Glycemic Dysregulation, Inflammation and Disease Outcomes in Patients Hospitalized with COVID-19: Beyond Diabetes and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Population
2.2. Data Collection
2.3. Study Variables and Endpoints
2.4. Statistical Analysis
3. Results
3.1. Patients with Diabetes
3.2. Patients with Obesity
3.3. Patients without Diabetes or Obesity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 24 April 2023).
- Manique, I.; Abegão Matias, A.; Bouça, B. Does the Hyperglycemia Impact on COVID-19 Outcomes Depend upon the Presence of Diabetes?—An Observational Study. Metabolites 2022, 12, 1116. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Gillies, C.L. Prevalence of co-morbidities and their association with mortality in patients with COVID-19: A systematic review and meta-analysis. Diabetes Obes. Metab. 2020, 22, 1915–1924. [Google Scholar] [CrossRef] [PubMed]
- Reiterer, M.; Rajan, M.; Gomez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5. [Google Scholar] [CrossRef]
- Dissanayake, H. COVID-19 and metabolic syndrome. Best Pract. Research. Clin. Endocrinol. Metab. 2023, 101753, in press. [Google Scholar] [CrossRef]
- Arulanandam, B.; Beladi, H.; Chakrabarti, A. Obesity and COVID-19 mortality are correlated. Sci. Rep. 2023, 13, 5895. [Google Scholar] [CrossRef]
- Morse, J.; Gay, W.; Korwek, K.M. Hyperglycaemia increases mortality risk in non-diabetic patients with COVID-19 even more than in diabetic patients. Endocrinol Diabetes Metab. 2021, 4, e00291. [Google Scholar] [CrossRef]
- Huang, I.; Lim, M.A.; Pranata, R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—A systematic review, meta-analysis, and meta-regression. Diabetes Metab. Syndr. 2020, 14, 395–403. [Google Scholar] [CrossRef]
- Gerganova, A.; Assyov, Y.; Kamenov, Z. Stress Hyperglycemia, Diabetes Mellitus and COVID-19 Infection: Risk Factors, Clinical Outcomes and Post-Discharge Implications. Front. Clin. Diabetes Healthc. 2022, 3, 826006. [Google Scholar] [CrossRef]
- Roncon, L.; Zuin, M.; Rigatelli, G.; Zuliani, G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020, 127, 104354. [Google Scholar] [CrossRef]
- Khunti, K.; Del Prato, S.; Mathieu, C.; Kahn, S.E.; Gabbay, R.A.; Buse, J.B. COVID-19, Hyperglycemia, and New-Onset Diabetes. Diabetes Care 2021, 44, 2645–2655. [Google Scholar] [CrossRef]
- Dennis, J.M.; Mateen, B.A.; Sonabend, R.; Thomas, N.J.; Patel, K.A.; Hattersley, A.T.; Denaxas, S.; McGovern, A.P.; Vollmer, S.J. Type 2 Diabetes and COVID-19–Related Mortality in the Critical Care Setting: A National Cohort Study in England, March–July 2020. Diabetes Care 2020, 44, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Scisciola, L.; Sardu, C.; Trotta, M.C.; De Feo, M.; Maiello, C.; Mascolo, P.; De Micco, F.; Turriziani, F.; Municino, E.; et al. Glycated ACE2 receptor in diabetes: Open door for SARS-CoV-2 entry in cardiomyocyte. Cardiovasc. Diabetol. 2021, 20, 99. [Google Scholar] [CrossRef]
- Tzeravini, E.; Stratigakos, E.; Siafarikas, C.; Tentolouris, A.; Tentolouris, N. The Role of Diabetes and Hyperglycemia on COVID-19 Infection Course-A Narrative Review. Front. Clin. Diabetes Healthc. 2022, 3, 812134. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; Monteiro, L.B.; de Souza, G.F.; Muraro, S.P.; Virgilio-da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Lontchi-Yimagou, E.; Sobngwi, E.; Matsha, T.E.; Kengne, A.P. Diabetes mellitus and inflammation. Curr. Diabetes Rep. 2013, 13, 435–444. [Google Scholar] [CrossRef]
- Hayden, M.R. An Immediate and Long-Term Complication of COVID-19 May Be Type 2 Diabetes Mellitus: The Central Role of β-Cell Dysfunction, Apoptosis and Exploration of Possible Mechanisms. Cells 2020, 9, 2475. [Google Scholar] [CrossRef]
- Yates, T.; Razieh, C.; Zaccardi, F.; Rowlands, A.V. Obesity, walking pace and risk of severe COVID-19 and mortality: Analysis of UK Biobank. Int. J. Obes. 2021, 45, 1155–1159. [Google Scholar] [CrossRef]
- Kwok, S.; Adam, S.; Ho, J.H.; Iqbal, Z.; Turkington, P.; Razvi, S.; Le Roux, C.W.; Soran, H.; Syed, A.A. Obesity: A critical risk factor in the COVID-19 pandemic. Clin. Obes. 2020, 10, e12403. [Google Scholar] [CrossRef]
- Maccioni, L.; Weber, S.; Elgizouli, M.; Stoehlker, A.S.; Geist, I.; Peter, H.H.; Vach, W.; Nieters, A. Obesity and risk of respiratory tract infections: Results of an infection-diary based cohort study. BMC Public Health 2018, 18, 271. [Google Scholar] [CrossRef] [PubMed]
- Raeisi, T.; Mozaffari, H.; Sepehri, N.; Darand, M.; Razi, B.; Garousi, N.; Alizadeh, M.; Alizadeh, S. The negative impact of obesity on the occurrence and prognosis of the 2019 novel coronavirus (COVID-19) disease: A systematic review and meta-analysis. Eat. Weight Disord. Stud. Anorex. Bulim. Obes. 2022, 27, 893–911. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.B.; Verma, A. COVID-19 and Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers: What Is the Evidence? JAMA 2020, 323, 1769–1770. [Google Scholar] [CrossRef] [PubMed]
- Kassir, R. Risk of COVID-19 for patients with obesity. Obesity reviews: An official journal of the International Association for the Study of Obesity. Obes. Rev. 2020, 21, e13034. [Google Scholar] [CrossRef] [PubMed]
- Rico-Martin, S.; Calderon-Garcia, J.F.; Basilio-Fernandez, B.; Clavijo-Chamorro, M.Z.; Sanchez Munoz-Torrero, J.F. Metabolic Syndrome and Its Components in Patients with COVID-19: Severe Acute Respiratory Syndrome (SARS) and Mortality. A Systematic Review and Meta-Analysis. J. Cardiovasc. Dev. Dis. 2021, 8, 162. [Google Scholar] [CrossRef]
- Gualtieri, P.; Marchetti, M.; Renzo, L.D.; De Santis, G.L.; Palma, R.; Colica, C.; Frank, G.; De Lorenzo, A.; Di Lorenzo, N. Impact of COVID-19 on the Destiny of Bariatric Patients. Nutrients 2022, 15, 163. [Google Scholar] [CrossRef]
- Zhu, L.; She, Z.G.; Cheng, X.; Qin, J.J.; Zhang, X.J.; Cai, J.; Lei, F.; Wang, H.; Xie, J.; Wang, W.; et al. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020, 31, 1068–1077.e3. [Google Scholar] [CrossRef]
- Jing, X.; Chen, J.; Dong, Y.; Han, D.; Zhao, H.; Wang, X.; Gao, F.; Li, C.; Cui, Z.; Liu, Y.; et al. Related factors of quality of life of type 2 diabetes patients: A systematic review and meta-analysis. Health Qual. Life Outcomes 2018, 16, 189. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Blundell, S.; Harris, T.; Cook, D.G.; Critchley, J. Diabetes and infection: Assessing the association with glycaemic control in population-based studies. Lancet. Diabetes Endocrinol. 2016, 4, 148–158. [Google Scholar] [CrossRef]
- Aluganti Narasimhulu, C.; Singla, D.K. Mechanisms of COVID-19 pathogenesis in diabetes. Am. J. Physiol. Heart Circ. Physiol. 2022, 323, H403–H420. [Google Scholar] [CrossRef]
- Li, S.; Wang, J. Diabetes Mellitus and Cause-Specific Mortality: A Population-Based Study. Curr. Opin. Hematol. 2019, 43, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Gubbi, S. COVID-19 pandemic, coronaviruses, and diabetes mellitus. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E736–E741. [Google Scholar] [CrossRef]
- Hodgson, K.; Morris, J.; Bridson, T.; Govan, B.; Rush, C.; Ketheesan, N. Immunological mechanisms contributing to the double burden of diabetes and intracellular bacterial infections. Immunology 2015, 144, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Lau, A.; So, H.C. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care 2020, 43, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.R.; Cao, Q.D.; Hong, Z.S.; Tan, Y.Y.; Chen, S.D.; Jin, H.J.; Tan, K.S.; Wang, D.Y.; Yan, Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020, 7, 11. [Google Scholar] [CrossRef]
- Gao, T.; Zhu, L. Highly pathogenic coronavirus N protein aggravates inflammation by MASP-2-mediated lectin complement pathway overactivation. medRxiv 2022, 7, 318. [Google Scholar] [CrossRef]
- Abbasi, F.; Reaven, G.M. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: Triglycerides × glucose versus triglyceride/high-density lipoprotein cholesterol. Metab. Clin. Exp. 2011, 60, 1673–1676. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef]
- Rohani-Rasaf, M.; Mirjalili, K.; Vatannejad, A.; Teimouri, M. Are lipid ratios and triglyceride-glucose index associated with critical care outcomes in COVID-19 patients? PLoS ONE 2022, 17, e0272000. [Google Scholar] [CrossRef] [PubMed]
- Babic, N.; Valjevac, A.; Zaciragic, A.; Avdagic, N.; Zukic, S.; Hasic, S. The Triglyceride/HDL Ratio and Triglyceride Glucose Index as Predictors of Glycemic Control in Patients with Diabetes Mellitus Type 2. Med. Arch. 2019, 73, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, S.; AlMasoudi, A.S.; AlBuhayri, A.H.; AlAtwi, H.M.; AlHwiti, S.S.; Alaidi, H.M.; Alshehri, A.M.; Alanazi, N.A.; Aljabri, A.; Al-Gayyar, M.M. Effect of COVID-19 on Glycemic Control, Insulin Resistance, and pH in Elderly Patients with Type 2 Diabetes. Cureus 2023, 15, e35390. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.K.; Feng, Y.; Yuan, M.Y.; Yuan, S.Y.; Fu, H.J.; Wu, B.Y.; Sun, G.Z.; Yang, G.R.; Zhang, X.L.; Wang, L.; et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Schoen, K.; Horvat, N.; Guerreiro, N.F.C.; de Castro, I.; de Giassi, K.S. Spectrum of clinical and radiographic findings in patients with diagnosis of H1N1 and correlation with clinical severity. BMC Infect. Dis. 2019, 19, 964. [Google Scholar] [CrossRef]
- Banik, G.R.; Alqahtani, A.S.; Booy, R.; Rashid, H. Risk factors for severity and mortality in patients with MERS-CoV: Analysis of publicly available data from Saudi Arabia. Virol. Sin. 2016, 31, 81–84. [Google Scholar] [CrossRef]
- Lippi, G.; Plebani, M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 58, 1131–1134. [Google Scholar] [CrossRef]
- Wang, A.; Zhao, W.; Xu, Z.; Gu, J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020, 162, 108118. [Google Scholar] [CrossRef]
- Knapp, S. Diabetes and infection: Is there a link?—A mini-review. Gerontology 2013, 59, 99–104. [Google Scholar] [CrossRef]
- Kohio, H.P.; Adamson, A.L. Glycolytic control of vacuolar-type ATPase activity: A mechanism to regulate influenza viral infection. Virology 2013, 444, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Sánchez, F.J.; López-Carmona, M.D.; Martínez-Marcos, F.J.; Pérez-Belmonte, L.M.; Hidalgo-Jiménez, A.; Buonaiuto, V.; Suárez Fernández, C.; Freire Castro, S.J.; Luordo, D.; Pesqueira Fontan, P.M.; et al. Admission hyperglycaemia as a predictor of mortality in patients hospitalized with COVID-19 regardless of diabetes status: Data from the Spanish SEMI-COVID-19 Registry. Ann. Med. 2021, 53, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ma, P.; Zhang, S.; Song, S.; Wang, Z.; Ma, Y.; Xu, J.; Wu, F.; Duan, L.; Yin, Z.; et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: A multi-centre retrospective study. Diabetologia 2020, 63, 2102–2111. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Huang, J.; Zhu, G.; Wang, Q.; Lv, Q.; Huang, Y.; Yu, Y.; Si, X.; Yi, H.; Wang, C.; et al. Elevation of blood glucose level predicts worse outcomes in hospitalized patients with COVID-19: A retrospective cohort study. BMJ Open Diabetes Res. Care 2020, 8, e001476. [Google Scholar] [CrossRef]
- Geetha, H.S.; Singh, G.; Sekar, A.; Gogtay, M.; Singh, Y.; Abraham, G.M.; Trivedi, N. Hyperglycemia in COVID-19 infection without diabetes mellitus: Association with inflammatory markers. World J. Clin. Cases 2023, 11, 1287–1298. [Google Scholar] [CrossRef]
- Stefan, N.; Birkenfeld, A.L.; Schulze, M.B. Global pandemics interconnected—Obesity, impaired metabolic health and COVID-19. Nat. Rev. Endocrinol. 2021, 17, 135–149. [Google Scholar] [CrossRef]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 3611–3616. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, B.; Chen, D.; Hu, X.; Xu, X.; Shen, W.J.; Hu, C.; Li, J.; Qu, S. COVID-19 May Increase the Risk of Insulin Resistance in Adult Patients Without Diabetes: A 6-Month Prospective Study. Endocr. Pract. 2021, 27, 834–841. [Google Scholar] [CrossRef]
- Duman, T.T.; Aktas, G.; Atak, B.M.; Kocak, M.Z.; Erkus, E.; Savli, H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr. Health Sci. 2019, 19, 1602–1606. [Google Scholar] [CrossRef]
- Coppelli, A.; Giannarelli, R.; Aragona, M.; Penno, G.; Falcone, M.; Tiseo, G.; Ghiadoni, L.; Barbieri, G.; Monzani, F.; Virdis, A.; et al. Hyperglycemia at Hospital Admission Is Associated with Severity of the Prognosis in Patients Hospitalized for COVID-19: The Pisa COVID-19 Study. Diabetes Care 2020, 43, 2345–2348. [Google Scholar] [CrossRef] [PubMed]
- Wingert, A.; Pillay, J.; Gates, M. Risk factors for severity of COVID-19: A rapid review to inform vaccine prioritisation in Canada. BMJ Open 2021, 11, e044684. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Melamed, R.; Paz, F.; Jepsen, S.; Smith, C.; Saavedra, R.; Mulder, M.; Masood, A.; Huelster, J.; Kirkland, L.; Guenther, A.; et al. Prognostic factors and outcomes in COVID-19 patients requiring prolonged mechanical ventilation: A retrospective cohort study. Ther. Adv. Respir. Dis. 2022, 16, 17534666221086415. [Google Scholar] [CrossRef]
- Izcovich, A.; Ragusa, M.A.; Tortosa, F.; Lavena Marzio, M.A.; Agnoletti, C.; Bengolea, A.; Ceirano, A.; Espinosa, F.; Saavedra, E. Prognostic factors for severity and mortality in patients infected with COVID-19: A systematic review. PLoS ONE 2020, 15, e0241955. [Google Scholar] [CrossRef]
- Cugnata, F.; Scarale, M.G.; De Lorenzo, R.; Simonini, M.; Citterio, L.; Querini, P.R.; Castagna, A.; Di Serio, C.; Lanzani, C. Profiling COVID-19 patients with respect to level of severity: An integrated statistical approach. Sci. Rep. 2023, 13, 5498. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Rajput, R.; Bhatia, M.; Arora, P.; Sood, V. Clinical Predictors of COVID-19 Severity and Mortality: A Perspective. Front. Cell. Infect. Microbiol. 2021, 11, 674277. [Google Scholar] [CrossRef]
- Liu, F.; Long, X.; Zhang, B.; Zhang, W.; Chen, X.; Zhang, Z. ACE2 Expression in Pancreas May Cause Pancreatic Damage After SARS-CoV-2 Infection. Clin. Gastroenterol. Hepatol. 2020, 18, 2128–2130.e2. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Xie, Y. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021, 594, 259–264. [Google Scholar] [CrossRef]
- Capes, S.E.; Hunt, D.; Malmberg, K.; Gerstein, H.C. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: A systematic overview. Lancet 2000, 355, 773–778. [Google Scholar] [CrossRef]
- Mondal, S.; DasGupta, R.; Lodh, M.; Garai, R.; Choudhury, B.; Hazra, A.K.; Mondal, A.; Ganguly, A. Stress hyperglycemia ratio, rather than admission blood glucose, predicts in-hospital mortality and adverse outcomes in moderate-to severe COVID-19 patients, irrespective of pre-existing glycemic status. Diabetes Res. Clin. Pract. 2022, 190, 109974. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Singh, R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020, 167, 108382. [Google Scholar] [CrossRef]
- Zahedi, M.; Kordrostami, S.; Kalantarhormozi, M.; Bagheri, M. A Review of Hyperglycemia in COVID-19. Cureus 2023, 15, e37487. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Uhl, S.; Zhang, T.; Xue, D.; Li, B.; Vandana, J.J.; Acklin, J.A.; Bonnycastle, L.L.; Narisu, N.; Erdos, M.R.; et al. SARS-CoV-2 infection induces beta cell transdifferentiation. Cell Metab. 2021, 33, 1577–1591.e7. [Google Scholar] [CrossRef]
- Wu, C.T.; Lidsky, P.V.; Xiao, Y.; Lee, I.T.; Cheng, R.; Nakayama, T.; Jiang, S.; Demeter, J.; Bevacqua, R.J.; Chang, C.A.; et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021, 33, 1565–1576.e5. [Google Scholar] [CrossRef]
- Stehouwer, C.D.A. Microvascular Dysfunction and Hyperglycemia: A Vicious Cycle with Widespread Consequences. Diabetes 2018, 67, 1729–1741. [Google Scholar] [CrossRef]
| Cohort | Patients with Diabetes | Patients with Obesity | Patients without Diabetes or Obesity | |||
|---|---|---|---|---|---|---|
| Characteristics | Frequency (n) | Mean/SD | Frequency (n) | Mean/SD | Frequency (n) | Mean/SD |
| Sex (male/female) | 165/105 | 107/94 | 369/262 | |||
| Age (years) | 73.45/12.31 | 59.69/16.33 | 63.85/18.34 | |||
| BMI (kg/m2) | 25.54/2.93 | 34.24/4.35 | 25.64/2.79 | |||
| Plasma glucose (mg/dL) | 177.92/82.37 | 126.22/45.06 | 119.73/35.08 | |||
| WBC count (#/μL) | 8205.65/7312.56 | 7218.68/3762.28 | 7244.40/4032.51 | |||
| NLR | 7.91/11.50 | 6.19/5.08 | 7.41/7.93 | |||
| CRP (mg/L) | 118.05/121.33 | 124.11/117.38 | 112.02/115.81 | |||
| PCT (ng/mL) | 0.73/3.08 | 1.36/11.04 | 0.53/2.24 | |||
| IL-6 (pg/mL) | 61.32/156.61 | 34.52/39.38 | 56.90/174.61 | |||
| PFR | 264.73/123.21 | 260.56/109.97 | 281.78/123.93 | |||
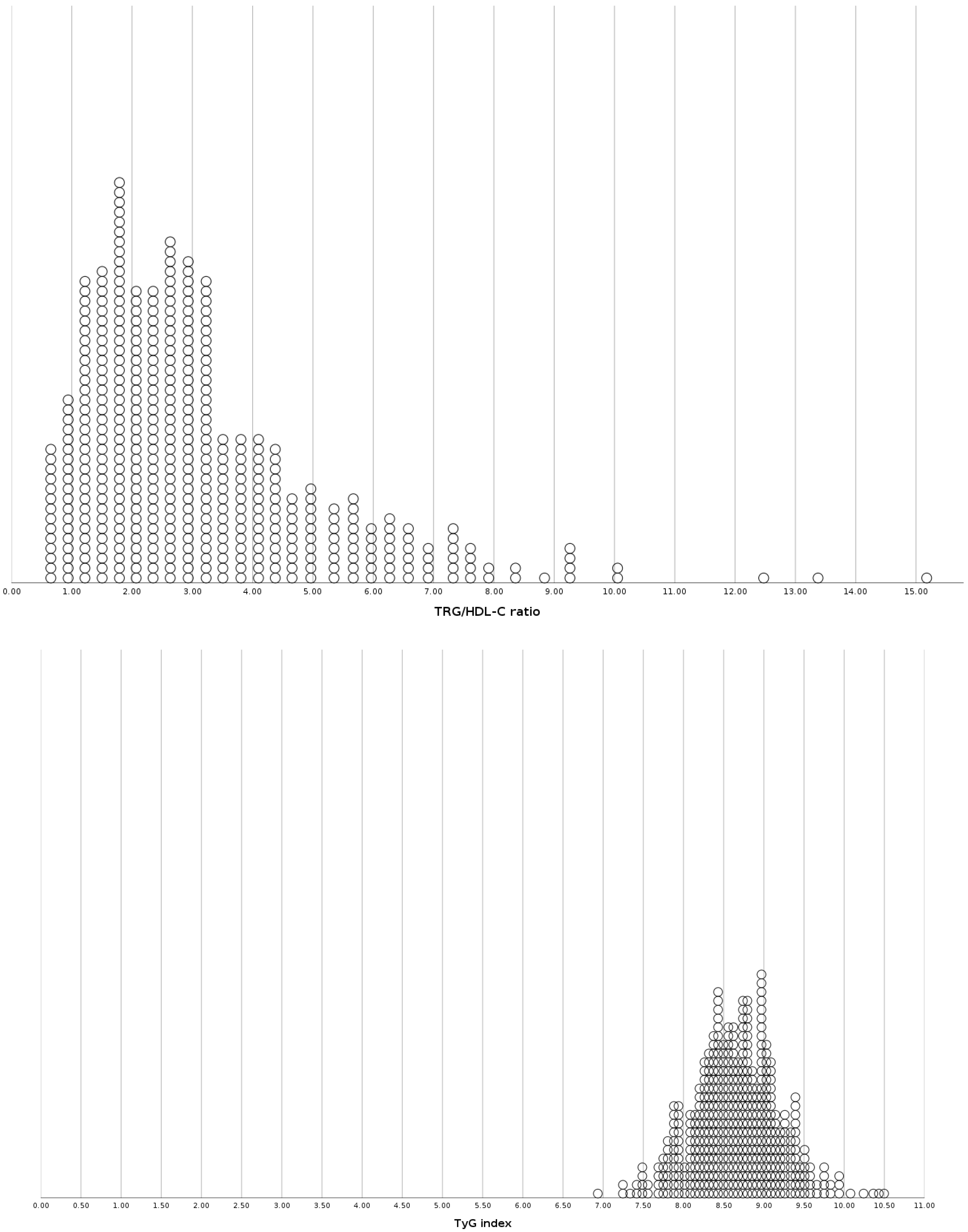
| TRG/HDL-C ratio | 4.66/8.85 | 3.51/2.92 | 3.20/2.18 | |||
| TyG index | 9.11/0.72 | 8.75/0.59 | 8.66/0.55 | |||
| Hyperglycemia on admission | 165 | 46 | 118 | |||
| Hyperglycemia during hospitalization | 229 | 100 | 317 | |||
| Comorbidities | ||||||
| CAD | 80 | 21 | 118 | |||
| AH | 192 | 86 | 248 | |||
| Dyslipidemia | 153 | 51 | 191 | |||
| COPD | 29 | 15 | 45 | |||
| Thyroid disease | 31 | 27 | 64 | |||
| Autoimmune disease | 15 | 14 | 43 | |||
| Cancer | 30 | 9 | 58 | |||
| Smoking (active) | 36 | 25 | 88 | |||
| COVID-19 vaccination | 124 | 67 | 186 | |||
| Outcomes | ||||||
| LoS > 7 days | 145 | 111 | 320 | |||
| Intubation | 14 | 5 | 31 | |||
| Patient death | 39 | 12 | 68 | |||
| Outcomes | LoS > 7 Days | Intubation | Death | |||
|---|---|---|---|---|---|---|
| Variables | OR | p-Value | OR | p-Value | OR | p-Value |
| WBC count (#/μL) | 1.00 | 0.407 | 1.000 | 0.959 | 1.000 | 0.004 |
| NLR | 1.01 | 0.235 | 1.002 | 0.951 | 1.035 | 0.005 |
| CRP (mg/L) | 1.01 | 0.186 | 1.003 | 0.028 | 1.004 | 0.001 |
| PCT (ng/mL) | 0.985 | 0.763 | 0.967 | 0.846 | 0.956 | 0.663 |
| IL-6 (pg/mL) | 1.004 | 0.074 | 1.002 | 0.038 | 1.002 | 0.078 |
| PFR | 0.996 | 0.001 | 0.990 | 0.005 | 0.994 | 0.002 |
| CT BoD | 4.980 | <0.001 | 17.634 | 0.009 | 2.753 | 0.057 |
| NLR > 3.1 | 1.099 | 0.737 | 2.36 | 0.271 | 2.932 | 0.035 |
| CRP > 100 | 1.787 | 0.022 | 3.321 | 0.051 | 1.913 | 0.073 |
| PCT > 0.5 | 1.554 | 0.278 | 2.654 | 0.198 | 1.876 | 0.189 |
| IL-6 > 24 | 2.840 | 0.001 | 2.925 | 0.129 | 5.582 | <0.001 |
| PFR < 200 | 4.413 | <0.001 | 6.636 | 0.007 | 5.984 | <0.001 |
| Hyperglycemia on admission | 1.719 | 0.034 | 1.964 | 0.389 | 1.896 | 0.111 |
| Hyperglycemia during hospitalization | 3.704 | 0.001 | 0.995 | 0.995 | 1.097 | 0.861 |
| TRG/HDL-C ratio | 1.209 | 0.005 | 0.992 | 0.890 | 0.999 | 0.973 |
| TyG index | 2.065 | 0.003 | 1.492 | 0.383 | 1.784 | 0.039 |
| Outcomes | LoS > 7 Days | Intubation | Death | |||
|---|---|---|---|---|---|---|
| Variables | OR | p-Value | OR | p-Value | OR | p-Value |
| WBC count (#/μL) | 1.000 | 0.189 | 1.000 | 0.221 | 1.000 | 0.144 |
| NLR | 1.055 | 0.087 | 0.947 | 0.630 | 1.051 | 0.373 |
| CRP (mg/L) | 1.003 | 0.021 | 1.00 | 0.927 | 1.000 | 0.956 |
| PCT (ng/mL) | 0.929 | 0.354 | 0.199 | 0.700 | 1.041 | 0.112 |
| IL-6 (pg/mL) | 1.012 | 0.032 | 1.000 | 0.984 | 1.006 | 0.451 |
| PFR | 0.995 | 0.001 | 0.980 | 0.023 | 0.991 | 0.025 |
| CT BoD | 9.938 | <0.001 | * | * | 1.311 | 0.835 |
| NLR > 3.1 | 2.179 | 0.019 | 1.312 | 0.812 | 4.933 | 0.148 |
| CRP > 100 | 1.624 | 0.096 | 1.521 | 0.653 | 1.314 | 0.674 |
| PCT > 0.5 | 0.731 | 0.572 | * | * | 1.658 | 0.605 |
| IL-6 > 24 | 1.831 | 0.063 | 3.226 | 0.317 | 2.383 | 0.248 |
| PFR < 200 | 3.073 | 0.001 | * | * | 4.750 | 0.025 |
| Hyperglycemia on admission | 1.442 | 0.333 | * | * | 0.509 | 0.433 |
| Hyperglycemia during hospitalization | 2.316 | 0.009 | * | * | 0.587 | 0.467 |
| TRG/HDL-C ratio | 0.948 | 0.376 | 0.812 | 0.582 | 1.134 | 0.125 |
| TyG index | 1.292 | 0.374 | 0.542 | 0.432 | 0.818 | 0.756 |
| Outcomes | LoS > 7 Days | Intubation | Death | |||
|---|---|---|---|---|---|---|
| Variables | OR | p-Value | OR | p-Value | OR | p-Value |
| WBC count (#/μL) | 1.000 | 0.346 | 1.000 | 0.259 | 1.000 | 0.002 |
| NLR | 1.024 | 0.034 | 1.038 | 0.024 | 1.062 | <0.001 |
| CRP (mg/L) | 1.003 | 0.001 | 1.004 | 0.002 | 1.005 | <0.001 |
| PCT (ng/mL) | 1.061 | 0.272 | 1.047 | 0.448 | 1.156 | 0.008 |
| IL-6 (pg/mL) | 1.000 | 0.858 | 1.001 | 0.087 | 1.003 | <0.001 |
| PFR | 0.997 | <0.001 | 0.989 | <0.001 | 0.994 | <0.001 |
| CT BoD | 4.757 | <0.001 | 28.430 | <0.001 | 5.535 | <0.001 |
| NLR > 3.1 | 1.511 | 0.022 | 5.552 | 0.021 | 5.724 | <0.001 |
| CRP > 100 | 2.179 | <0.001 | 2.579 | 0.015 | 3.022 | <0.001 |
| PCT > 0.5 | 1.393 | 0.279 | 2.263 | 0.109 | 4.742 | <0.001 |
| IL-6 > 24 | 2.323 | <0.001 | 2.496 | 0.041 | 3.705 | <0.001 |
| PFR < 200 | 2.442 | <0.001 | 5.100 | <0.001 | 3.546 | <0.001 |
| Hyperglycemia on admission | 1.023 | 0.916 | 2.390 | 0.029 | 1.667 | 0.103 |
| Hyperglycemia during hospitalization | 1.685 | 0.002 | 3.352 | 0.010 | 1.425 | 0.226 |
| TRG/HDL-C ratio | 1.249 | <0.001 | 1.186 | 0.023 | 1.369 | <0.001 |
| TyG index | 2.418 | <0.001 | 2.983 | 0.002 | 3.203 | <0.001 |
| Group of Patients | Patients with Diabetes | Patients with Obesity | Patients without Diabetes or Obesity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycemic Status | Hyperglycemia on Admission | Hyperglycemia during Hospitalization | Hyperglycemia on Admission | Hyperglycemia during Hospitalization | Hyperglycemia on Admission | Hyperglycemia during Hospitalization | ||||||
| Variables | OR | p-Value | OR | p-Value | OR | p-Value | OR | p-Value | OR | p-Value | OR | p-Value |
| WBC count (#/μL) | 1.000 | 0.161 | 1.000 | 0.916 | 1.000 | 0.068 | 1.000 | 0.995 | 1.000 | < 0.001 | 1.000 | 0.018 |
| NLR | 1.105 | 0.001 | 1.079 | 0.083 | 1.068 | 0.038 | 1.041 | 0.193 | 1.066 | < 0.001 | 1.021 | 0.075 |
| CRP (mg/L) | 1.002 | 0.105 | 1.000 | 0.753 | 1.000 | 0.980 | 1.002 | 0.236 | 1.002 | 0.004 | 1.002 | 0.042 |
| PCT (ng/mL) | 1.256 | 0.236 | 3.472 | 0.186 | 0.797 | 0.541 | 0.507 | 0.175 | 1.004 | 0.934 | 1.012 | 0.792 |
| IL-6 (pg/mL) | 1.001 | 0.561 | 1.003 | 0.388 | 1.002 | 0.690 | 0.999 | 0.740 | 1.000 | 0.381 | 1.000 | 0.718 |
| PFR | 0.998 | 0.110 | 0.997 | 0.065 | 0.998 | 0.200 | 1.000 | 0.799 | 0.997 | 0.008 | 1.000 | 0.595 |
| CT BoD > 50% | 1.220 | 0.608 | 7.482 | 0.059 | 1.643 | 0.254 | 1.221 | 0.604 | 1.586 | 0.119 | 1.316 | 0.255 |
| NLR > 3.1 | 0.997 | 0.992 | 0.755 | 0.499 | 1.885 | 0.172 | 1.260 | 0.512 | 2.402 | 0.002 | 1.369 | 0.105 |
| CRP > 100 mg/L | 1.638 | 0.060 | 1.793 | 0.116 | 0.716 | 0.350 | 1.321 | 0.357 | 1.882 | 0.003 | 1.441 | 0.035 |
| PCT > 0.5 ng/mL | 2.162 | 0.080 | 2.786 | 0.186 | 0.711 | 0.630 | 0.206 | 0.015 | 1.516 | 0.215 | 0.997 | 0.993 |
| IL-6 > 24 pg/mL | 0.919 | 0.782 | 1.239 | 0.634 | 0.745 | 0.453 | 0.900 | 0.752 | 0.583 | 0.027 | 0.807 | 0.283 |
| PFR < 200 | 1.633 | 0.105 | 3.285 | 0.022 | 1.124 | 0.760 | 1.000 | 1.000 | 2.131 | 0.001 | 1.352 | 0.139 |
| TRG/HDL-C ratio | 0.992 | 0.649 | 1.313 | 0.203 | 1.048 | 0.441 | 0.968 | 0.582 | 1.007 | 0.890 | 1.064 | 0.200 |
| TyG index | 13.838 | <0.001 | 4.005 | 0.001 | 34.014 | <0.001 | 4.190 | <0.001 | 9.408 | <0.001 | 3.612 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liontos, A.; Biros, D.; Kavakli, A.; Matzaras, R.; Tsiakas, I.; Athanasiou, L.; Samanidou, V.; Konstantopoulou, R.; Vagias, I.; Panteli, A.; et al. Glycemic Dysregulation, Inflammation and Disease Outcomes in Patients Hospitalized with COVID-19: Beyond Diabetes and Obesity. Viruses 2023, 15, 1468. https://doi.org/10.3390/v15071468
Liontos A, Biros D, Kavakli A, Matzaras R, Tsiakas I, Athanasiou L, Samanidou V, Konstantopoulou R, Vagias I, Panteli A, et al. Glycemic Dysregulation, Inflammation and Disease Outcomes in Patients Hospitalized with COVID-19: Beyond Diabetes and Obesity. Viruses. 2023; 15(7):1468. https://doi.org/10.3390/v15071468
Chicago/Turabian StyleLiontos, Angelos, Dimitrios Biros, Aikaterini Kavakli, Rafail Matzaras, Ilias Tsiakas, Lazaros Athanasiou, Valentini Samanidou, Revekka Konstantopoulou, Ioannis Vagias, Aikaterini Panteli, and et al. 2023. "Glycemic Dysregulation, Inflammation and Disease Outcomes in Patients Hospitalized with COVID-19: Beyond Diabetes and Obesity" Viruses 15, no. 7: 1468. https://doi.org/10.3390/v15071468
APA StyleLiontos, A., Biros, D., Kavakli, A., Matzaras, R., Tsiakas, I., Athanasiou, L., Samanidou, V., Konstantopoulou, R., Vagias, I., Panteli, A., Pappa, C., Kolios, N.-G., Nasiou, M., Pargana, E., Milionis, H., & Christaki, E. (2023). Glycemic Dysregulation, Inflammation and Disease Outcomes in Patients Hospitalized with COVID-19: Beyond Diabetes and Obesity. Viruses, 15(7), 1468. https://doi.org/10.3390/v15071468









