, D.; Vargas-Vázquez, A.; Gutiérrez-Vargas, R.I.; García-Rodríguez, G.; López-Gatell, H.; Valdés-Ferrer, S.I.; et al. Protection of hybrid immunity against SARS-CoV-2 reinfection and severe COVID-19 during periods of Omicron variant predominance in Mexico. Front. Public Health 2023, 11, 1146059. [Google Scholar] [CrossRef]
- Ochoa-Hein, E.; ELeal-Morán, P.; Nava-Guzmán, K.A. Significant Rise in SARS-CoV-2 Reinfection Rate in Vaccinated Hospital Workers during the Omicron Wave: A Prospective Cohort Study. Rev. Invest. Clin. 2022, 74, 175–180. [Google Scholar] [CrossRef]
- Malhotra, S.; Mani, K.; Lodha, R.; Bakhshi, S.; Mathur, V.P.; Gupta, P.; Kedia, S.; Sankar, M.J.; Kumar, P.; Kumar, A.; et al. COVID-19 infection, and reinfection, and vaccine effectiveness against symptomatic infection among health care workers in the setting of omicron variant transmission in New Delhi, India. Lancet Reg. Health 2022, 3, 100023. [Google Scholar]
- COVID-19 Forecasting Team. Past SARS-CoV-2 infection protection against reinfection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Niyomnaitham, S.; Toh, Z.Q.; Licciardi, P.V.; Wongprompitak, P.; Srisutthisamphan, K.; Copeland, K.K.; Chokephaibulkit, K. Immunogenicity of a single dose of BNT162b2, ChAdOx1 nCoV-19, or CoronaVac against SARS-CoV-2 delta and omicron variants among previously infected adults: A randomized trial. J. Infect. 2022, 85, 436–480. [Google Scholar] [CrossRef] [PubMed]
- Epsi, N.J.; Richard, S.A.; Lindholm, D.A.; Mende, K.; Ganesan, A.; Huprikar, N.; Lalani, T.; Fries, A.; Maves, R.; Colombo, R.; et al. Understanding “hybrid immunity”: Comparison and predictors of humoral immune responses to severe acute respiratory syndrome coronavirus 2 infection (SARS-CoV-2) and coronavirus disease 2019 (COVID-19) Vaccines. Clin. Infect. Dis. 2022, 3, e439–e449. [Google Scholar] [CrossRef] [PubMed]
- Goldblatt, D. SARS-CoV-2: From herd immunity to hybrid immunity. Nat. Rev. Immunol. 2022, 22, 333–334. [Google Scholar] [CrossRef] [PubMed]
- Carazo, S.; Skowronski, D.M.; Brisson, M.; Barkati, S.; Sauvageau, C.; Brousseau, N.; Gilca, R.; Fafard, J.; Talbot, D.; Ouakki, M.; et al. Protection against omicron (B.1.1.529) BA.2 reinfection conferred by primary omicron BA.1 or pre-omicron SARS-CoV-2 infection among health-care workers with and without mRNA vaccination: A test-negative case-control study. Lancet Infect. Dis. 2022, 23, 45–55. [Google Scholar] [CrossRef]
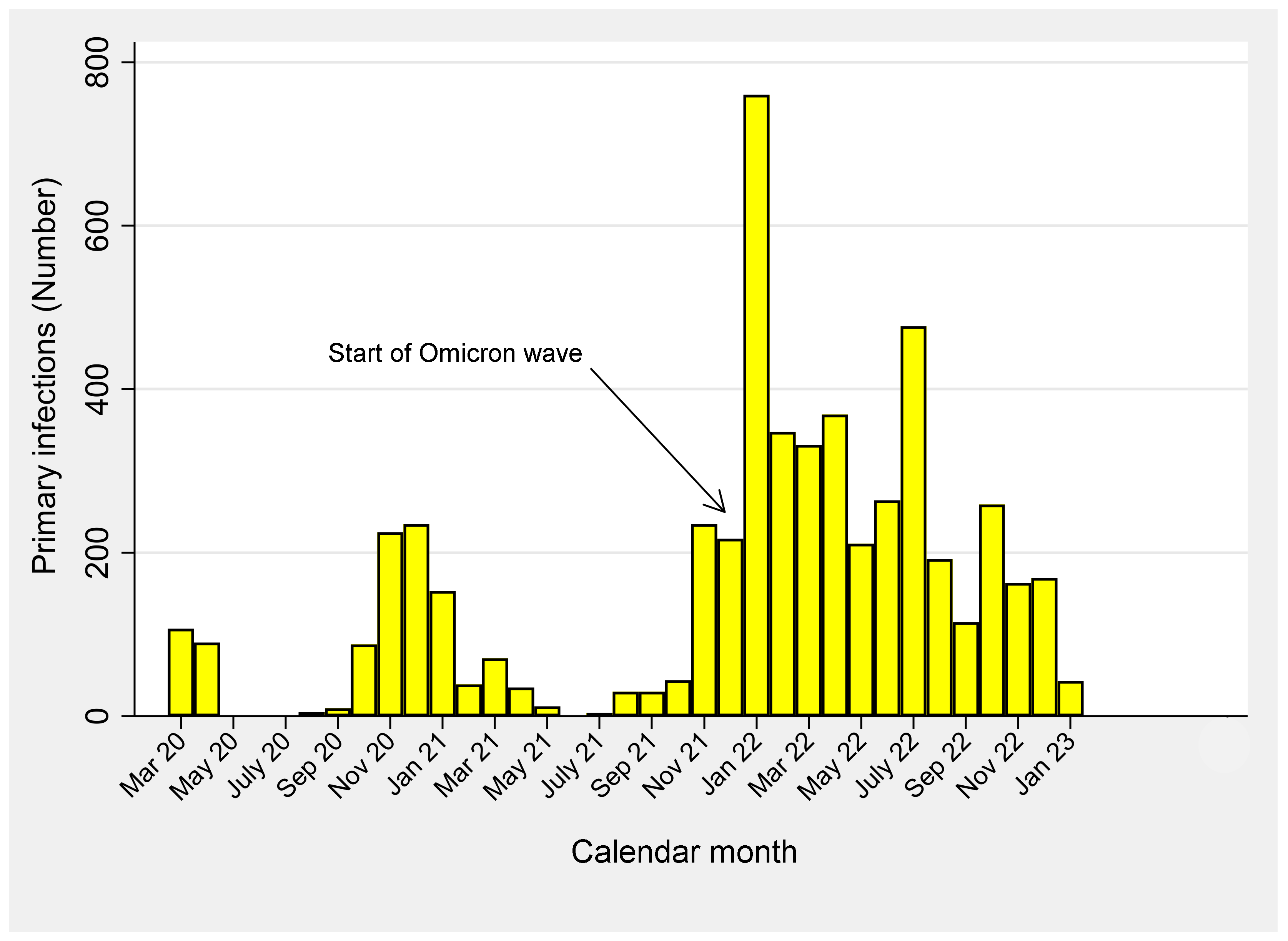
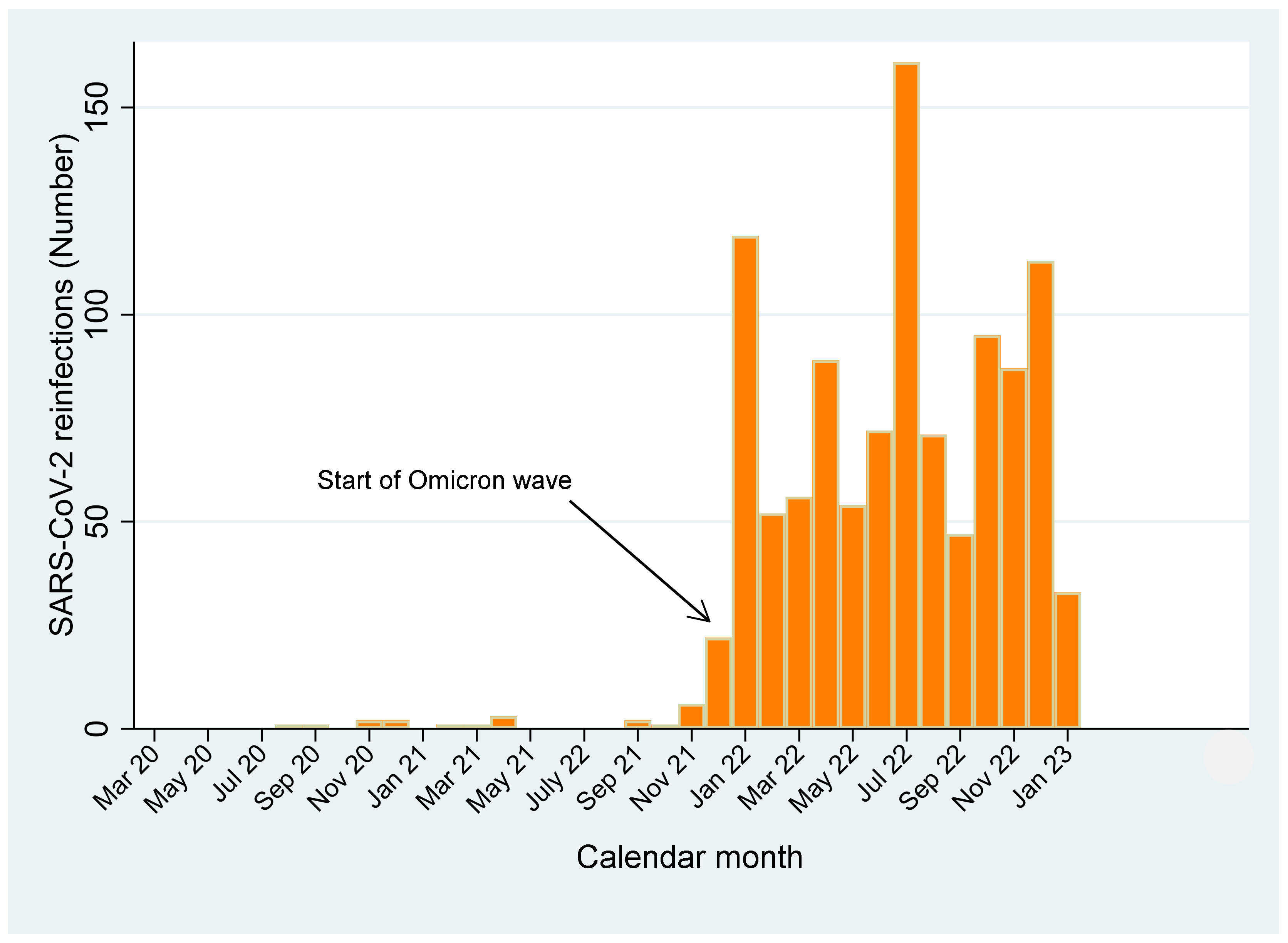

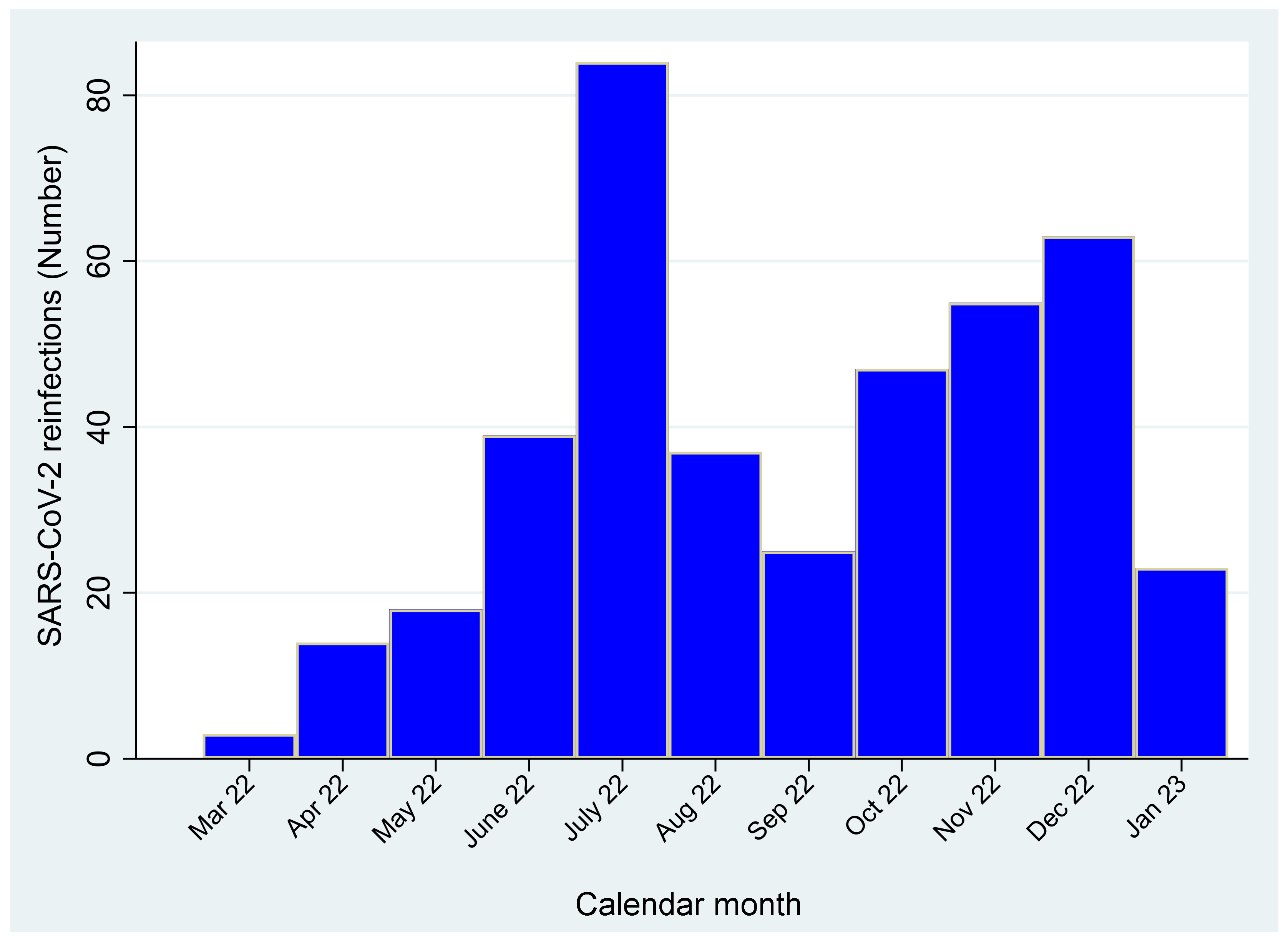
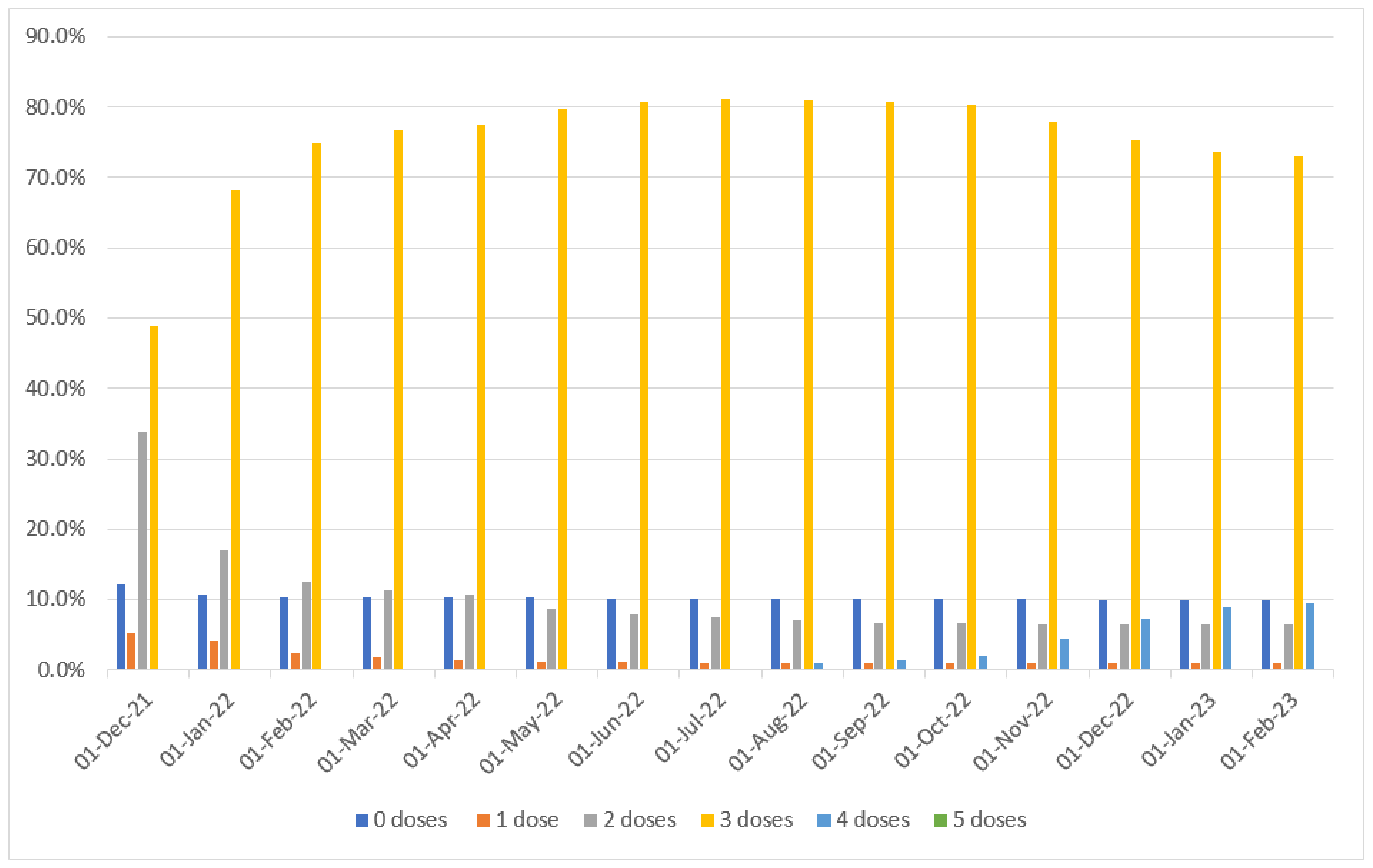
| Terms | Total | SARS-CoV-2 Infections | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| Only One | Two | |||||||
| TOTAL (row %) | 8205 (100) | 4262 (51.9) | 1091 (13.3) | |||||
| Primary SARS-CoV-2 Infections | Before 1 December 2021 | 1434 (26.8) | 751 (17.6) | 683 (62.6) | <0.001 | |||
| After 30 November 2021 | 3919 (73.2) | 3511 (82.4) | 408 (37.4) | |||||
| SARS-CoV-2 Reinfections | Before 1 December 2021 | 20 (1.8) | NA | 20 | ||||
| After 30 November 2021 | 1071 (98.2) | NA | 1071 | |||||
| Sex | Females | 5572 (67.9) | 2954 (60.3) | 809 (74.1) | <0.001 | |||
| Males | 2633 (32.1) | 1308 (30.7) | 282 (25.9) | |||||
| Age (years) | Mean± SD | 46.6 ± 12.0 | 46.3 ± 11.7 | 44.9 ± 10.9 | ||||
| Median (IQR) | 48 (36; 57) | 48 (36; 56) | 46 (36; 54) | <0.001 | ||||
| <30 | 866 (10.6) | 451 (10.6) | 115 (10.5) | <0.001 | ||||
| 30–39 | 1738 (21.2) | 889 (21.2) | 258 (23.7) | |||||
| 40–49 | 1725 (21.0) | 952 (21.0) | 290 (26.6) | |||||
| 50–59 | 2851 (34.8) | 1365 (34.8) | 367 (33.6) | |||||
| 60+ | 1025 (12.5) | 605 (12.5) | 61 (5.6) | |||||
| Employment | Administrative clerks | 1401 (17.1) | 615 (14.4) | 131 (12.0) | <0.001 | |||
| Medical doctors | 1203 (14.7) | 659 (15.5) | 107 (9.8) | |||||
| Nurses | 2908 (35.4) | 1604 (37.6) | 465 (42.6) | |||||
| Academics | 263 (3.2) | 76 (1.8) | 6 (0.6) | |||||
| Nurse aids | 1233 (15.0) | 658 (15.4) | 233 (21.4) | |||||
| Technicians | 324 (4.0) | 165 (3.9) | 42 (3.9) | |||||
| Health technicians | 873 (10.6) | 485 (11.4) | 107 (9.8) | |||||
| Dpt. | Administrative services | 1195 (14.4) | 584 (13.7) | 116 (10.1) | <0.001 | |||
| Medical and geriatric ward | 2617 (31.9) | 1237 (29.0) | 323 (12.3) | |||||
| Surgical ward | 1199 (14.6) | 692 (16.2) | 142 (13.0) | |||||
| Community services | 1636 (19.9) | 890 (20.9) | 251 (23.0) | |||||
| A&E | 764 (9.3) | 426 (10.0) | 149 (13.7)) | |||||
| Health services (radiology, labs) | 593 (7.2) | 322 (7.6) | 73 (6.7)) | |||||
| Non-clinical work and other | 115 (1.4) | 56 (1.3) | 18 (15.7) | |||||
| COVID-19 ward | 86 (1.1) | 55 (1.3) | 19 (1.7)) | |||||
| N. doses of COVID-19 vaccine | 0 | 818 (10.0) | 223 (5.2) | 188 (17.2) | <0.001 | |||
| 1 | 79 (1.0) | 32 (0.8) | 33 (3.0) | <0.001 | ||||
| 2 | 530 (6.5) | 215 (5.0) | 135 (12.4) | <0.001 | ||||
| 3 | 5988 (73.0) | 3441 (80.7) | 688 (63.1) | <0.001 | ||||
| 4 | 773 (9.4) | 348 (8.2) | 47 (4.3) | <0.001 | ||||
| 5 | 17 0.2) | 3 (0.1) | 0 | - | ||||
| Swabs tests (Number) | 1 March 2020–31 January 2023 (range: 5–130) | Mean± SD | 37.8 ± 19.9 | 41.2 ± 18.4 | 47.4 ± 18.0 | |||
| Median (IQR) | 37 (23: 51) | 40 (28; 53) | 46 (35; 58) | <0.001 | ||||
| 1 December 2021–31 January 2023 (range: 2–70) | Mean± SD | 12.9 ± 8.8 | 14.4 ± 8.5 | 16.2 ± 8.0 | ||||
| Median (IQR) | 12 (6–18) | 13 (8–19) | 15 (11; 20) | <0.001 | ||||
| 1st Vaccine Dose | Comirnaty | 6875 (94.6) | 3785 (95.1) | 832 (92.5) | 0.005 | |||
| Spikevax | 319 (4.4) | 161 (4.1) | 58 (6.5) | |||||
| Others * | 71 (1.0) | 34 (0.8) | 7 (1.0) | |||||
| 2nd Vaccine Dose | Comirnaty | 6594 (94.9) | 3664 (95.9) | 686 (92.0) | <0.001 | |||
| Spikevax | 298 (4.3) | 139 (3.6) | 54 (7.2) | |||||
| Others * | 56 (0.8) | 16 (0.5) | 5 (0.8) | |||||
| 3rd Vaccine Dose | Comirnaty | 6095 (91.2) | 3473 (91.7) | 658 (89.5) | 0.116 | |||
| Spikevax | 558 (8.4) | 274 (7.2) | 64 (8.7) | |||||
| Others * | 28 (0.4) | 42 (1.1) | 13 (1.8) | |||||
| DATE | 0 Doses | 1 Dose | 2 Doses | 3 Doses | 4 Doses | 5 Doses |
|---|---|---|---|---|---|---|
| 1 December 2021 | 989 | 428 | 2773 | 4015 | 0 | 0 |
| 15 December 2021 | 920 | 362 | 1695 | 5228 | 0 | 0 |
| 1 January 2022 | 887 | 323 | 1394 | 5601 | 0 | 0 |
| 15 January 2022 | 873 | 259 | 1240 | 5833 | 0 | 0 |
| 1 February 2022 | 853 | 195 | 1022 | 6135 | 0 | 0 |
| 15 February 2022 | 847 | 158 | 944 | 6256 | 0 | 0 |
| 1 March 2022 | 847 | 139 | 922 | 6297 | 0 | 0 |
| 15 March 2022 | 849 | 127 | 903 | 6326 | 0 | 0 |
| 1 April 2022 | 845 | 116 | 877 | 6361 | 6 | 0 |
| 15 April 2022 | 842 | 108 | 806 | 6443 | 6 | 0 |
| 1 May 2022 | 840 | 99 | 708 | 6548 | 10 | 0 |
| 15 May 2022 | 837 | 95 | 668 | 6594 | 11 | 0 |
| 1 June 2022 | 833 | 90 | 644 | 6625 | 13 | 0 |
| 15 June 2022 | 832 | 89 | 630 | 6638 | 16 | 0 |
| 1 July 2022 | 831 | 84 | 617 | 6656 | 17 | 0 |
| 15 July 2022 | 830 | 82 | 594 | 6671 | 28 | 0 |
| 1 August 2022 | 830 | 79 | 581 | 6641 | 74 | 0 |
| 15 August 2022 | 987 | 77 | 537 | 6503 | 101 | 0 |
| 1 September 2022 | 829 | 77 | 553 | 6626 | 120 | 0 |
| 15 September 2022 | 829 | 77 | 548 | 6623 | 128 | 0 |
| 1 October 2022 | 828 | 77 | 543 | 6597 | 160 | 0 |
| 15 October 2022 | 817 | 77 | 537 | 6503 | 260 | 0 |
| 1 November 2022 | 824 | 78 | 533 | 6398 | 371 | 1 |
| 15 November 2022 | 822 | 78 | 532 | 6296 | 473 | 4 |
| 1 December 2022 | 818 | 79 | 532 | 6178 | 589 | 9 |
| 15 December 2022 | 818 | 79 | 531 | 6111 | 655 | 11 |
| 1 January 2023 | 818 | 79 | 531 | 6037 | 728 | 12 |
| 15 January 2023 | 818 | 79 | 531 | 6007 | 756 | 14 |
| 31 January 2023 | 818 | 79 | 530 | 5988 | 773 | 17 |
| TERM | MALES | FEMALES | TOTAL | p-Value | ||
|---|---|---|---|---|---|---|
| All HCWs (N = 1071) | Range | 100; 1010 | 92; 1025 | 92; 1025 | ||
| M ± SD | 450.7 ± 227.6 | 382.7 ± 215.3 | 400.3 ± 220.5 | |||
| Median (IQR) | 425 (272; 643) | 328 (193; 536) | 356 (202; 567) | <0.001 | ||
| Primary SARS-CoV-2 event | Pre-Omicron (N = 683) | Range | 100; 1010 | 92; 1025 | 92; 1025 | |
| M ± SD | 535.8 ± 206.8 | 502.1 ± 204.3 | 512.3 ± 205.5 | |||
| Median (IQR) | 524 (396; 687) | 497.5 (371; 655) | 508 (379; 664) | 0.069 | ||
| Omicron (N = 408) | Range | 104; 378.3 | 92; 373 | 92; 378.3 | ||
| M ± SD | 225.7 ± 76.8 | 216.6 ± 73.3 | 218.3 ± 74.0 | |||
| Median (IQR) | 205 (172; 287.5) | 199.5 (159; 281.7) | 200 (161; 282.5) | 0.388 | ||
| Vaccination status | Unvaccinated (N = 193) | Range | 100; 744 | 92; 857 | 92; 857 | |
| M ± SD | 249.4 ± 149.9 | 282.7 ± 167 | 239.6 ± 143.1 | |||
| Median (IQR) | 185 (154; 300) | 242 (151; 390) | 181 (154; 294) | 0.143 | ||
| >14 days after 1st dose and before 2nd dose (N = 158) | Range | 300; 753.54 | 101; 981 | 101; 981 | ||
| M ± SD | 530.3 ± 125.3 | 499.9 ± 147.9 | 505.8 ± 141.7 | |||
| Median (IQR) | 503 (413; 620) | 479 (405; 598.5) | 484.5 (406; 602) | 0.397 | ||
| >7 days after 2nd dose and Before 3rd dose (N = 136) | Range | 104; 802 | 97; 800 | (97; 802 | ||
| M ± SD | 424 ± 217.7 | 363.4 ± 206.3 | 379.1 ± 210.2 | |||
| Median (IQR) | 425 (248; 633) | 360 (171; 541) | 361 (175; 574.5) | 0.161 | ||
| >7 days after 3rd dose and before 4th dose (N = 680) | Range | 108.3; 1010 | 92; 1025 | 92; 1025 | ||
| M ± SD | 496.7 ± 225.1 | 424.5 ± 219.6 | 443.7 ± 223.2 | |||
| Median (IQR) | 484 (315; 676) | 379 (246; 590) | 416 (258; 623.5) | <0.001 | ||
| >7 days after 4th dose and before 5th dose (N = 16) | Range | 263; 935 | 276; 1000 | 263; 1000 | ||
| M ± SD | 625.2 ± 329.7 | 541.2 ± 246.3 | 567.5 ± 266.5 | |||
| Median (IQR) | 704 (279; 932) | 539 (318; 752) | 575 (306.5; 767) | 0.827 | ||
| TERMS | SARS-CoV-2 Reinfections | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All (1 December 2021–31 January 2023) | Primary SARS-CoV-2 Infection before 1 December 2021 | Primary SARS-CoV-2 Infection after 30 November 2021 | |||||||||
| Cases (N) | P-d | Cases × 1000 P-d (95% CI) | Cases (N) | P-d | Cases × 1000 P-d (95% CI) | Cases (N) | P-d | Cases × 1000 P-d (95% CI) | |||
| All HCWs | 1009 | 2,048,703.1 | 0.5 (0.5; 0.5) | 605 | 434,168.5 | 1.4 (1.3; 1.5) | 404 | 1,614,534.6 | 0.3 (0.2; 0.3) | ||
| Sex | Females | 755 | 1,434,299.5 | 0.5 (0.5; 0.6) | 425 | 292,239.5 | 1.5 (1.3; 1.6) | 330 | 1,142,060 | 0.3 (0.3; 0.3) | |
| Males | 254 | 614,403.1 | 0.4 (0.4; 0.5) | 180 | 141,929.1 | 1.3 (1.1; 1.5) | 74 | 472,474.5 | 0.2 (0.1; 0.2) | ||
| Age (years) | <30 | 105 | 214,568.3 | 0.5 (0.4; 0.6) | 71 | 47,003 | 1.5 (1.2; 1.9) | 34 | 167,565.3 | 0.2 (0.1; 0.3) | |
| 30–39 | 236 | 435,343.2 | 0.5 (0.5; 0.6) | 131 | 99,979 | 1.3 (1.1; 1.6) | 105 | 335,464.2 | 0.3 (0.3; 0.4) | ||
| 40–49 | 271 | 467,634.7 | 0.6 (0.5; 0.7) | 159 | 103,209.5 | 1.5 (1.3; 1.8) | 112 | 364,425.2 | 0.3 (0.3; 0.4) | ||
| 50–59 | 341 | 720,223 | 0.5 (0.4; 0.5) | 210 | 139,682 | 1.5 (1.3; 1.7) | 131 | 580,541 | 0.2 (0.2; 0.3) | ||
| 60+ | 56 | 210,833.9 | 0.3 (0.2; 0.3) | 34 | 44,295 | 0.8 (0.5; 1.1) | 22 | 166,538.9 | 0.1 (0.1; 0.2) | ||
| Job task | Administrative clerks | 122 | 291272.5 | 0.4 (0.4; 0.5) | 68 | 48,691.5 | 1.4 (1.1; 1.8) | 54 | 242,581 | 0.2 (0.2; 0.3) | |
| Medical doctors | 97 | 303,653.8 | 0.3 (0.3; 0.4) | 66 | 59,747 | 1.1 (0.9; 1.4) | 31 | 243906.8 | 0.1 (0.1; 0.2) | ||
| Nurses | 432 | 782,791.7 | 0.6 (0.5; 0.6) | 255 | 176,082.1 | 1.4 (1.3; 1.6) | 177 | 606,764.6 | 0.3 (0.3; 0.3) | ||
| Nurse aids | 3 | 33,035 | 0.1 (0.0; 0.3) | 1 | 5586 | 0.2 (0.0; 1.3) | 2 | 27,449 | 0.1 (0.0; 0.3) | ||
| Academics | 211 | 327,992.7 | 0.6 (0.6; 0.7) | 139 | 87,369 | 1.6 (1.3; 1.9) | 72 | 240,450.7 | 0.3 (0.2; 0.4) | ||
| Health technicians | 40 | 78,958.5 | 0.5 (0.4; 0.7) | 24 | 17,072 | 1.4 (0.9; 2.1) | 16 | 61,886.5 | 0.3 (0.2; 0.4) | ||
| Technicians | 104 | 230,944 | 0.5 (0.4; 0.5) | 52 | 39,448 | 1.3 (1.0; 1.7) | 52 | 191,496 | 0.3 (0.2; 0.4) | ||
| Dpt. | Administrative services | 110 | 273,524 | 0.4 (0.3; 0.5) | 63 | 45,848 | 1.4 (1.1; 1.8) | 47 | 227,676 | 0.2 (0.2; 0.3) | |
| Medical and geriatric ward | 294 | 594,444.7 | 0.5 (0.4; 0.6) | 177 | 143,007 | 1.2 (1.1; 1.4) | 117 | 451,437.7 | 0.1 (0.1; 0.2) | ||
| Surgical ward | 132 | 324,351 | 0.4 (0.3; 0.5) | 83 | 61,235 | 1.4 (1.1; 1.7) | 49 | 263,116 | 0.1 (0.1; 0.2) | ||
| Community services | 235 | 434,933.1 | 0.5 (0.5; 0.6) | 128 | 88,390.5 | 1.4 (1.2; 1.7) | 107 | 346,542.6 | 0.2 (0.2; 0.2) | ||
| A&E | 132 | 210,566 | 0.6 (0.5; 0.7) | 90 | 50,856 | 1.8 (1.4; 2.2) | 42 | 159,710.0 | 0.2 (0.1; 0.2) | ||
| Health services (radiology, labs) | 71 | 154,940.9 | 0.5 (0.4; 0.6) | 42 | 28,510 | 1.5 (1.1; 2.0) | 29 | 126,429.9 | 0.1 (0.1; 0.2) | ||
| Non-clinical unit and other | 16 | 27,678 | 0.6 (0.4; 0.9) | 8 | 6197 | 1.3 (0.6; 2.6) | 8 | 21,481 | 0.2 (0.1; 0.4) | ||
| COVID-19 unit | 19 | 28,265.3 | 0.7 (0.4; 1.1) | 14 | 10,124 | 1.4 (0.8; 2.3) | 5 | 18,141.3 | 0.2 (0.1; 0.5) | ||
| Doses of COVID-19 vaccine | 0 | 193 | 129,391.5 | 1.5 (1.3; 1.7) | 98 | 36,134 | 2.2 (2.1; 3.3) | 95 | 93,257.5 | 1.0 (0.8; 1.2) | |
| 1 | 29 | 19,606 | 1.5 (1.0; 2.1) | 25 | 14,692 | 1.7 (1.1; 2.5) | 4 | 4914 | 0.8 (0.3; 2.2) | ||
| 2 | 118 | 123,654 | 1.0 (0.8; 1.1) | 76 | 62,066 | 1.2 (1.0; 1.5) | 42 | 61,588 | 0.7 (0.5; 0.9) | ||
| 3 | 652 | 1,625,118.6 | 0.4 (0.4; 0.4) | 397 | 293,277.5 | 1.4 (1.2; 1.5) | 255 | 1,333,841.1 | 0.2 (0.2; 0.2) | ||
| 4 | 17 | 149,652 | 0.1 (0.1; 0.2) | 9 | 27,999 | 0.3 (0.2; 0.6) | 8 | 121,653 | 0.1 (0.0; 0.1) | ||
| Terms | Multivariable Cox Regression Analysis aHR (95% CI) | ||||
|---|---|---|---|---|---|
| All Reinfections (5271 obs.) | With Primary Event before 1 December 2021 (1356 obs.) | With Primary Event after 30 November 2021 (3915 obs.) | |||
| Sex | Females | Reference | Reference | Reference | |
| Males | 0.84 (0.73; 0.98) | 0.95 (0.79; 1.15) | 0.57 (0.43; 0.75) | ||
| Age (years) | <30 | Reference | Reference | Reference | |
| 30–39 | 1.11 (0.88; 1.41) | 0.84 (0.62; 1.13) | 1.63 (1.10; 2.41) | ||
| 40–49 | 1.13 (0.90; 1.43) | 0.91 (0.68; 1.22) | 1.51 (1.02; 2.24) | ||
| 50–59 | 0.85 (0.67; 1.06) | 0.89 (0.67; 1.18) | 0.94 (0.64; 1.39) | ||
| ≥60 | 0.53 (0.38; 0.74) | 0.55 (0.36; 0.85) | 0.58 (0.33; 1.02) | ||
| Employment | Administrative clerks | Reference | Reference | Reference | |
| Medical doctors | 0.90 (0.67; 1.21) | 0.91 (0.61; 1.35) | 0.70 (0.43; 1.11) | ||
| Nurses | 1.22 (0.96; 1.53) | 0.88 (0.63; 1.23) | 1.24 (0.87; 1.76) | ||
| Academics | 0.20 (0.06; 0.63) | 0.08 (0.01; 0.61) | 0.32 (0.08; 1.33) | ||
| Nurse Aids | 1.38 (1.07; 1.77) | 0.94 (0.65; 1.34) | 1.23 (0.83; 1.80) | ||
| Technicians | 1.27 (0.87; 1.86) | 1.02 (0.61; 1.71) | 1.25 (0.66; 2.35) | ||
| Health technicians | 0.96 (0.72; 1.28) | 0.83 (0.54; 1.28) | 1.09 (0.72; 1.65) | ||
| Dpt. | Administrative services | Reference | Reference | Reference | |
| Community services | 1.09 (0.84; 1.39) | 0.83 (0.54; 1.28) | 1.18 (0.81; 1.72) | ||
| Health services (radiology, labs) | 0.87 (0.66; 1.17) | 0.81 (0.57; 1.16) | 0.90 (0.58; 1.42) | ||
| Surgical wards | 1.10 (0.85; 1.41) | 0.79 (0.53; 1.18) | 1.33 (0.91; 1.95) | ||
| Medical and geriatric wards | 1.29 (0.97; 1.71) | 1.07 (0.72; 1.59) | 1.19 (0.75; 1.90) | ||
| COVID-19 wards | 1.12 (0.81; 1.55) | 1.07 (0.67; 1.71) | 1.07 (0.65; 1.76) | ||
| A&E | 1.17 (0.68; 2.01) | 0.87 (0.39; 1.92) | 1.50 (0.69; 3.27) | ||
| Non-clinical units | 1.33 (0.80; 2.22) | 0.78 (0.42; 1.47) | 0.86 (0.32; 2.27) | ||
| Doses of COVID-19 vaccine | 0 | Reference | Reference | Reference | |
| 1 | 0.84 (0.56; 1.25) | 0.60 (0.38; 0.94) | 0.28 (0.09; 0.88) | ||
| 2 | 0.49 (0.38; 0.63) | 0.31 (0.22; 0.42) | 0.41 (0.27; 0.63) | ||
| 3 | 0.24 (0.20; 0.28) | 0.38 (0.30; 0.48) | 0.16 (0.13; 0.20) | ||
| 4 | 0.08 (0.05; 0.14) | 0.11 (0.05; 0.22) | 0.07 (0.04; 0.15) | ||
| Swab tests during Omicron wave (linear term) | 1.00 (1.00; 1.03) | 1.04 (1.03; 1.05) | 1.00 (1.00; 1.03) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cegolon, L.; Magnano, G.; Negro, C.; Larese Filon, F., on behalf of the ORCHESTRA Working Group. SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses 2023, 15, 1551. https://doi.org/10.3390/v15071551
Cegolon L, Magnano G, Negro C, Larese Filon F on behalf of the ORCHESTRA Working Group. SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses. 2023; 15(7):1551. https://doi.org/10.3390/v15071551
Chicago/Turabian StyleCegolon, Luca, Greta Magnano, Corrado Negro, and Francesca Larese Filon on behalf of the ORCHESTRA Working Group. 2023. "SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023" Viruses 15, no. 7: 1551. https://doi.org/10.3390/v15071551
APA StyleCegolon, L., Magnano, G., Negro, C., & Larese Filon, F., on behalf of the ORCHESTRA Working Group. (2023). SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses, 15(7), 1551. https://doi.org/10.3390/v15071551







